Abstract
Background and Purpose
The antibiotic azithromycin is a suggested alternative to erythromycin for treating patients with delayed gastric emptying. However, although hypothesized to activate motilin receptors, supportive evidence is unavailable. This was investigated using recombinant and naturally expressed motilin receptors in human stomach, comparing azithromycin with erythromycin.
Experimental Approach
[125I]-motilin binding and calcium flux experiments were conducted using human recombinant motilin receptors in CHO cells. Neuromuscular activities were studied using circular muscle of human gastric antrum, after electrical field stimulation (EFS) of intrinsic nerves.
Key Results
Azithromycin (1–100 μM) and erythromycin (3–30 μM) concentration-dependently displaced [125I]-motilin binding to the motilin receptor (52 ± 7 and 58 ± 18% displacement at 100 and 30 μM respectively). Azithromycin, erythromycin and motilin concentration-dependently caused short-lived increases in intracellular [Ca2+] in cells expressing the motilin receptor. EC50 values were, respectively, 2.9, 0.92 and 0.036 μM (n = 3 each); and maximal activities were similar. In human stomach, EFS evoked cholinergically mediated contractions, attenuated by simultaneous nitrergic activation. Azithromycin and erythromycin lactobionate (30–300 μM each) facilitated these contractions (apparent Emax values of 2007 ± 396 and 1924 ± 1375%, n = 3–4 each concentration, respectively). These actions were slow in onset and faded slowly. The higher concentrations also evoked short-lived muscle contraction. Contractions to a submaximally effective concentration of carbachol were unaffected by either drug.
Conclusions and Implications
Azithromcyin activates human recombinant motilin receptors in therapeutically relevant concentrations, similar to erythromycin. In humans, gastric antrum azithromycin caused long-lasting facilitation of cholinergic activity. These actions explain the gastric prokinetic activity of azithromycin.
Keywords: azithromycin, erythromycin, motilin, human, stomach
Introduction
Azithromycin is an antibiotic drug with additional properties, including immunomodulatory activity (Vrančić et al., 2012) and an ability to stimulate gastro-duodenal motility (Sifrim et al., 1994; Chini et al., 2012). Furthermore, azithromycin is increasingly suggested as a potential treatment of upper gastrointestinal (GI) motility disorders such as gastroesophageal reflux, or as a means of reducing esophageal acid exposure in patients receiving lung transplant or suffering from hiatus hernia (Mertens et al., 2009; Rohof et al., 2012). Notably, the activity of azithromycin did not appear to fade over a prolonged period of dosing (Mertens et al., 2009). However, the mechanisms by which this complex drug stimulates gastric motility are unknown.
Other antibiotic drugs, which are structurally related to the basic macrolide template (14-member macrocyclic lactone ring attached to two deoxy sugars) and which also increase gastric motility and/or induce GI symptoms, are often said to activate the motilin receptor; indeed, for some molecules, evidence is available to support such claims (Omura, 2002; Abu-Gharbieh et al., 2004) This follows the original discovery that the macrolide erythromycin is an effective agonist at the motilin receptor (Peeters et al., 1989), now recognized as the mechanism by which this drug stimulates gastric emptying. Furthermore, at doses that are usually lower than those used when erythromycin is given as an antibiotic agent, the drug can relieve symptoms in patients with gastroparesis, help control blood glucose levels in diabetic patients, facilitate enteral feeding and assist endoscopy procedures requiring rapid intubation or prior removal of gastric contents (Sanger, 2008; Chen and Tsai, 2012). However, erythromycin has other actions. Clinically, the drug may provoke cardiac proarrhythmia and inhibit cytochrome P450 activity (Abu-Gharbieh et al., 2004). In other studies, erythromycin has been shown to inhibit both neuronal and purinergic P2X channel functions (Furness et al., 1999; Zhao et al., 2000). The search for an alternative gastric prokinetic agent is, therefore, of high importance (Sanger and Alpers, 2008).
For some macrolide-based antibiotic drugs that could be used instead of erythromycin, such as azithromycin in particular, the evidence to support an action at the motilin receptor is surprisingly weak or absent. However, if azithromycin is to be used clinically as an alternative gastric prokinetic agent, it is essential to understand its mechanisms of action. A classic method of investigating how drugs might activate the motilin receptor is to transfect the human motilin receptor into a host cell and measure changes in intracellular calcium evoked in response to the drug (Thielemans et al., 2005). However, increasing evidence is emerging to indicate that this method is insufficient to characterize how drugs interact with the motilin receptor that is expressed in its natural environment. For example, motilin receptor agonists usually appear to have short-lasting activity at the recombinant receptor yet some show sustained ability to increase gastric emptying in human volunteers when repeatedly dosed, and/or long-lasting ability to facilitate cholinergic activity in isolated stomach preparations (Westaway and Sanger, 2009; Sanger et al., 2012; see below).
The primary mechanism by which motilin receptor agonists are thought to stimulate gastrointestinal motility is by facilitation of ACh release from the cholinergic motor nerves of the gut (Coulie et al., 1998; Dass et al., 2003; Sanger, 2008). This has been demonstrated in vitro using human (Broad et al., 2012) and rabbit (Van Assche et al., 1997; Dass et al., 2003; Jarvie et al., 2007; Sanger et al., 2009) isolated stomach, in which motilin receptor agonists are shown to enhance the amplitude of cholinergically mediated contractions evoked by electrical stimulation of the intrinsic nerves. Surprisingly, different motilin receptor agonists achieve this facilitation in different ways, with long-lasting actions of erythromycin and GSK962040 (a selective, small molecule motilin receptor agonist) contrasting markedly with shorter-lasting activity of motilin (Dass et al., 2003; Jarvie et al., 2007; Sanger et al., 2009; Broad et al., 2012). Thus, for azithromycin, it is now important to ask two questions. First, is this drug a motilin receptor agonist? Second, if motilin receptor agonist activity is proved, how might such activity translate in terms of either a short- or long-lasting ability to facilitate cholinergic activity in the human stomach? To address these questions, comparisons were also made with the actions of erythromycin.
Methods
Nomenclature
The term ‘motilin receptor’ has been used and not the earlier names ‘GPR38’ or ‘MTLR1’, in accordance with agreed nomenclature (Alexander et al., 2011).
Binding studies with the recombinant human motilin receptor
Studies were conducted at a contract research organization (CEREP, Paris, France), using [125I]-motilin (0.05 nM; Kd 0.26 nM), CHO cells stably expressing the human motilin receptor and a modified method of Feighner et al. (1999). Compounds were dissolved in ethanol (final solvent concentration kept constant) and incubated with the cells for 120 min at room temperature. Displacement of binding was assessed using scintillation counting, with non-specific binding determined using 1 μM [13Nleu]-motilin. The results are expressed as a % inhibition of control-specific binding obtained in the presence of the test compounds.
Functional studies with the recombinant human motilin receptor
CHO-K1 cells stably expressing the human motilin receptor were obtained as a gift from GlaxoSmithKline, and parental cells were obtained from ACTT (LCG standards, Middlesex, UK). The cells were grown to 90% confluence in incubators at 37°C and passaged every 3–4 days. The cells expressing motilin were grown in DMEM/F12 + Glutamax (Invitrogen, Paisley, UK), containing 10% FBS (Invitrogen) and 1 mg·mL−1 geneticin (Invitrogen). Parental cells were grown in F12 Hams (Invitrogen) containing 10% FBS (Invitrogen).
On the day of the assay, the cells were removed from the flasks and re-suspended in fluo-4 direct calcium assay kit assay buffer (Invitrogen) containing probenicid; 100 μL of cells was seeded at 100 000 cells per well into flat black-walled clear-bottomed 96-well plates. Following 10 min incubation at 37°C, 100 μL fluo-4 direct calcium assay reagent was added to the wells. The cells were then incubated for 60 min at 37°C; 20 μL test compound was added prior to reading on a fluorescent plate reader, an excitation wavelength of 494 nm and an emission wavelength of 516 nm. Assays were performed in triplicate for each concentration. N = number of assays. All compounds were prepared from stock solutions in ethanol (azithromycin, erythromycin) or H2O (motilin) and diluted in PBS.
Functional studies in human isolated stomach
Segments of human stomach were obtained from patients undergoing surgery for obesity or cancer. The study was approved by the local ethics committee (REC reference number 10/H0703/71, SSA reference number 10/H0703/76), and written informed consent was obtained from all patients. The segments were transferred to the research laboratories within 2 h after resection in Krebs' solution (containing in mM: NaCl 121.5, CaCl2 2.5, KH2PO4 1.2, KCl 4.7, MgSO4 1.2, NaHCO3 25, glucose 5.6) equilibrated with 5% CO2 and 95% O2.
Immediately on arrival in the laboratory, segments were cut open, and the mucosa was removed by blunt dissection and discarded. The methods used to prepare and electrically stimulate the stomach have previously been described (Broad et al., 2012). In brief, strips were cut parallel to the circular muscle fibres and used immediately or after overnight storage at 4°C in fresh, oxygenated Krebs' solution. The strips were mounted in tissue baths containing Krebs' solution (37°C; gassed with 5% CO2 in O2), and changes in muscle tension were recorded using isometric force transducers (AD Instruments, Chalgrove, UK) and a data acquisition system (Biopac Systems Inc., Goleta, CA, USA). The strips were given 2 g tension and allowed to recover for 60 min (with bath solutions changed every 15 min) before being stimulated via two parallel platinum ring electrodes connected to a stimulator (STG2008, Scientifica, Uckfield, UK). The stimulation parameters were 50 V (c. 200 mA), 0.5 ms bipolar pulse duration, 5 Hz, given for 10 s every 1 min. Electrical field stimulation (EFS) was applied continuously until consistent responses were obtained, during which time the bath solution was changed every 15 min.
The effects of azithromycin and erythromycin were investigated by using only a single concentration in each strip. Changes in amplitude of responses to EFS were determined by measuring at least three EFS-induced responses at a given time point and were expressed as a percentage of the mean of at least three pre-drug EFS-induced responses (100%). Any effect of azithromycin and erythromycin on baseline tension was expressed as a % of the pre-drug EFS-induced contraction. To investigate the effects of the drugs on contractions evoked by carbachol, a preliminary concentration–effect curve was established (data not shown) and 1 μM carbachol selected as the concentration that evoked a contraction approximately 50% of maximum. In subsequent experiments, consistent responses were obtained with 1 μM carbachol (5 min contact time followed by washout, repeated at 15 min intervals). Fifteen minutes before the last application, azithromycin, (300 μM) or erythromycin (30 or 100 μM) was added to the bath.
Statistics
Data are expressed as medians and ranges or as the mean ± SEM; n values are the numbers of patients. Data were analysed using GraphPad Prism 5. Curves were fitted using a three-parameter (log) agonist response curve. The binding assay was analysed using a one-site fit Ki curve. Differences between the medians were determined using the Mann–Whitney U-test. Concentration-dependent effects between the drugs were compared using two-way anova with Bonferroni post tests. Changes from baseline EFS were compared using paired t-tests. P < 0.05 is considered as statistically significant.
Drugs
All drugs were freshly prepared prior to use. Azithromycin (Tocris, UK) was dissolved in ethanol to 100 mM. Erythromycin (Sigma, UK) was dissolved in ethanol to 10 mM. Erythromycin lactobionate (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was dissolved in ethanol to 100 mM. Motilin (Tocris), was dissolved in distilled water (dH2O) at 100 μM. Carbachol was dissolved in dH2O.
Results
Binding to the recombinant human motilin receptor
Azithromycin and erythromycin (1–100 and 3–30 μM, respectively) displaced [125I]-motilin binding to the human recombinant receptor in a concentration-dependent manner (Figure 1). At the highest concentrations tested (100 and 30 μM, respectively), binding was displaced by 52 ± 7% and 58 ± 18% (n = 3 each). Ki values were 20 and 19 μM respectively.
Figure 1.
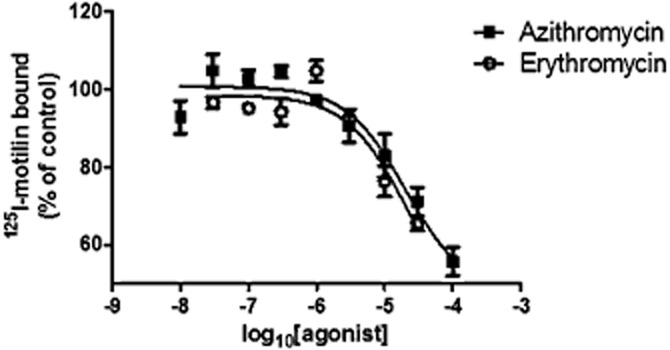
Radioligand binding studies were conducted using [125I]-motilin and CHO cells stably expressing the human motilin receptor; non-specific binding was determined using 1 μM [13Nleu]-motilin. The results are expressed as a % inhibition of control-specific binding obtained in the presence of azithromycin or erythromycin, performed in triplicate.
Functional studies with the recombinant human motilin receptor
Azithromycin caused concentration-dependent increases in intracellular [Ca2+] in cells expressing the motilin receptor and had no effect in parental cells. The EC50 was 2.9 μM (n = 3), and the relative efficacy was 121 ± 15% compared with 300 nM motilin (Figure 2). Similarly, erythromycin (EC50 0.92 μM, relative efficacy 98 ± 9%, n = 3) and motilin (EC50 36 nM, n = 3) caused concentration-dependent increases in intracellular [Ca2+] in cells expressing the motilin receptor, with no effects in parental cells. The vehicles used did not affect intracellular [Ca2+] (data not shown; n = 3). Notably, the responses to each of the motilin receptor agonists were not maintained during the continued presence of the agonists, the times taken for the responses to azithromycin, erythromycin and motilin to fade by 50% (t½) being, respectively, 34 ± 5 s, 36 ± 1 s and 39 ± 4 s (at the maximally effective concentrations of, respectively, 30 μM, 30 μM and 300 nM; n = 3 each).
Figure 2.
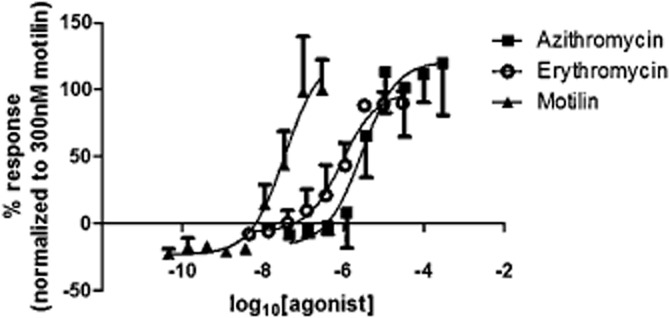
The effect of azithromycin (0.03–300 μM), erythromycin (0.003–30 μM) and motilin (0.03–300 nM) on CHO-K1 cells transfected with the human motilin receptor. Responses are measured as the maximum change in fluorescence in response to compound application, expressed as a percentage of the response to 300 nM motilin. There was no response to the agonists in the parental cell line. Each concentration was performed in triplicate in the assay plate, and each experiment was performed three times.
Human tissue characterization
Table 1 summarizes the patient details. Overall, the median age of patients undergoing bariatric surgery for obesity was 46 years (range 25–86; n = 35), and the male to female ratio was 1:3.9; the median age of patients undergoing surgery for cancer was 58 (40–75; n = 4), and the male to female ratio was 1:1. EFS (5 Hz, given for 10 s every 1 min) was applied during the recovery phase as previously described (Broad et al., 2012). For tissues studied on the day of surgery or after overnight storage, consistent responses were achieved, respectively, 186 (range: 104–314) and 256 (103–425 min) (n = 13 [80 strips] and 32 [205 strips] patients; different strips from six patients were used fresh or after overnight storage) after suspension in the tissue baths. This difference was not statistically significant (P = 0.06; Mann–Whitney U-test). Following recovery and using identical conditions, responses to EFS have previously been shown to be prevented by application of 1 μM tetrodotoxin and 1 μM atropine, and increased by 300 μM l-NAME (Broad et al., 2012). In these experiments, the contractions evoked by EFS 5 Hz was 3.5 ± 1.7% of that evoked by a maximally effective concentration of carbachol (10 μM, n = 4; data not shown).
Table 1.
Details of patients and tissues used in the functional studies
| Region (n) | Disease | Fresh/overnight | n (strips) | Male : Female ratio | Median age (years) | Recovery time (min) |
|---|---|---|---|---|---|---|
| Antrum | Obesity | Fresh | 13 (80) | 1:3.3 | 50 (30–72) | 186 (104–314) |
| Overnight | 28 (179) | 1:3.5 | 46 (25–86) | 241 (103–411) | ||
| Cancer | Overnight | 4 (26) | 1:1 | 58 (40–75) | 333.5 (325–425) |
Recovery times are defined as the time taken to achieve consistent responses to 5 Hz EFS following suspension in the tissue baths. Data are expressed as median values with ranges in parenthesis, obtained using preparations from 35 patients with obesity and 4 patients with cancer. Tissues from some patients were used on the day of surgery and after overnight storage; n = number of patients with numbers of strips in parenthesis.
Responses to azithromycin and erythromycin in human gastric antrum
There was no effect of the vehicle used to dissolve the maximally effective concentrations of both drugs (ethanol; 0.3% final volume) on baseline muscle tension or on the magnitude of the contractions evoked by EFS (a change of −3 ± 15% initial EFS-evoked contraction; n = 3; P > 0.05, paired t-test).
Azithromycin 30–300 μM concentration-dependently facilitated EFS-evoked contractions (Figures 3 and 4; Table 2), with an apparent Emax of 2007 ± 396% at 300 μM; lower concentrations (0.1–10 μM) had no consistent activity. This excitatory action of azithromycin was slow in onset and, after reaching maximal activity, began to slowly fade during the continued presence of the drug (Figure 3; Table 2). For example, at 300 μM, the time to reach maximum was 45 ± 6 min, and the time for this action to subsequently decline by 50% (t½) was 22 ± 3 min. Interestingly, the fade of activity did not occur in a regular manner, with large and small EFS-evoked contractions appearing at irregular intervals in strips from three of three patients investigated at 300 μM and in two of five patients investigated at 100 μM (Figure 3 for 300 μM). In addition, the higher concentrations of 100 and 300 μM also evoked a relatively short-lasting muscle contraction (equivalent to respectively, 89 ± 22% and 463 ± 149% of the contraction amplitude evoked by EFS prior to addition of azithromycin; Table 2), which appeared disconnected from the increase in EFS-evoked contractions, reaching maximum more quickly (e.g. 13 ± 1 min at 300 μM) and fading completely before the maximum enhancement of the EFS-evoked contraction had occurred (e.g. after 15 ± 1 min at 300 μM). Finally, azithromycin (300 μM) had no effect on the magnitude of contractions evoked by the submaximally effective concentration of carbachol (1 μM; contractions were 78 ± 10% of the responses before azithromycin addition; n = 3, P > 0.05 paired t-test).
Figure 3.
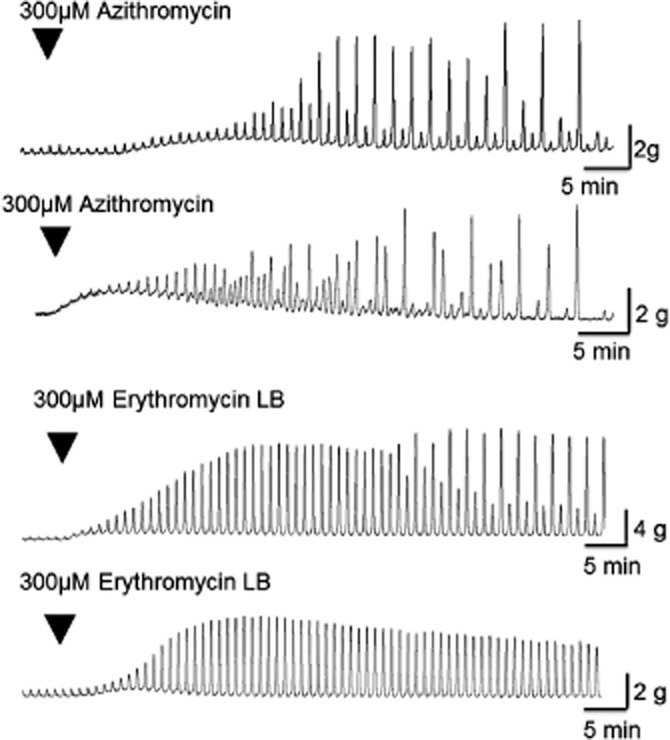
Trace examples showing the abilities of azithromycin (300 μM) and erythromycin lactobionate (300 μM) to increase cholinergically mediated contractions evoked by EFS in circular muscle strips from human isolated gastric antrum. The contractions shown are in response to EFS (50 V, 0.5 ms bipolar pulse duration, 5 Hz, given for 10 s, every 1 min), and once added to the bathing solution, the drugs remained in contact with the tissue for the remainder of the experiment. The excitatory action of both drugs was slow in onset and, after reaching maximal activity, began to slowly fade during the continued presence of the drug. Fade occurred in an irregular manner in strips from three of three patients exposed to azithromycin 300 μM and in strips from two of three patients exposed to erythromycin lactobionate 300 μM.
Figure 4.
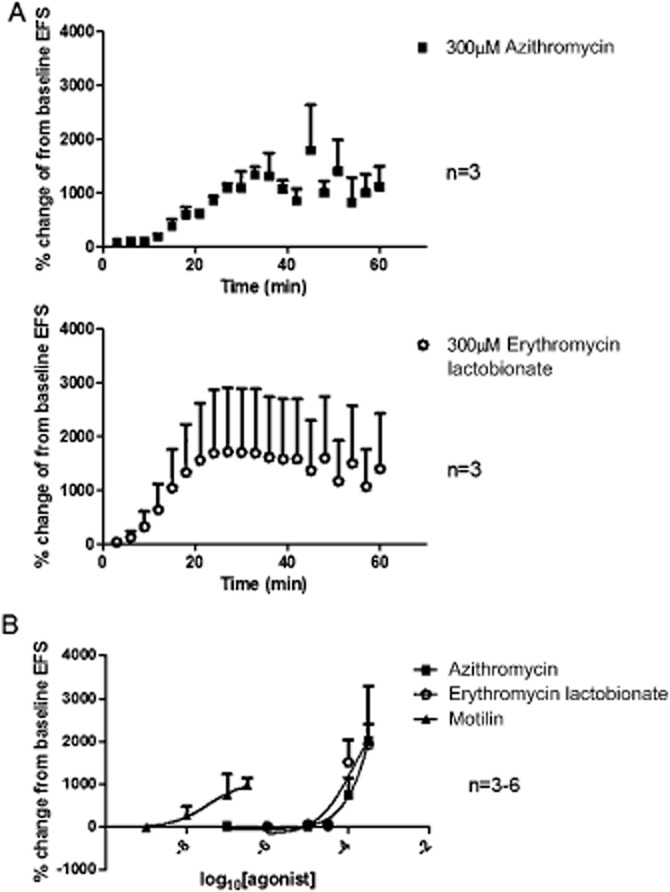
Facilitation by azithromycin and erythromycin lactobionate of cholinergically mediated contractions evoked by EFS in circular muscle preparations of human gastric antrum, shown as time-dependent changes (A) and as concentration–response curves (B). EFS was given at 50 V, 0.5 ms bipolar pulse duration, 5 Hz, given for 10 s, every 1 min. N = number of patients. Panel A shows the time-dependent changes in tension following addition of azithromycin (300 μM) and erythromycin (300 μM). These data were obtained by measuring at least three EFS-evoked responses at a given time points, tending to ‘smooth’ the irregularity of the contractions during the period when the excitatory effects of the drugs are fading. Panel B compares the concentration–response curves for azithromycin erythromycin lactobionate with that to motilin (previously described using similar conditions: Broad et al., 2012; Emax 1041 ± 592%, pEC50 7.5 ± 1.3). In each experiment, only a single concentration of compound was tested and changes in the amplitude of contractions were calculated as a % of at least three contractions recorded immediately before addition of compound (n = 3–5 each concentration).
Table 2.
Excitatory actions of azithromycin and erythromycin in human isolated gastric antrum
| Baseline muscle contraction | Increase in neuronally mediated contraction amplitude | ||||||
|---|---|---|---|---|---|---|---|
| Drug concentration (μM) | Contraction (% baseline EFS) | Time to max (min) | Time to fade | Increase (% baseline EFS) | Time to max (min) | t½ (min) | n |
| Azithromycin | |||||||
| 300 | 463 ± 149* | 13 ± 1 | 15 ± 1 | 2007 ± 396 | 45 ± 6 | 22 ± 3 | 3 |
| 100 | 89 ± 22 | 20 ± 5 | 25 ± 3 | 753 ± 401 | 46 ± 7 | 40 ± 5** | 5 |
| 30 | 5 ± 10 | – | – | 64 ± 27 | 33 ± 6 | 24 ± 3 | 4 |
| 10 | −14 ± 18 | – | – | 6 ± 5 | – | – | 4 |
| Erythromycin lactobionate | |||||||
| 300 | 150 ± 76* | 20 ± 12 | 20† | 1924 ± 1375 | 35 ± 10 | 29 ± 5 | 3 |
| 100 | 87 ± 32 | 22 ± 4 | 12, 29† | 1414 ± 381 | 47 ± 3 | 21, 21†† | 4 |
| 30 | 21 ± 31 | – | – | 31 ± 15 | 38, 39 ¥ | – | 3 |
| 10 | −24 ± 20 | – | – | 54 ± 48 | 48, 68 ¥ | – | 3 |
Data are expressed as mean values ± standard error of the mean; n = number of patients.
Azithromycin is causes a significantly greater increase in baseline muscle tension than erythromycin lactobionate at 300 μM (P < 0.01; two-way anova plus Bonferroni post test). The rest of the parameters were not significantly different between azithromycin and erythromycin lactobionate (P > 0.05; two-way anova plus Bonferroni post test).
n = 4; 1 strip investigated did not fade during the experiment.
n = 1; 2 strips investigated did not fade during the experiment.
n = 2; 2 strips investigated did not fade during the experiment.
n = 2, 1 strip in each did not increase during the experiment.
Erythromycin lactobionate 30–300 μM also facilitated EFS-evoked contractions (Figures 3 and 4; Table 2), with apparent Emax of 1924 ± 1375%; the lower concentration of 10 μM was without consistent effect. As with azithromycin, this activity of erythromycin was slow in onset and then faded slowly during the continuous presence of the drug (e.g. response at 300 μM erythromycin reached maximum 35 ± 10 min after application, with the t½ being reached after 29 ± 5 min); the fade of activity occurred in an irregular manner in strips from two of three patients at 300 μM and in strips from two of four patients at 100 μM (Figure 3 for 300 μM). Erythromycin (100 and 300 μM) also increased baseline muscle tension, equivalent to 87 ± 32% and 150 ± 76% of the contraction amplitude to EFS prior to addition of erythromycin (Figure 3), reached after 22 ± 4 and 20 ± 12 min respectively. However, this effect only faded in three of seven strips investigated at 100–300 μM (Table 2). Erythromycin (30 μM; base) or (100 μM; lacotobionate) had no effects on the magnitude of the contractions evoked by carbachol 1 μM [contractions were, respectively, 100 ± 7% and 92 ± 12% of the contractions before erythromycin addition; n = 3, P > 0.05, paired t–test and n = 2 (4 strips)].
Figure 4 shows the concentration–response curves for azithromycin and erythromycin alongside the curve for motilin (previously reported; Broad et al., 2012).
Discussion
The present experiments support the suggestion but show for the first time that azithromycin can both bind to and activate the human recombinant motilin receptor, stably expressed in CHO cells. Activation was demonstrated by measuring changes in intracellular [Ca2+] evoked by azithromycin in CHO-K1 cells stably transfected with the human motilin receptor. In this assay, both azithromycin and erythromycin acted as full agonists, relative to motilin, and each had similar potency, being approximately 85- and 29-fold less than that of motilin itself respectively. The results also confirm previous studies with erythromycin and motilin in similar motilin receptor-transfected cells (Sanger et al., 2009). Notably, for each of the motilin receptor agonists, the duration of the responses were short-lived (t½ values ranging from 34 to 39 s) and not predictive of the much longer durations of action observed in the human stomach (see below).
Interestingly, the studies using the recombinant receptor also show that the potencies of azithromycin and erythromycin as motilin receptor agonists were greater in the functional studies than that suggested by the radioligand binding experiments. For erythromycin, these findings contrast with Feighner et al. (1999) who found a greater potency in binding studies (using HEK293 cell membranes containing a transient expression of the receptor) compared with functional studies in the same cell type (using an aequorin-based Ca2+ release assay). The reasons for this discrepancy are not clear, although one possibility must be that the use of different expression systems, receptor densities, cell types and assay techniques contribute to the lack of consistency between the different studies. Nevertheless, the experiments with azithromycin fulfil the aim of demonstrating the ability of this drug to bind and activate the human recombinant motilin receptor, prior to investigating its actions at the motilin receptors, which are naturally expressed by the human stomach.
In circular muscle preparations of human and rabbit isolated gastric antrum, different motilin receptor agonists have been shown to facilitate cholinergic activity by acting pre-junctionally to increase ACh release (human: motilin, GSK962040; Broad et al., 2012); rabbit: motilin, erythromycin, GSK962040; Van Assche et al., 1997; Dass et al., 2003; Jarvie et al., 2007; Sanger et al., 2009); this ability to facilitate the actions of the major excitatory motor neurotransmitter of the stomach is thought to be the most likely mechanism by which motilin receptor agonists stimulate gastric emptying (Coulie et al., 1998; Sanger, 2008). In the present experiments, azithromycin (30–300 μM) and erythromycin (3–300 μM) also facilitated cholinergically mediated contractions evoked by EFS in human gastric antrum; the excitatory activity was not matched by significant changes in submaximal contractions to carbachol, indicating a pre-junctional ability to facilitate ACh release. This action of azithromycin is, therefore, consistent with the ability of this drug to stimulate gastric antrum motility in volunteers (Sifrim et al., 1994). Similarly i.v. azithromycin increased gastric emptying in pilot studies on patients with chronic gastrointestinal pain and refractory gastroparesis (Moshiree et al., 2010), with the 500 mg dose evoking a significantly greater increase in the motility index compared with erythromycin 250 mg i.v. Larson et al. (2010) found that azithromycin and erythromycin (250 mg i.v. each; n = 60 patients) both increased gastric emptying in patients with gastroparesis. Enhancement of gastric motility is also consistent with the ability of azithromycin (250 mg, three times a week) to reduce the number of reflux events in 12 lung transplant patients (Mertens et al., 2009).
It needs to be noted that relatively high concentrations of azithromycin and other motilin receptor agonists have an additional ability to directly contract gastrointestinal (GI) muscle; such observations are consistent with the expression of motilin receptors on GI muscle cells in addition to the enteric nervous system (Miller et al., 2000; Broad et al., 2012). Thus, in previous experiments with human and rabbit isolated stomach (see above references), the concentrations of motilin, erythromycin and GSK962040, which caused muscle contractions, were higher than those that facilitated the cholinergically mediated contractions. Furthermore, these contractions were short-lived, compared with the longer ability of erythromycin and GSK962040 to facilitate cholinergic function. Similarly, in the present experiments, azithromycin consistently evoked short-lived contractions only at the higher concentrations tested (100 and 300 μM), which faded before the facilitation of cholinergic activity had reached its maximum. These contractions were not large, compared with the maximum ability of carbachol to cause contraction of the muscle (e.g. the contractions to EFS were only ∼3.5% of the maximum contraction caused by carbachol and the highest concentration of azithromycin tested [300 μM] evoked a contraction which was only ∼4.6 times the contraction evoked by EFS) and have previously been noted in response to azithromycin in rabbit isolated duodenal muscle (Chiragh et al., 2006). For erythromycin, a similar ability to cause muscle contraction was also observed, although compared with azithromycin this activity appeared longer lasting.
The ability of azithromycin to facilitate human gastric cholinergic activity showed interesting similarities and differences compared to motilin. First, the maximum facilitation caused by azithromycin 300 μM (∼2000%) was approximately twice the maximum facilitation caused by motilin in similar experiments with human gastric antrum (∼1000%; Broad et al., 2012). Interestingly, this difference was not predicted by the present studies using the recombinant motilin receptor, in which statistically similar intrinsic activities of motilin and azithromycin were noted. Second, the time taken to reach maximum was over twice that previously reported for motilin (∼18 min with 100 nM motilin [Broad et al., 2012] and 45 min with 300 μM azithromycin). Once maximum activity had been achieved, the response began to slowly fade in an irregular manner, with large contractions appearing at intervals between smaller contractions. This pattern of activity has previously been observed using motilin, but the mechanism is not known; the possibility of a re-alignment between the periodicity of muscular slow-wave activity and the frequency of EFS has been suggested (Broad et al., 2012). In human isolated gastric antrum, slow and sometimes irregular fading of the increased cholinergic activity was also observed in response to erythromycin (present experiments) but not by the non-macrolide motilin receptor agonist GSK962040, which in similar experiments consistently facilitated cholinergic activity in a manner that did not significantly fade over the 70 min observation period (Broad et al., 2012).
In summary, the serendipitous discovery of an ability of azithromycin to stimulate gastric motility is now supported by demonstrating that this drug can activate the motilin receptor and facilitate cholinergic activity in human isolated stomach. The effective concentrations (30–300 μM) have clinical relevance because similar concentrations have been found in blood plasma after therapeutic dosing with azithromycin. For example, in healthy human volunteers receiving 500 mg azithromycin once daily over 3 days, the peak blood plasma concentrations were around 80 μM (Wildfeuer et al., 1994). Thus, the present experiments now provide a rational basis for the use of azithromycin as a gastric prokinetic agent as well as explain the mechanism of the GI side effects of this drug when used for non-GI purposes. Compared with erythromycin, the present experiments suggest that the efficacies of the two macrolides are broadly similar. However, it has been argued that as a gastric prokinetic agent azithromycin is preferable because at therapeutic doses, it seems to be without an ability to inhibit cytochrome P450 enzyme CYP3A4 activity (Hopkins, 1991), a property of erythromycin (Zhou et al., 2004). Nevertheless, both drugs have been associated with a risk of cardiovascular death (Ray et al., 2004; 2012), and the argument that erythromycin should be used sparingly as a treatment of non-infectious disease because of growing bacterial resistance (Abrahamsson, 2007; Hawkyard and Koerner, 2007) must also apply to azithromycin.
Acknowledgments
We thank Dr Adam Góralczyk, Mr Kesava Mannur and Dr Joanne Chin-Aleong for providing the specimens used in this study, and GSK (Mike Bird and Mark Wigglesworth) for the gift of the CHO-K1 cells expressing the human motilin receptor. Professor Daniel Sifrim made valuable comments during construction of this manuscript, for which he is gratefully thanked. GJS was initially funded by a ‘skills gap’ award from the Medical Research Council, UK.
Glossary
- CHO-K1
CHO type K1 cells
- EC50
concentration which provides a response equal to 50% of the maximum
- EFS
electrical field stimulation
- Emax
maximum response to agonist
- GI
gastrointestinal
- MMC
migrating motor complex
Conflict of interest
GJS has received funding from GlaxoSmithKline to study the mechanisms of action of the motilin receptor agonist GSK962040.
References
- Abrahamsson H. Treatment options for patients with severe gastroparesis. Gut. 2007;56:877–883. doi: 10.1136/gut.2005.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Gharbieh E, Vasina V, Poluzzi E, De Ponti F. Antibacterial macrolides: a drug class with a complex pharmacological profile. Pharmacol Res. 2004;50:211–222. doi: 10.1016/j.phrs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad J, Mukherjee S, Samadi M, Martin JE, Dukes GE, Sanger GJ. Regional- and agonist-dependent facilitation of human neurogastrointestinal functions by motilin receptor agonists. Br J Pharmacol. 2012;167:763–774. doi: 10.1111/j.1476-5381.2012.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Tsai CY. Ghrelin and motilin in the gastrointestinal system. Curr Pharm Des. 2012;18:4755–4765. doi: 10.2174/138161212803216915. [DOI] [PubMed] [Google Scholar]
- Chini P, Toskes PP, Waseem S, Hou W, McDonald R, Moshiree B. Effect of azithromycin on small bowel motility in patients with gastrointestinal dysmotility. Scand J Gastroenterol. 2012;47:422–427. doi: 10.3109/00365521.2012.654402. [DOI] [PubMed] [Google Scholar]
- Chiragh S, Begum A, Karim S. Prokinetic effect of clarithromycin and azithromycin – in vitro study on rabbit duodenum. Biomedica. 2006;22:130–134. [Google Scholar]
- Coulie B, Tack J, Peeters T, Janssens J. Involvement of two different pathways in the motor effects of erythromycin on the gastric antrum in humans. Gut. 1998;43:395–400. doi: 10.1136/gut.43.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass NB, Hill J, Muir A, Testa T, Wise A, Sanger GJ. The rabbit motilin receptor: molecular characterisation and pharmacology. Br J Pharmacol. 2003;140:948–954. doi: 10.1038/sj.bjp.0705505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighner SD, Tan CP, McKee KK, Palyha OC, Hreniuk DL, Pong S-S, et al. Receptor for motilin identified in the human gastrointestinal system. Science. 1999;284:2184–2188. doi: 10.1126/science.284.5423.2184. [DOI] [PubMed] [Google Scholar]
- Furness JB, Clark MJ, Wright T, Bertrand PP, Bornstein JC, Verlinden M. An action of erythromycin in the intestine that is not mediated via motilin receptors. Clin Exp Pharmacol Physiol. 1999;26:100–104. doi: 10.1046/j.1440-1681.1999.03002.x. [DOI] [PubMed] [Google Scholar]
- Hawkyard CV, Koerner RJ. The use of erythromycin as a gastrointestinal prokinetic agent in adult critical care: benefits versus risks. J Antimicrob Chemother. 2007;59:347–358. doi: 10.1093/jac/dkl537. [DOI] [PubMed] [Google Scholar]
- Hopkins S. Clinical toleration and safety of azithromycin. Am J Med. 1991;91:40S–45S. doi: 10.1016/0002-9343(91)90401-i. [DOI] [PubMed] [Google Scholar]
- Jarvie EM, North-Laidler V, Corcoran S, Bassil A, Sanger GJ. Differences between the abilities of tegaserod and motilin receptor agonists to stimulate gastric motility in vitro. Br J Pharmacol. 2007;150:455–462. doi: 10.1038/sj.bjp.0707118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JM, Tavakkoli A, Drane WE, Toskes PP, Moshiree B. Advantages of azithromycin over erythromycin in improving the gastric emptying half-time in adult patients with gastroparesis. J Neurogastroenterol Motil. 2010;16:407–413. doi: 10.5056/jnm.2010.16.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens V, Blondeau K, Pauwels A, Farre R, Vanaudenaerde B, Vos R, et al. Azithromycin reduces gastroesophageal reflux and aspiration in lung transplant recipients. Dig Dis Sci. 2009;54:972–979. doi: 10.1007/s10620-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Miller P, Roy AS, Pierre S, Dagenais M, Lapointe R, Poitras P. Motilin receptors in the human antrum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G18–G23. doi: 10.1152/ajpgi.2000.278.1.G18. [DOI] [PubMed] [Google Scholar]
- Moshiree B, McDonald R, Hou W, Toskes PP. Comparison of the effect of azithromycin versus erythromycin on antroduodenal pressure profiles of patients with chronic functional gastrointestinal pain and gastroparesis. Dig Dis Sci. 2010;55:675–683. doi: 10.1007/s10620-009-1038-3. [DOI] [PubMed] [Google Scholar]
- Omura S. Macrolide Antibiotics: Chemistry, Biology and Practice. 2nd edn. San Diego, CA: Academic Press; 2002. [Google Scholar]
- Peeters T, Matthijs G, Depoortere I, Cachet T, Hoogmartens J, Vantrappen G. Erythromycin is a motilin receptor agonist. Am J Physiol. 1989;257:G470–G474. doi: 10.1152/ajpgi.1989.257.3.G470. [DOI] [PubMed] [Google Scholar]
- Ray WA, Murray KT Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohof WO, Bennink RJ, Ruigh AA, Hirsch DP, Zwinderman AH, Boeckxstaens GE. Effect of azithromycin on acid reflux, hiatus hernia and proximal acid pocket in the postprandial period. Gut. 2012;61:1670–1677. doi: 10.1136/gutjnl-2011-300926. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. Motilin, ghrelin and related neuropeptides as targets for the treatment of GI diseases. Drug Discov Today. 2008;13:234–239. doi: 10.1016/j.drudis.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Alpers D. Development of drugs for gastrointestinal motor disorders: translating science to clinical need. Neurogastroenterol Motil. 2008;20:177–184. doi: 10.1111/j.1365-2982.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Westaway SM, Barnes AA, MacPherson DT, Muir AI, Jarvie EM, et al. GSK962040: a small molecule, selective motilin receptor agonist, effective as a stimulant of human and rabbit gastrointestinal motility. Neurogastroenterol Motil. 2009;21:657–666. doi: 10.1111/j.1365-2982.2008.01270.x. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Wang Y, Hobson A, Broad J. Motilin: toward a new understanding of the gastrointestinal neuropharmacology and therapeutic use of motilin receptor agonists. Br J Pharmacol. 2012 doi: 10.1111/bph.12075. DOI: 10.1111/bph.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifrim D, Matsuo H, Janssens J, Vantrappen G. Comparison of the effects of midecamycin acetate and azithromycin on gastrointestinal motility in man. Drugs Exp Clin Res. 1994;20:121–126. [PubMed] [Google Scholar]
- Thielemans L, Depoortere I, Perret J, Robberecht P, Liu Y, Thijs T, et al. Desensitization of the human motilin receptor by motilides. J Pharmacol Exp Ther. 2005;313:1397–1405. doi: 10.1124/jpet.104.081497. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Depoortere I, Thijs T, Janssens JJ, Peeters TL. Concentration-dependent stimulation of cholinergic motor nerves or smooth muscle by [Nle13]motilin in the isolated rabbit gastric antrum. Eur J Pharmacol. 1997;337:267–274. doi: 10.1016/s0014-2999(97)01317-4. [DOI] [PubMed] [Google Scholar]
- Vrančić M, Banjanac M, Nujić K, Bosnar M, Murati T, Munić V, et al. Azithromycin distinctively modulates classical activation of human monocytes in vitro. Br J Pharmacol. 2012;165:1348–1360. doi: 10.1111/j.1476-5381.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway SM, Sanger GJ. The identification and rationale for drugs which act at the motilin receptor. Prog Med Chem. 2009;48:31–80. doi: 10.1016/s0079-6468(09)04802-4. [DOI] [PubMed] [Google Scholar]
- Wildfeuer A, Laufen H, Zimmermann T. Distribution of orally administered azithromycin in various blood compartments. Int J Clin Pharmacol Ther. 1994;32:356–360. [PubMed] [Google Scholar]
- Zhao DM, Xue HH, Chida K, Suda T, Oki Y, Kanai M, et al. Effect of erythromycin on ATP-induced intracellular calcium response in A549 cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L726–L736. doi: 10.1152/ajplung.2000.278.4.L726. [DOI] [PubMed] [Google Scholar]
- Zhou S, Chan E, Lim LY, Boelsterli UA, Li SC, Wang J, et al. Therapeutic drugs that behave as mechanism-based inhibitors of cytochrome P450 3A4. Curr Drug Metab. 2004;5:415–442. doi: 10.2174/1389200043335450. [DOI] [PubMed] [Google Scholar]


