Abstract
Background and Purpose
Alterations in transcription factors that regulate the development and maintenance of dopamine (DA) neurons (such as Nurr1 and Pitx3) play an important role in the pathogenesis of addiction diseases. We have examined the effects of acute and chronic morphine and morphine withdrawal on TH expression and activity as well as expression of Nurr1, Pitx3 and Ago2 in the ventral tegmental area (VTA) and nucleus accumbens (NAc) of the rat.
Experimental Approach
Rats were injected acutely with morphine and decapitated 1 or 2 h later. Another set of rats were made dependent on morphine by implantation of two morphine pellets. Precipitated withdrawal was induced by injection of naloxone. Ago2, Pitx3, Nurr1, total TH (tTH), TH phosphorylated at Ser31 and at Ser40, and 3,4-Dihydroxyphenylacetic acid, and DA determination in the VTA and/or NAc were measured using immunoblotting, HPLC and immunofluorescence.
Key Results
Acute morphine produced a marked increase in TH activity and DA turnover in the NAc, concomitantly with increased Nurr1 and Pitx3 expression in the VTA. In contrast, precipitated morphine withdrawal decreased TH activation, TH expression and did not increase DA turnover in the NAc. These effects paralleled decreases in Ago2 expression, which was accompanied by increased Nurr1 and Pitx3, TH activity and normalized TH protein levels in the VTA.
Conclusions and Implications
The combined decrease in Ago2 and increases in Nurr1 and Pitx3 might represent some of the mechanisms that served to protect against accumbal TH regulation observed in morphine withdrawn rats, which may be critical for DA bioavailability to influence behaviour.
Keywords: opiate dependence, TH, DA turnover, Nurr1, Pitx3, Ago2
Introduction
Multiple adaptive changes in molecular and cellular function in the mesocorticolimbic dopamine (DA) system have been shown after repeated opiate administration, and are thought to be linked to the persistent craving and relapse in animals and human addicts (Nestler, 2001). Substantial evidence indicates that the ventral tegmental area (VTA) of the mesocorticolimbic DA system has a key role in mechanisms of opiate dependence. Nurr1 is an orphan member of the nuclear receptor superfamily of transcription factors, which is critical for the generation of the DA neurons of the substantia nigra and VTA. It is essential for transcription of a set of genes involved in DA metabolism, such as TH (the rate limiting enzyme in DA synthesis), DA transporter (DAT) and vesicular monoamine transporter (Vmat2) (Jankovic et al., 2005; Reddy et al., 2011). Another critical transcription factor for the development and for the survival and maintenance of DA neurons is the homeobox protein Pitx3 (Kim et al., 2007). The gene encoding for Pitx3 is expressed exclusively in midbrain DA neurons (Smidt et al., 1997) and activates the transcription of genes directly involved in the differentiation of dopaminergic neurons (Hwang et al., 2009; Reddy et al., 2011). Determining alterations of Nurr1 and or Pitx3 in the adult brain is of particular importance because previous studies suggested an association of these proteins with addiction pathology.
In recent years, studies highlight post-transcriptional modifications mediated by micro-RNAs (miRNA) in addiction (Kim et al., 2007; He et al., 2010). Some of them regulate the differentiation, maturation and function of dopaminergic neurons by down-regulating the transcription of Nurr1 and Pitx3 (Kim et al., 2007). miRNAs associate with specific members of a large protein family, the Argonautes (Ago), directing them to specific targets mRNAs to repress protein expression (Ambros, 2004). Among the Ago family members, only Ago2 seems to have an important role in miRNA generation and execution of miRNA-mediated gene silencing (Liu et al., 2004; Hutvagner and Simard, 2008).
Given the important implications of DA neurotransmission in addiction disorders and the complexity of opiates-induced neuroadaptive responses in the brain reward dopaminergic system, the study was focused on identifying the DA markers that are altered in association with acute and chronic morphine exposure as well as with morphine withdrawal in the VTA and nucleus accumbens (NAc) because those opioid-related conditions generate strong cellular responses despite their very different neurobiological effects relevant to reward function. For that, we have exposed rats to acute and chronic morphine administration and analysed TH and dopaminergic activity in the mesolimbic system as well as expression of Ago2 and transcription factors Nurr1 and Pitx3. Here, we report differences in the response to acute and chronic morphine when analysed TH levels and activity, transcription factors protein expression as well as Ago2 levels in drug reward pathways, suggesting that Nurr1, Pitx3 and Ago2 regulation might play a role in controlling adaptation to chronic morphine and to morphine withdrawal-induced depression of DA neurons activity in the NAc. Furthermore, present results revealed a striking segregation in response to morphine of areas engaged in the opiate-related responses.
Methods
Subjects
Male Wistar rats (220–240 g; age, 8–10 weeks; Harlan, Barcelona, Spain; n = 83 at the beginning of the experiment) were housed two or three per cage (length, 45 cm; width, 24 cm; height, 20 cm) on arrival in a room with controlled temperature (22 ± 2°C) and humidity (50 ± 10%), with free access to water and food (Harlan Teklad standard rodent chow; Harlan Interfauna Ibérica, Barcelona, Spain). Animals were adapted to a standard 12 h light–dark cycle (lights on: 08:00–20:00 h) for 7 days before the beginning of the experiments. All surgical and experimental procedures were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC), and were approved by the local Committees for animal research (REGA ES300305440012). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Drug treatment and experimental procedure
Following habituation, rats were implanted subcutaneously (s.c.) with placebo pellets (lactose) for 6 days. On day 7, rats were injected intraperitoneally (i.p.) with either morphine HCl (20 mg·kg−1 i.p.) or an equivalent volume of 0.9% saline and decapitated 1 or 2 h later. Another set of rats were made dependent on morphine by implantation (s.c.) of two 75 mg morphine pellets under light ether anaesthesia (Navarro-Zaragoza et al., 2012). Control rats received placebo pellets containing the excipient without morphine. This procedure has been shown to produce consistent plasma morphine concentrations beginning a few hours after the implantation of the pellets and a full withdrawal syndrome after acute injection of opiate antagonists (Frenois et al., 2002). Dependence on morphine remained constant for 15 days (Gold et al., 1994). Seven days after the implantation of morphine or placebo pellets, precipitated withdrawal was induced by s.c. injection of naloxone (1 mg·kg−1; in a volume of 1 mL·kg−1 body weight). The experimental conditions investigated for opiate withdrawal-induced physical signs of dependence, Ago2, Pitx3, Nurr1, total TH (tTH), TH phosphorylated at Ser31 and at Ser40, 3,4-Dihydroxyphenylacetic acid (DOPAC), and DA determination in the VTA and/or NAc are shown in (Table 1). Body weight loss was determined as the difference between the weight determined immediately before naloxone injection and a second determination made 120 min later. The weight gain of the rats was checked during treatment to ensure that the morphine was liberated correctly from the pellets because it is known that chronic morphine treatment induces a decrease in body weight gain due to lower caloric intake (Houshyar et al., 2004; Núñez et al., 2009). Two hours after acute administration of saline, morphine or naloxone, rats were decapitated (between 10:00 and 12:00 h to avoid circadian variations in plasma levels of the hormones), the brains were rapidly removed, and stored immediately at −80°C until use for Western blot analysis of Ago2, Pitx3, Nurr1, total TH (tTH), TH phosphorylated at Ser31 and at Ser40, and for determination of DA and DOPAC. A second set of animals from each treatment group was used for immunofluorescence staining of Pitx3-TH and Nurr1-TH colocalization.
Table 1.
Treatments and number of animal used for each assay
| Assay | |||
|---|---|---|---|
| Pretreatment | Acute treatment | NAc | VTA |
| Placebo | Saline | DA and DOPAC levels, DOPAC/DA ratio (n = 6), THpSer31 (n = 7), THpSer40 (n = 8), tTH (n = 7) | Ago2 (n = 6), Nurr1 (n = 6), Pitx3 (n = 6), tTH (n = 6) |
| Placebo | Morphine (1 h) | DA and DOPAC levels, DOPAC/DA ratio (n = 4) | |
| Placebo | Morphine (2 h) | DA and DOPAC levels, DOPAC/DA ratio (n = 5), THpSer31 (n = 8), THpSer40 (n = 8), tTH (n = 7) | Ago2 (n = 8), Nurr1 (n = 7), Pitx3 (n = 7), tTH (n = 8) |
| Placebo | Saline | DOPAC/DA ratio (n = 8), THpSer31 (n = 6), THpSer40 (n = 8), tTH (n = 7) | Ago2 (n = 7), Nurr1 (n = 6), Pitx3 (n = 6), THpSer31 (n = 8), THpSer40 (n = 6), tTH (n = 8), Nurr1/tTH and Pitx3/tTH colocalization (IF) (n = 3) |
| Placebo | Naloxone | DOPAC/DA ratio (n = 5), THpSer31 (n = 6), THpSer40 (n = 7), tTH (n = 6) | Ago2 (n = 8), Nurr1 (n = 8), Pitx3 (n = 7), THpSer31 (n = 7), THpSer40 (n = 7), tTH (n = 8) |
| Morphine | Saline | DOPAC/DA ratio (n = 6), THpSer31 (n = 6), THpSer40 (n = 7), tTH (n = 7) | Ago2 (n = 8), Nurr1 (n = 8), Pitx3 (n = 7), THpSer31 (n = 8), THpSer40 (n = 7), tTH (n = 7), Nurr1/tTH and Pitx3/tTH colocalization (IF) (n = 3) |
| Morphine | Naloxone | DOPAC/DA ratio (n = 6), THpSer31 (n = 6), THpSer40 (n = 7), tTH (n = 6) | Ago2 (n = 8), Nurr1 (n = 8), Pitx3 (n = 6), THpSer31 (n = 7), THpSer40 (n = 7), tTH (n = 8), Nurr1/tTH and Pitx3/tTH colocalization (IF) (n = 3) |
DOPAC, 3,4-Dihydroxyphenylacetic acid.
Electrophoresis and Western blotting
Animals were killed by rapid decapitation. The brains were removed, placed (with its ventral surface facing up) on a plaque over crushed ice, and tissue samples of the VTA and NAc were dissected out. Regions were identified based on standard anatomical landmarks and stereotaxic coordinates (plane of sections: VTA, 4900–6300 μm caudal to bregma; NAc, 2400–900 μm rostral to bregma (Paxinos and Watson, 2007). For VTA dissection, one 1–1.5 mm slice was made by cutting between the rostral boundary of the pons (at a 30° angle from the ventral rostral end down toward the dorsal caudal end) and the caudal boundary of the mamillary bodies. The section obtained was placed on a cold slide under a head magnifier and then, the VTA was bilaterally dissected doing a cut dorsal to the paranigral nucleus, ventral to the red nucleus, lateral to the interfascicular nucleus, and medial to the medial lemniscus. Billateral NAc was dissected from the remaining rostral brain section, scalped blade as described in Heffner et al. (Heffner et al., 1980), with a triangle centred over each anterior commissure and according to boundaries defined by Paxinos and Watson (Paxinos and Watson, 2007).
Brain regions were places in individual wells, frozen immediately on dry ice and stored at −80°C until assaying. Samples were placed in homogenization buffer (Núñez et al., 2007), homogenized and sonicated for 30 s prior to centrifugation at 10 000 g for 10 min at 4°C. Samples containing equal quantities of total proteins (20–30 mg, depending on the protein of interest) were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Western analysis was performed with the following primary antibodies: rabbit monoclonal anti-Argonaute 2 (1:1000; #2897, Cell Signaling Technology Inc., Danvers, MA, USA), rabbit polyclonal anti-Pitx3 (1:750; ab30734, Abcam, Cambridge, MA, USA), rabbit polyclonal anti-Nurr1 (1:500; sc-991, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-tTH (1:10 000; AB152, Millipore), rabbit polyclonal anti-tyrosine-hydroxylase phosphorylated at Ser31 (pSer31; 1:1000; AB5423, Millipore), rabbit polyclonal anti-tyrosine-hydroxylase phosphorylated at Ser40 (pSer40; 1:1000; AB5935, Millipore). Anti-rabbit IgG, HRP-linked (1:5000; sc-2004, Santa Cruz Biotechnology) was used as secondary antibody. After washing, immunoreactivity (IR) was detected with an enhanced chemiluminescent/chemifluorescent Western blot detection system (ECL Plus, GE Healthcare, Chalfont St. Giles, UK) and visualized by a Typhoon 9410 variable mode Imager (GE Healthcare). We used β-actin as our loading control for all the experiments. Before re-probing, blots were stripped by incubation with stripping buffer (glycine 25 mM and SDS 1%, pH 2) for 1 h at 37°C. Blots were subsequently reblocked and probed with rabbit polyclonal anti-β-actin (1:1000; #4967, Cell Signaling Technology Inc.). The ratios of Ago2/β-actin, Pitx3/β-actin, Nurr1/β-actin, tTH/β-actin, pSer31/β-actin and pSer40/β-actin were plotted and analysed. Protein levels were corrected for individual levels.
Double immunofluorescence study
Two hours after saline or naloxone injections, rats were anesthetized with pentobarbital (100 mg·kg−1 i.p) and quickly perfused through the ascending aorta with cold saline and subsequently with a cold fixative solution containing 4% paraformaldehyde in 0.1 M borate buffer, pH 9.5, pH 7.4. Brains were removed and kept in the same fixative solution containing sucrose (30%) for 3 h. After that, brains were placed in PBS containing 30% sucrose overnight at 4°C. A series of 30 μm coronal sections of the midbrain including the VTA were cut on freezing microtome, collected in cryoprotectant and stored at −20°C until processing. Brain sections were rinsed in PBS, and for detecting Nurr1- and Pitx3-expressing neurons grafted in the brain sections, an antigen retrieval procedure was applied by treating sections with citrate buffer (10 mM citric acid in 0.05% Tween 20, pH 6.0) at 60°C for 20 min before the blocking procedure. Sections were blocked in 2% normal horse serum/0.3% Triton-X-100 in PBS for 1 h at room temperature. Sections were then incubated for 48 h at 4°C with the following primary antibodies: Rabbit polyclonal anti-Nurr1 (1:500; sc-991, Santa Cruz Biotechnology); rabbit anti-Pitx3 [1:1000; a gift of Dr Marten Smidt, The Netherlands; (Smidt et al., 2000; 2004)]; goat polyclonal anti-tTH (1:4000; ab101853, Abcam). Alexa Fluor 488 Donkey Anti-Rabbit IgG (1:1000; A-21206, Invitrogen, Eugene, OR, USA) and Alexa Fluor 594 Donkey Anti-Goat IgG (1:1000; A-11058, Invitrogen) labelled secondary antibodies were applied for 4 h. Sections were incubated in DAPI (1:100 000) for 1 min, and mounted in ProLong® Gold antifade reagent (Invitrogen).
Image analysis
Images were obtained using a Nikon Confocal Microscope (Nikon Corporation, Tokyo, Japan) using 408-nm excitation for DAPI, 488-nm excitation for Alexa Fluor 488 and 543-nm excitation for Alexa Fluor 594. Emitted light was detected in the range of 450 nm for DAPI, 515–530 nm for Alexa Fluor 488 and 605 nm for Alexa Fluor 594. Every channel was captured separately to avoid spectral crosstalking. Images were deconvolved using Huygens Essential 3.6 by Scientifica Volume Imaging. Colocalization analysis was performed in images previously processed with Huygens (SVI, Hilversum, The Netherlands) and carried out using the JAcoP plugin (Bolte and Cordelierès, 2006) for Image J (http://rsbweb.nih.gov/ij/). Three to four images were analysed per animal.
Estimation of DA and its metabolite DOPAC in the NAc
One and 2 h after saline or morphine injection to placebo-treated rats, DA and its metabolite DOPAC were determined in the NAc by HPLC with electrochemical detection as described previously (Navarro-Zaragoza et al., 2010). Tissue samples of the NAc were dissected, weighed, placed in perchloric acid (0.1 M), homogenized and centrifuged at 10 000 g and 4°C for 20 min and the supernatants taken for analysis and filtered through 0.22 mm GV (Millipore Bedford, MA, USA). Ten microlitres of each sample were injected into a 5 mm C18 reversed-phase column (Waters, Milford, MA, USA) through a Rheodyne syringe-loading injector (Waters). Electrochemical detection was accomplished with an electrode set at a potential of +0.65 V versus the reference electrode. The mobile phase was delivered at 0.9 mL·min−1 flow rate, and consisted of a 90:10 (v/v) mixture of water and methanol and chromatographic data were analysed with chromatography equipment (Empower 2 software, Waters). DA and DOPAC were identified according to the retention times of a standard mixture of DA and DOPAC. Standard curves (ranging from 122.5 to 490 pg) at the beginning and the end of each experiment were used to quantify DA and DOPAC levels for each experimental group. The amounts of DA and its metabolite were quantified by calculating with peak areas. The content of DA and DOPAC in the NAc is expressed as ng·g−1 wet weight of tissue. The DA turnover was determined as the DA ratio, which was calculated as: DA ratio = DOPAC/DA. The ratio DOPAC/DA was used as indices of transmitter metabolism.
Materials
Morphine HCl and morphine base were supplied from Alcaliber Laboratories (Madrid, Spain) in cooperation with the Área de Estupefacientes y Psicotropos, Agencia Española del Medicamento y de Productos Sanitarios (Madrid, Spain). Pellets of morphine and lactose (control) were prepared in the Department of Pharmacy and Pharmaceutics Technology (School of Pharmacy, Granada, Spain); naloxone HCl was purchased from Sigma-Aldrich (Sigma Chemical Co., St Louis, MO, USA). Morphine HCl and naloxone HCl doses are expressed as the weight of the salt. Protease inhibitors were purchased from Boehringer Mannheim, (Mannheim, Germany); phosphatase inhibitor Cocktail Set was purchased from Calbiochem (Darmstadt, Germany); HPLC reagents were purchased from Sigma Chemical Co. Morphine HCl and naloxone were prepared fresh each day by reconstitution in sterile saline (0.9% NaCl; ERN Laboratories, Barcelona, Spain).
Statistical analysis
Data are presented as mean ± SEM and were analysed by one-way or two-way anova with pretreatment (placebo, morphine) and acute treatment (vehicle, naloxone) as independent variables. The Newman–Keuls post hoc test was used for individual group comparisons. To compare two groups, Student's t-test was used. Differences with a P < 0.05 were considered significant.
Nomenclature
Drug/molecular target nomenclature conforms to BJP's Guide to Receptors and Channels (Alexander et al. 2011).
Results
Morphine- and morphine withdrawal-induced changes in body weight
Student's t-test showed that rats treated with morphine had a significantly (t79 = 12.29; P < 0.0001) lower weight gain than that observed in animals receiving placebo pellets (Figure 1A). The body weight loss after saline or naloxone injection to placebo-pelleted and morphine-dependent rats was also recorded as a sign of opiate withdrawal (Figure 1B). In agreement with our previous results (Navarro-Zaragoza et al., 2011) two-way anova revealed that morphine pretreatment (F(1,60) = 183.73, P < 0.0001), naloxone injection (F(1,60) = 224.25, P < 0.0001) and the interaction between pretreatment and acute treatment (F(1,60) = 127.06, P < 0.0001) had a significant effect on body weight loss. Post hoc analysis showed that naloxone injection to morphine-dependent animals significantly increased (P < 0.001) the body weight loss when compared with the placebo-pelleted group also receiving naloxone and with morphine-treated rats given saline.
Figure 1.
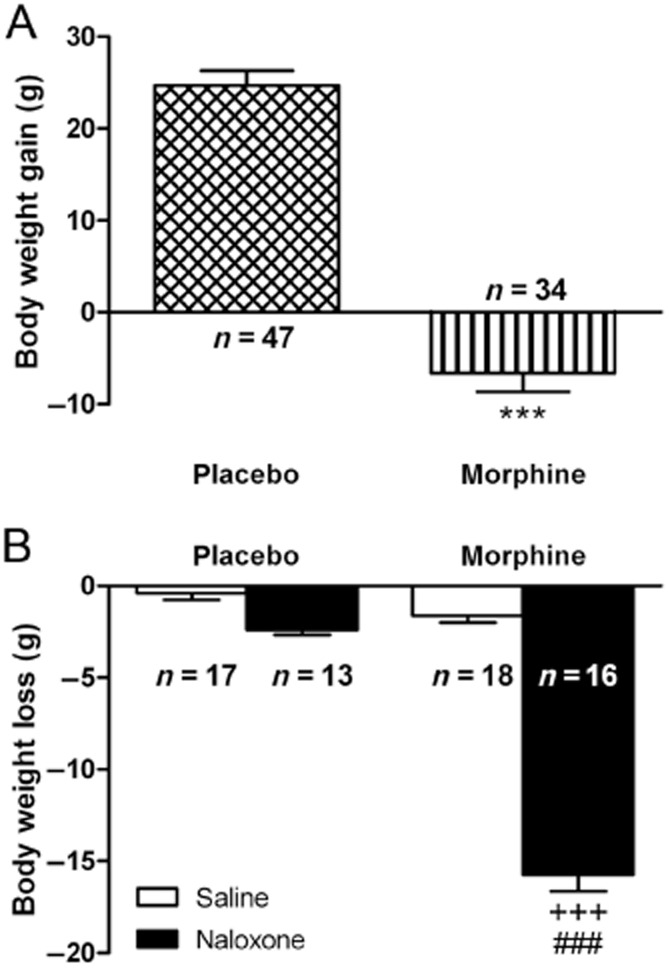
(A) Body weight gain 7 days after placebo or morphine pellets implantation. (B) Effects naloxone-induced morphine withdrawal on body weight loss. Rats were implanted with morphine or placebo pellets for 7 days. Weight loss was checked immediately before and 2 h after naloxone injection. Data shown are means ± SEM. There were a decrease in body weight gain in morphine-pelleted rats (***P < 0.001 vs. placebo). Post hoc test revealed that there was an increase in body weight loss during morphine withdrawal. +++P < 0.001 versus placebo + naloxone; ###P < 0.001 versus morphine + saline.
All morphine-dependent animals receiving naloxone displayed behaviour and somatic signs characteristic of opioid withdrawal, as previously was shown using the same method of morphine dependence induction (Navarro-Zaragoza et al., 2012): wet-dog shakes, piloerection, teeth-chattering, diarrhoea, ptosis, body tremor, paw tremor, salivation, rinorrhoea, cromodacriorrhoea, sniffing and irritability.
Acute morphine-induced DOPAC production and DA turnover elevation in the NAc
anova (F(2,14) = 0.8547, P = 0.4498) revealed that administration of morphine (20 mg·kg−1 i.p.) did not significantly increase DA levels in the NAc 1 or 2 h after administration, compared with saline control group (Figure 2A). By contrast, anova revealed significant (F(2,14) = 13.65, P = 0.0008) differences in DOPAC production after acute morphine administration. As shown in Figure 2B, post hoc comparisons showed that morphine administration significantly elevated DOPAC production in the NAc at 1 (P < 0.01) and 2 h (P < 0.001) after administration, compared with the control group receiving saline. Results for the anova for DA turnover (as revealed by DOPAC/DA ratio) in the NAc showed a significant effect of morphine administration (F(2,14) = 19.05, P = 0.0002). As shown in Figure 2C, rats injected with morphine showed significant higher DA turnover (both at 1 and 2 h) than the saline-injected group (P < 0.001).
Figure 2.
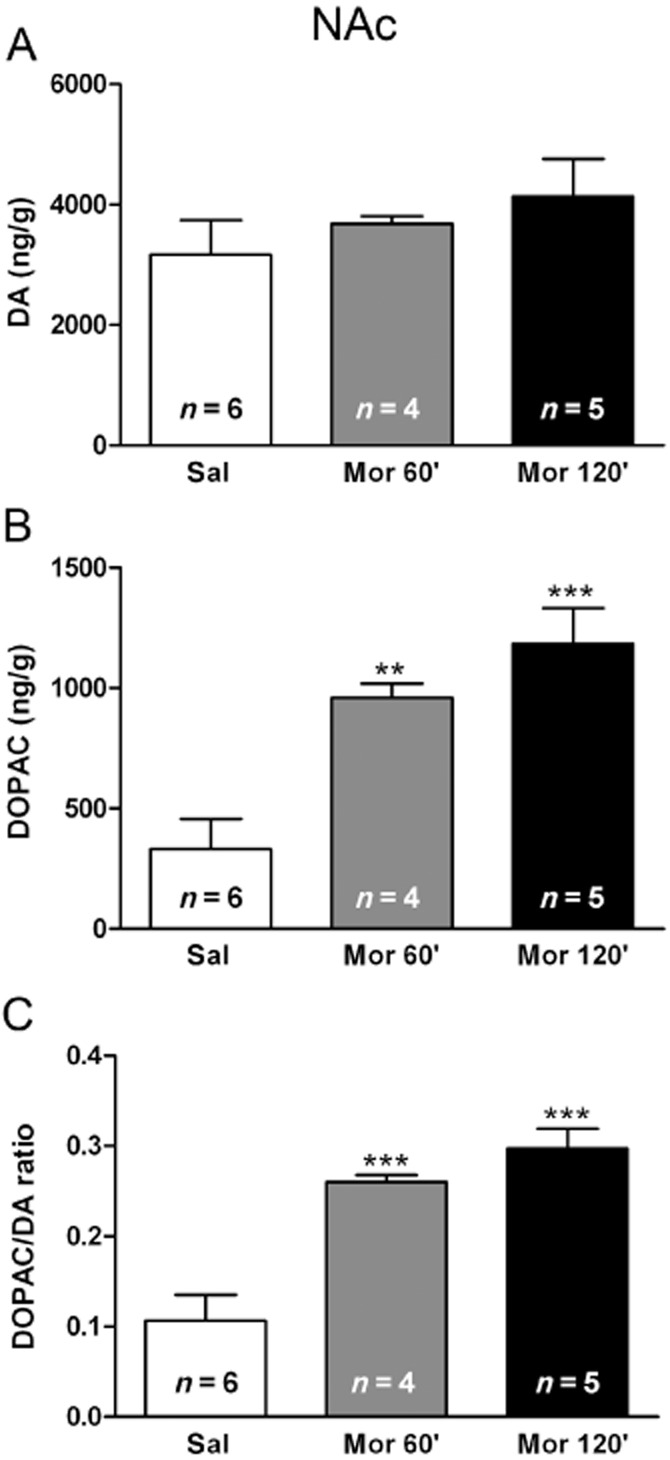
Effects of acute morphine administration on DA and 3,4-Dihydroxyphenylacetic acid (DOPAC) levels at the NAc and on DA turnover (as estimated by the DOPAC/DA ratio). Data represent the mean ± SEM 1 and 2 h after morphine or saline injection to control pellets-treated rats. Newman–Keuls post hoc comparison test revealed a significant increase in DOPAC production and DA turnover in morphine (mor)-treated rats. **P < 0.01; ***P < 0.001 versus control pellets (placebo) + saline (sal).
Acute morphine administration induced TH phosphorylation in the NAc
Student's t-test (t13 = 2.488; P < 0.05) showed that rats treated with morphine showed significant increases in pSer31-TH levels in the NAc (Figure 3A). No changes in pSer40-TH levels were found in this nucleus after morphine administration (t14 = 1.288; Figure 3B) compared with saline-injected rats. As shown in Figure 3C, tTH IR in the NAc from rats treated with morphine did not significantly differ (t12 = 0.3762; P = 0.7134) from that in the placebo group injected with saline, indicating that the amount of total protein was not changed after acute morphine administration.
Figure 3.
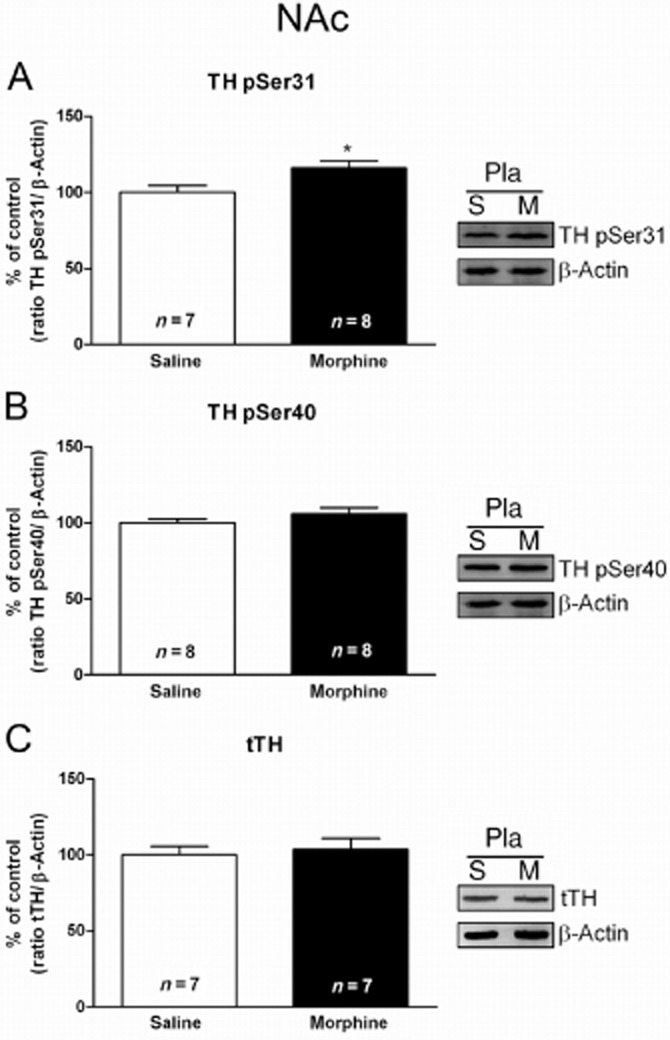
Morphine administration stimulates TH phosphorylation at Ser31, but not Ser40 in the NAc. Semi-quantitative analysis and representative immunoblots of pSer31-TH (A), pSer40-TH (B) and total TH (tTH; C) in NAc tissue isolated from placebo-treated rats 120 min after acute administration of saline or morphine (20 mg·kg−1 i.p.). Each bar corresponds to mean ± SEM (% of control). Student's t-test revealed a significant increase in pSer31-TH after morphine administration (*P < 0.05 vs. placebo plus saline). No significant difference was observed in the pSer40-TH or tTH protein levels.
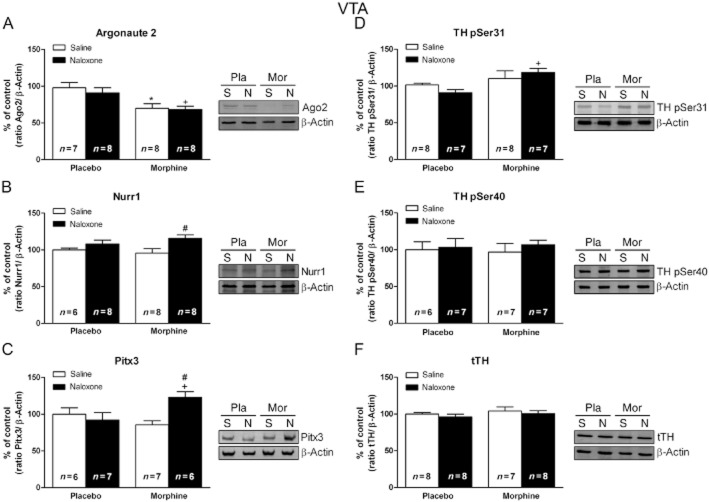
Effects of acute morphine on Ago2, Nurr1, Pitx3 and tTH protein levels in the VTA
The effects of morphine on Ago2, Nurr1, Pitx3 and tTH protein levels are shown in Figure 4. Morphine injection did not modify Ago2 protein levels in the VTA (Figure 4A). A single dose of morphine caused significant (t11 = 2.391; P < 0.05) increased Nurr1 protein levels in the VTA in rats decapitated 2 h after drug injection, as shown in Figure 4B. Similar to this effect, morphine administration caused also increases in Pitx3 protein expression (t10 = 2.242; P < 0.05; Figure 4C). However, morphine injection did not modify tTH IR in the VTA (Figure 4D).
Figure 4.
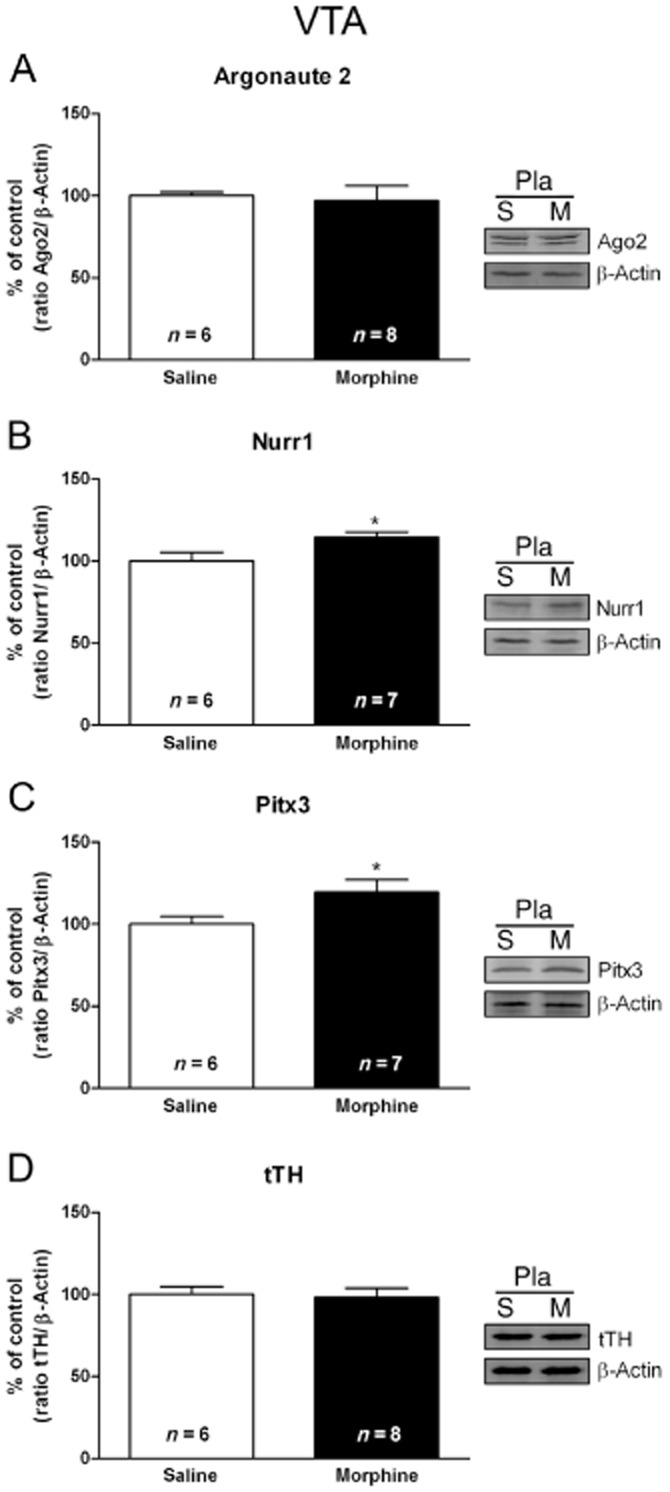
Nurr1 and Pitx3 are sensitive to acute morphine (m) administration in the rat VTA. Semi-quantitative analysis and representative Western blots using protein extracts from saline- (s) treated control and morphine-treated rats showed that injection of morphine (20 mg·kg−1 i.p.) leads to increased Nurr1 (B) and Pitx3 (C) expression in the VTA. No significant modifications were observed in the Argonaute 2 (A) or tTH (D) protein levels. Each bar corresponds to mean ± SEM (% of control) 120 min after morphine or saline injection to control pellets-treated rats. *P < 0.05 versus placebo (pla) plus saline.
Chronic morphine- and morphine withdrawal-induced changes in TH levels and activity and in DA turnover in the NAc
Two-way anova for pSer31-TH revealed a main effect of morphine pretreatment (F(1,20) = 5.52; P = 0.0292), naloxone administration (F(1,20) = 6.77; P = 0.0171) with main acute treatment–pretreatment interaction (F(1,20) = 9.97; P = 0.0050). These experiments demonstrated that, in the NAc, acute naloxone treatment had no effect on animals chronically treated with placebo and that phosphorylation of TH at Ser31 was also unchanged after saline administration to morphine-dependent rats. However, rats chronically treated with morphine and given naloxone showed significant (P < 0.01) decreases in pSer31-TH levels (Figure 5A) 2 h after the opioid antagonist injection, compared with the corresponding control group receiving naloxone and the morphine-dependent animals receiving saline. The anova for pSer40-TH in the NAc (Figure 5B) showed significant effects of chronic morphine pretreatment (F(1,25) = 18.93; P = 0.0002), with no significant effects of naloxone treatment (F(1,25) = 3.06; P = 0.0925), or an interaction between acute treatment and pretreatment treatment (F(1,25) = 0.03; P = 0.8618). The anova for tTH revealed significant effects of morphine pretreatment (F(1,21) = 6.15; P = 0.0217), naloxone administration (F(1,21) = 8.41; P = 0.0085), and interaction between pretreatment and acute treatment (F(1,21) = 21.28; P = 0.0002). As shown in Figure 5C, tTH in the NAc from rats treated with morphine did not significantly differ from that in the placebo control group after saline administration, indicating that the amount of total protein was not changed during morphine dependence. However, 2 h after naloxone injection to morphine-dependent rats, there was a significant (P < 0.001) decrease in total protein levels, compared with placebo-pelleted animals also receiving naloxone and with morphine-dependent animal injected with saline.
Figure 5.
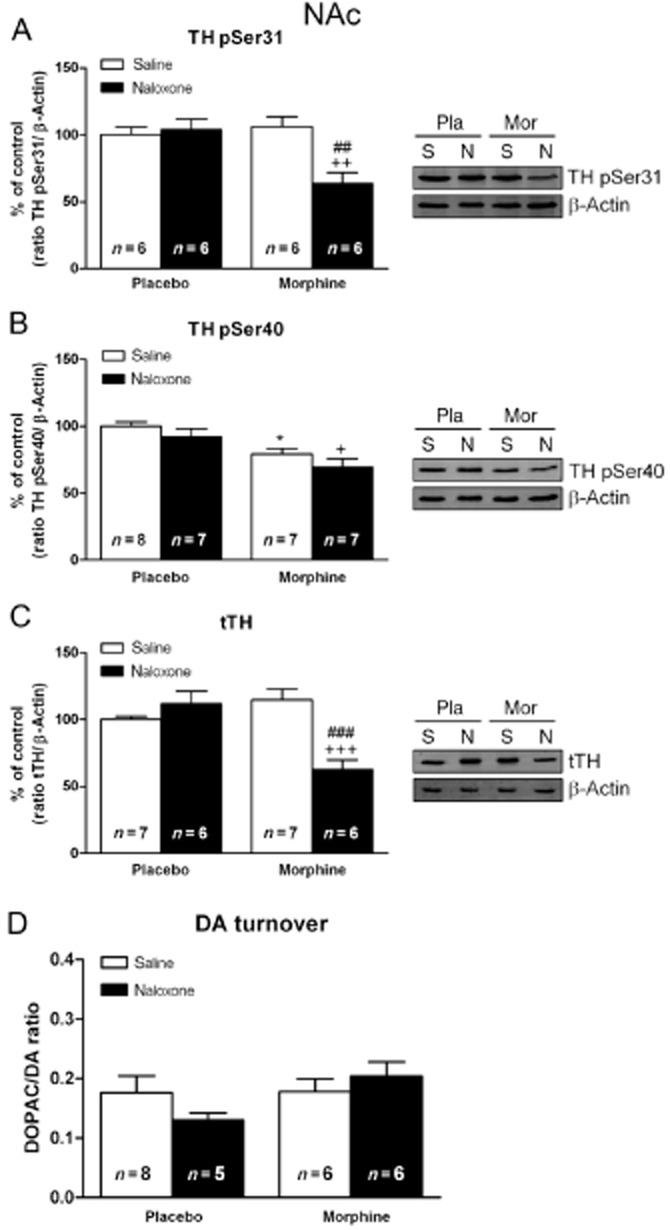
Morphine withdrawal decreased TH phosphorylation at Ser31 and Ser40, total TH (tTH) protein expression and DA turnover in the NAc. Semi-quantitative analysis and representative immunoblots of pSer31-TH (A), pSer40-TH (B), tTH (C) and DA turnover (D), in NAc tissue isolated from placebo or morphine-dependent rats 2 h after s.c. administration of saline or naloxone (1 mg·kg−1). Each bar corresponds to mean ± SEM (% of control). Newman–Keuls post hoc comparison test revealed a significant decrease in pSer31-TH, pSer40-TH and tTH in morphine-withdrawn rats. No significant changes in DA turnover were seen in morphine-dependent rats receiving naloxone. *P < 0.05 versus placebo (pla) plus saline (s); +P < 0.05, ++P < 0.01, +++P < 0.001 versus placebo plus naloxone (n); ##P < 0.01, ###P < 0.001 versus morphine (mor) plus saline.
Two-way anova for DA turnover revealed no significant effects of morphine pretreatment (F(1,21) = 2.31; P = 0.1433), naloxone injection (F(1,21) = 0.16; P = 0.6888), or an interaction between acute treatment and pretreatment (F(1,21) = 2.09; P = 1631) (Figure 5D).
Effects of morphine dependence and withdrawal on VTA Ago2, DA transcription factors expression, pSer31-TH, pSer40-TH and tTH levels
Two-way anova for Ago2 protein levels (Figure 6A) revealed a main effect of morphine pretreatment (F(1,24) = 13.10; P = 0.0014) with no significant effects of naloxone treatment (F(1,24) = 0.93; P = 0.3451) or an interaction between acute treatment and pretreatment (F(1,24) = 0.24; P = 0.6278). anova for Nurr1 showed significant effects of naloxone treatment (F(1,26) = 7.41; P = 0.0114) with no significant effects of chronic morphine pretreatment (F(1,26) = 0.1; P = 0.7545) or an interaction between acute treatment and pretreatment (F(1,26 = 1.36; P = 0.2539) (Figure 6B). The anova for Pitx3 revealed an interaction between acute treatment and pretreatment (F(1,22) = 7.7; P = 0.0110), with no significant effects of morphine pretreatment (F(1,22) = 1.03; P = 0.3221) or naloxone injection (F(1,22) = 3.25; P = 0.0851). As shown in Figure 6C, morphine withdrawal induced a significant (P < 0.05) increase in Pitx3 protein levels compared with both morphine-dependent rats and placebo animals receiving naloxone.
Figure 6.
Nurr1 and Pitx3 expression is activated in the VTA from morphine-withdrawn rats. Representative Western blots and semi-quantitative analysis of Argonaute 2 (A), Nurr1 (B), Pitx3 (C), pSer31-TH (D), pSer40-TH (E) and total TH (tTH; F) protein levels in the VTA tissue isolated from placebo (pla) or morphine (mor)-dependent rats after s.c. administration of saline (s) or naloxone (n). Each bar represents the mean optical density ± SEM; values are expressed as % of controls. Newman–Keuls' post hoc comparison test revealed a significant increase in Nurr1, Pitx3 and pSer31-TH in morphine-withdrawn rats at 2 h after naloxone injection, whereas there was a decrease in Argonaute 2 protein levels in morphine-dependent rats receiving saline and after naloxone-induced morphine withdrawal. No significant modifications were observed in the tTH protein levels #P < 0.05 versus morphine + saline; +P < 0.05 versus placebo + naloxone; *P < 0.05 versus placebo + saline.
Two-way anova for TH phosphorylated at Ser31 (Figure 6D) revealed a main effect of morphine pretreatment (F(1,22) = 9.02; P = 0.0061) with no significant effects of naloxone treatment (F(1,22) = 0.33; P = 0.8636) or an interaction between acute treatment and pretreatment (F(1,22) = 2.47; P = 0.1301). Two-way anova for TH phosphorylated at Ser40 (Figure 6E) revealed no significant effects of chronic morphine pretreatment (F(1,22) = 0; P = 0.9986), naloxone injection (F(1,22) = 0.40; P = 0.5349), or an interaction between acute treatment and pretreatment (F(1,22) = 0.09; P = 0.7685). The anova for tTH (Figure 6F) showed no significant effects of chronic morphine pretreatment (F(1,27) = 1.19; P = 0.2846), naloxone injection (F(1,27) = 0.88; P = 0.3552), or an interaction between acute treatment and pretreatment (F(1,27) = 0.00; P = 0.9692).
Immunohistochemical approach for the determination of Pitx3 and Nurr1 in the VTA TH-positive neurons
In the present study, we confirmed the co-localization of Pitx3 and Nurr1 with TH-positive neurons in the VTA. Representative images are shown in Figure 7. Double-labelling experiments showed that either Pitx3- and Nurr1-IR was highly colocalized with the selective DA neuron marker TH in the VTA. Importantly, Pitx3-IR was strongly (P < 0.001) detected in the nucleus of TH-positive cells in morphine-withdrawn rats, according to the correlation analysis based on Pearson's coefficient (Figure 7H'’’–J'’’).
Figure 7.
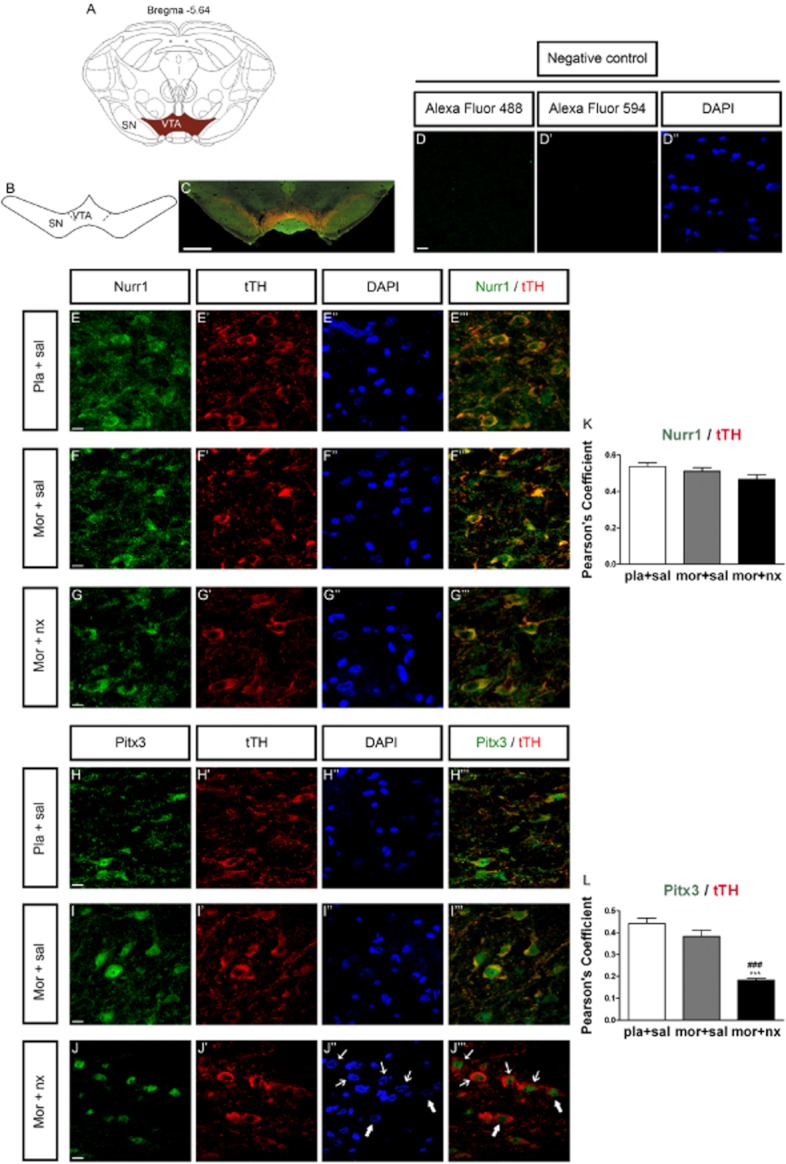
Effects of chronic morphine and naloxone-precipitated morphine withdrawal on Nurr1 and Pitx3 expression in TH neurons of the VTA. Over a 7 day period, control (pla) and morphine- (mor) dependent rats received saline (sal; s.c.) or 1 mg·kg−1 naloxone (nx; s.c.) on day 7. Three to four sections containing the VTA were selected per animal. The analysed region within VTA is schematically illustrated in A and B (diagram modified from Paxinos and Watson, 2007); coordinate is mm from Bregma. (C) Example of low magnification micrograph showing a midbrain coronal section of rats immunostained for TH and Pitx3; scale bar, 1 mm. (D–D'’), negative control (without the first antibody). Representative confocal images of Nurr1 (green) and tTH (red) (E–G’) and Pitx3 (green) and TH (red) (H–J’) immunostaining and DAPI (nuclear stain, blue; E'’–J'’) in the VTA. Merged images are shown in E'’’-G'’’ (Nurr1/tTH) and H'’’–J'’’ (Pitx3/tTH). Colocalization is shown by yellow neurons in the merged images of TH/Nurr1 and TH/Pitx3. Scale bars, 10 μm. (K, L) Colocalization analysis of TH and Pitx3 with JACoP. J'’’: nuclear translocation of Pitx3 in morphine-withdrawn rats. Arrows indicates that Pitx3-IR was drastically expressed in the nucleus of TH-positive neurons during morphine withdrawal. Correlation analysis was based on Pearson's coefficient. Results are shown as mean ± SD of Pearson's coefficient. ###P < 0.001 versus morphine plus saline.
Discussion
Abused drugs, such as opiates, ethanol, cocaine and nicotine, trigger an acute release of DA in NAc in animal models (Di Chiara and Bassareo, 2007), thus activating mesolimbic reward pathway. Consistent with this, our results showed DA release, in the context of DOPAC concentration and DA turnover were markedly increased in the NAc after acute morphine administration at different time-points. Short-term regulation of catecholamine biosynthesis occurs through phosphorylation of TH, which enhances enzymatic activity. In particular, increases in the phosphorylation of Ser31 and Ser40 accelerate TH activity, thereby stimulating production of neurotransmitter in catecholamine terminals (Haycock and Haycock, 1991; Nakashima et al., 2009). The results of present work provided evidence for TH phosphorylation after morphine administration in dopaminergic terminals innervating the NAc. Thus, we have shown that morphine increased the level of TH phosphorylation at Ser31, but not at Ser40 in rat NAc, concomitantly with enhanced DA turnover. Together, these data suggest that Ser31 phosphorylation of TH plays a significant role in regulating TH activity after acute morphine administration and might be directly involved in regulating DA turnover in the NAc and, consequently, upon DA-influenced behaviours, as has been recently proposed (Salvatore and Pruett, 2012). Thus, acutely administered opioids increase DA release in the NAc, an effect that is thought to mediate the reward (Di Chiara, 1999). On the other hand, a role for the transcription factors Pitx3 and Nurr1 in TH regulation has been previously shown (Reddy et al., 2011). Here we show that a single injection of morphine is, at least in part, sufficient for the activation of Pitx3 and Nurr1 protein expression in the VTA. The increased expression of these two transcription factors can be viewed as a homeostatic response to excess DA neurotransmission in the mesolimbic pathway caused by acute exposure to morphine, thus maintaining TH at normal levels and protecting against excessive DA release.
Adaptive changes in cellular and synaptic function in the mesocorticolimbic DA system after prolonged opiate administration are believed to play an important role in the development of tolerance to and dependence on opiates and to contribute to changes underlying the complex neurobiological syndrome of opiate withdrawal (Nestler, 2001; Georges et al., 2006). Present results showed tolerance to the excitatory effects of morphine at the NAc, as shown by the ineffectiveness of chronic morphine to activate (phosphorylate) TH. Thus, morphine-dependent rats implanted with pellets failed to increase TH activity. In addition, and in contrast to the effects of acute morphine, precipitated morphine withdrawal decreased both phosphorylation (activation) of TH and TH protein expression and did not increase DA turnover in rat NAc. These results are in agreement with previous data showing an association of opiate withdrawal with decreased activity of dopaminergic neurons innervating the NAc (Georges et al., 2006). DA impairment in the NAc during withdrawal could underlie the compulsive drug intake that characterizes addiction (Volkow et al., 2009).
Present data showed that, in contrast to the NAc, TH IR in the VTA did not decrease in morphine-withdrawn rats. DA levels in the NAc are in synaptic terminals of neurons arising in the VTA and are directly dependent on TH levels in these neurons. Because TH is at normal levels in the VTA during morphine withdrawal in spite of the impaired TH levels in NAc, we reasoned that there might be homeostatic mechanisms to elevate TH expression in DA cell bodies to replenish neurotransmitter levels lost during withdrawal. Nevertheless, these compartmental differences would reflect an overall autonomy of DA regulation between somatodendritic and terminal fields compartments, as has been shown recently (Salvatore and Pruett, 2012).
Binding sites for both Nurr1 and Pitx3 factors have been identified at the promoter region of TH gen (Jacobs et al., 2009). On the other hand, alterations in Nurr1 and Pitx3 transcription and protein levels have been detected after acute and chronic cocaine and methamphetamine administration (Leo et al., 2007; Krasnova et al., 2011), but it remained unclear whether a regulatory interaction exists between these transcription factors and opiate effects on TH in the mesolimbic pathway. Our result revealed an increase of Nurr1 and Pitx3 expression in morphine-withdrawn rats, suggesting that these transcription factors play a role in controlling adaptation to morphine withdrawal-induced depression of DA neurons activity in the NAc (as revealed by decreased TH expression). Because these transcription factors are required for proper neurotransmission and maintenance of DA neurons (Jankovic et al., 2005; Jacobs et al., 2009), the combined increases in Nurr1 and Pitx3 expression might represent, in part, some of the mechanisms that served to protect against the decreased dopaminergic neurotransmission observed in morphine-withdrawn rats, as has been proposed for TH regulation in response to stress (Tank et al., 2008) and after chronic methamphetamine administration (Krasnova et al., 2011). In fact, the morphine withdrawal-induced decreased tTH levels in the NAc were not seen in the VTA. In addition, significant increase in pSer31-TH levels occurred in the VTA. TH is an enzyme that catalyses the first reaction in catecholamine synthesis pathways. It has been shown that Ser31 phosphorylation could be critical for DA biosynthesis regulation in vivo (Salvatore et al., 2009). In agreement, pSer31-TH co-varies with DA tissue content. So, TH Ser31 phosphorylation could have considerable impact on DA regulation and, consequently, upon DA-influenced behaviours in neuronal compartments where DA synthesis may be critical to maintain normal DA bioavailability. Thus, both TH Ser31 phosphorylation and tTH protein content in the substantia nigra have significant correlation to locomotor activity (Salvatore et al., 2009). Taken together, these findings support the idea that modulation of TH activity not only in terminal fields, but also in the VTA, might have important consequences for behaviours modulated by DA. In fact, it has been shown that somatodendritic DA content and release is relevant to the mesoaccumbens pathway where the process of drug reinforcement may be influenced by increased DA tone in VTA (Nimitvilai and Brodie, 2010).
Our data contrast with previous results showing significant reduction of Nurr1 expression with age in post-mortem VTA of human heroin abusers, where elevated TH mRNA levels were seen (Horvath et al., 2007). Many factors may account for this discrepancy, such as differences in species, the possibility that subjects could have used other drugs that contributed in part to dopaminergic alteration and information regarding the entire medication history that may influence the dopaminergic markers.
It is well known that transcriptional factors are translocated to the nucleus when activated. So, we investigated, by immunostaining and confocal microscopy, if Nurr1 and/or Pitx3 are translocated to the nucleus of VTA TH-positive neurons. The results showed that significant Pitx3 nuclear translocation occurred in the morphine-withdrawn group. Because that translocation of Pitx3 to the nucleus was strictly correlated with normal TH protein expression and with increased TH activation in the VTA, it is tempting to speculate that the up-regulation of Nurr1 and Pitx3 that was seen at that level after naloxone-induced morphine withdrawal might be a compensatory mechanism to replenish TH levels in DA cell bodies, suggesting the possibility that TH is a direct target of Nurr1/Pitx3 activation. TH activation in the VTA would be viewed as the mechanism to elevate the depleted neurotransmitter levels. Our observations also point to complex regulatory network involved in the control of TH expression and activation in the midbrain as has recently been showed (Lenartowski and Goc, 2011).
It has been suggested that post-transcriptional regulation by miRNA may be a molecular mechanism contributing to opiate tolerance and drug addiction (He et al., 2010; Sanchez-Simon et al., 2010). Ago2, which is important in miRNA-mediated gene silencing, has recently been implicated along with several miRNAs, in cocaine-mediated gene expression and cocaine addiction (Schaefer et al., 2010). Here, we showed that chronic morphine administration as well as naloxone-induced morphine withdrawal induced a decrease of Ago2 in the VTA, which coincided with increased Nurr1 and Pitx3 expression. Based in the well known negative correlation between Ago2 and Nurr1 and Pitx3 expression, present results might suggest that deficiency in Ago2 during chronic morphine administration would indicate its potential involvement, beside miRNAs, in regulation of transcription factors important for the expression of TH, as has been proposed in cocaine addiction studies (Schaefer et al., 2010).
In conclusion, acute morphine-increased DA neural activity in the NAc and triggers adaptive changes in TH expression and activity as well as in transcription factors and Ago2 levels when administered chronically. To our knowledge, this is the first time that the effects of morphine and morphine dependence on Ago2 and transcription factors Nurr1 and Pitx3 have been studied in the context of dopaminergic function in the mesolimbic pathway. With the increasing understanding of Ago2 and miRNAs in epigenetic regulation, further research is needed to identify the underlying molecular mechanisms in TH disregulation during opiate administration.
Acknowledgments
This work was supported by the following grants: Ministerio de Ciencia e Imnovación (SAF/FEDER2009-07178, SAF/FEDER2010-17907), Spain; Red de Trastornos Adictivos (RD06/0001/1006 and RD06/0001/1001), Spain; Fundación Séneca, Agencia Regional de Ciencia y Tecnología Región de Murcia (15405/PI/10), Spain. D. G-P. was supported by a fellowship from the Ministerio de Ciencia e Imnovación (AP2009-2379).
Glossary
- Ago2
Argonaute2
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Conflict of interest
Authors have no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edn. Brit J Pharmacol. 2011;164(Suppl. 1):324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Bolte S, Cordelierès FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Frenois F, Cador M, Caille S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci. 2002;16:1377–1389. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G. No effects of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26:5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellets implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multipl-site phosphorylation in vivo and in synaptosomes. J Biol Chem. 1991;266:5650–5657. [PubMed] [Google Scholar]
- He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the μ opioid receptor. J Neurosci. 2010;30:10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13:453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- Horvath MC, Kovacs GG, Kovari V, Majtenyi K, Hurd YL, Keller E. Heroin abuse is characterized by discrete mesolimbic dopamine and opioid abnormalities and exaggerated nuclear receptor-related 1 transcriptional decline with age. J Neurosci. 2007;27:13371–13375. doi: 10.1523/JNEUROSCI.2398-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshyar H, Manalo S, Dallman MF. Time-dependent alterations in mRNA expression of brain neuropeptides regulating energy balance and hypothalamo-pituitary-adrenal activity after withdrawal from intermittent morphine treatment. J Neurosci. 2004;24:9414–9424. doi: 10.1523/JNEUROSCI.1641-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Hong S, Jeong JW, Choi S, Kim H, Kim J, et al. Vesicular monoamine transporter 2 and dopamine transporter are molecular targets of Pitx3 in the ventral midbrain dopamine neurons. J Neurochem. 2009;111:1202–1212. doi: 10.1111/j.1471-4159.2009.06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs FMJ, van Erp S, van der Linden AJA, von Oerthel L, Burbach JP, Smidt MP. Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development. 2009;136:531–540. doi: 10.1242/dev.029769. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Prog Neurobiol. 2005;77:128–138. doi: 10.1016/j.pneurobio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Hodges AB, Volkow ND, Cadet JL. Chronic methamphetamine administration causes differential regulation of transcription factors in the rat midbrain. PLoS ONE. 2011;6:e19179. doi: 10.1371/journal.pone.0019179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowski R, Goc A. Epigenetic, transcriptional and posttranscriptional regulation of the tyrosine hydroxylase gene. Int J Dev Neurosci. 2011;29:873–883. doi: 10.1016/j.ijdevneu.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Leo D, di Porzio U, Racagni G, Riva MA, Fumagalli F, Perrone-Capano C. Chronic cocaine administration modulates the expression of transcription factors involved in midbrain dopaminergic neuron function. Exp Neurol. 2007;203:472–480. doi: 10.1016/j.expneurol.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Hayashi N, Kaneko IS, Mori K, Sabban EL, Nagatsu T, et al. Role of N-terminus of tyrosine hydroxylase in the biosynthesis of catecholamines. J Neural Transm. 2009;116:1355–1362. doi: 10.1007/s00702-009-0227-8. [DOI] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Núñez C, Laorden ML, Milanes MV. Effects of corticotropin-releasing factor receptor-1 (CRF1R) antagonists on the brain stress system responses to morphine withdrawal. Mol Pharmacol. 2010;77:864–873. doi: 10.1124/mol.109.062463. [DOI] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Núñez C, Ruiz-Medina J, Laorden ML, Valverde O, Milanés MV. CRF(2) mediates the increased noradrenergic activity in the hypothalamic paraventricular nucleus and the negative state of morphine withdrawal in rats. Br J Pharmacol. 2011;162:851–862. doi: 10.1111/j.1476-5381.2010.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Hidalgo JM, Laorden ML, Milanés MV. Glucocorticoid receptors participate in the opiate withdrawal-induced stimulation of rats NTS noradrenergic activity and in the somatic signs of morphine withdrawal. Br J Pharmacol. 2012;166:2136–2147. doi: 10.1111/j.1476-5381.2012.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, Brodie MS. Reversal of prolonged dopamine inhibition of dopaminergic neurons of the ventral tegmental area. J Pharmacol Exp Ther. 2010;333:555–563. doi: 10.1124/jpet.109.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez C, Laorden ML, Milanes MV. Regulation of serine (Ser)-31 and Ser40 tyrosine hydroxylase phosphorylation during morphine withdrawal in the hypothalamic paraventricular nucleus and nucleus tractus solitarius-A2 cell group. Role of ERK1/2. Endocrinology. 2007;148:5780–5793. doi: 10.1210/en.2007-0510. [DOI] [PubMed] [Google Scholar]
- Núñez C, Földes A, Pérez-Flores D, García-Borrón JC, Laorden ML, Kovács KJ, et al. Elevated glucocorticoid levels are responsible for induction of tyrosine hydroxylase (TH) mRNA expression, phosphorylation and enzyme activity in the nucleus of the solitary tract (NTS-A2) during morphine withdrawal. Endocrinology. 2009;150:3118–3127. doi: 10.1210/en.2008-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Academic Press; 2007. [Google Scholar]
- Reddy SD, Rayala SK, Ohshiro K, Pakala SB, Kobori N, Dash P, et al. Multiple coregulatory control of tyrosine hydroxylase gene transcription. Proc Natl Acad Sci U S A. 2011;108:4200–4205. doi: 10.1073/pnas.1101193108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS ONE. 2012;7:e29867. doi: 10.1371/journal.pone.0029867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS, Spann SL, Dempsey C. Aging reveals a role for nigral tyrosine hydroxylase ser31 phosphorylation in locomotor activity generation. PLoS ONE. 2009;4:e8466. doi: 10.1371/journal.pone.0008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE. Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol. 2010;78:935–942. doi: 10.1124/mol.110.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Im HI, Veno MT, Fowler CD, Min A, Intrator A, et al. Argonaute 2 in dopamine 2 receptor-coexpressing neurons regulates cocaine addiction. J Exp Med. 2010;207:1843–1851. doi: 10.1084/jem.20100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt MP, van Schaick HSA, Lanctôt C, Tremblay JJ, Cox JJ, van der Kleij AAM, et al. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci U S A. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP. A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci. 2000;3:337–341. doi: 10.1038/73902. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Smits SM, Burbach JP. Homeobox gene Pitx3 and its role in the development of dopamine neurons of the substantia nigra. Cell Tissue Res. 2004;318:35–43. doi: 10.1007/s00441-004-0943-1. [DOI] [PubMed] [Google Scholar]
- Tank AW, Xu L, Chen X, Radcliffe P, Sterling CR. Post-transcriptional regulation of tyrosine hydroxylase expression in adrenal medulla and brain. Ann N Y Acad Sci. 2008;1148:238–248. doi: 10.1196/annals.1410.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl. 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]