Intravenous immunoglobulin (IVIg) therapy can benefit diverse autoimmune and inflammatory diseases via several mutually nonexclusive mechanisms[1,2]. Recent studies both in vitro and in vivo in experimental models have also demonstrated that IVIg can expand CD4+CD25+ regulatory T cells (Treg), the cells that play a critical role in maintaining immune tolerance[3,4]. Tregs maintain immune tolerance by suppressing the activation and function of both innate and adaptive immune cells while deficiency of Tregs is associated with autoimmune and inflammatory conditions[5,6]. Since IVIg therapy in autoimmune patients is associated with restoration of immune tolerance, we hypothesize that this effect of IVIg is in part via expansion of Tregs in these patients, the bonafide immune regulators.
To test our hypothesis, we analyzed Treg in paired blood samples of autoimmune rheumatic patients before and 72–96 hr after high-dose IVIg therapy (Tegeline®, 2g/kg per month)[1]. The patients broadly belonged to two groups: idiopathic inflammatory myopathy (8 patients with age ranging from 22 to 57 yr; 3 male patients) and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (3 patients with age ranging from 61 to 68 yr; 2 males). The patients’ details are provided in Table 1. The local ethical committee approval was obtained for collecting the blood samples and informed consent was taken from the patients. Peripheral blood mononuclear cells were isolated from the blood samples by ficoll-density gradient and CD4+CD25high T cells were analyzed by flow cytometry by using fluorescence-conjugated monoclonal antibodies (BD Biosciences, France).
Table 1.
Summary of data for autoimmune rheumatic patients
| Number | Disease | Sex/Age (years) | Associated symptoms |
|---|---|---|---|
| 1 | Dermatomyositis | F/49 | Proximal muscle weakness, skin rash |
| 2 | Dermatomyositis | M/35 | Proximal muscle weakness, skin rash |
| 3 | Polymyositis | M/42 | Proximal muscle weakness, polyarthritis, interstitial lung disease |
| 4 | Granulomatosis with polyangiitis | M/62 | Polyarthritis, peripheral neuropathy, CNS involvement, pulmonary nodules, anti-proteinase 3 ANCA |
| 5 | Microscopic polyangiitis | F/61 | Arthralgias, myalgias, peripheral neuropathy, anti-myeloperoxidase ANCA |
| 6 | Dermatomyositis | F/22 | Proximal muscle weakness, interstitial lung disease, typical skin involvement with Gottron papules |
| 7 | Inflammatory myopathy associated with diffuse systemic sclerosis | F/38 | Proximal muscle weakness, severe gastrointestinal tract involvement with gastroparesis and colectasis |
| 8 | Inclusion body myositis | M/57 | Myalgias and proximal and distal asymmetrical muscle weakness |
| 9 | Granulomatosis with polyangiitis | M/68 | Skin, peripheral nerves, joint involvement, anti-proteinase 3 and anti-myeloperoxidase ANCA, tritruncular coronaropathy and dilatation. |
| 10 | Polymyositis | F/43 | Proximal muscle weakness and myocardial involvement |
| 11 | Dermatomyositis | F/45 | Proximal muscle weakness, skin rash |
We found that, six patients including the three with ANCA-associated vasculitis had substantial increase in the percentage of Tregs following IVIg therapy (2.2±0.3% before IVIg therapy and 7.9±1.8% post-IVIg therapy), 3 patients with myositis had marginal enhancement in Tregs (1.1±0.6% before IVIg therapy and 1.7±0.7% post-IVIg therapy) and in two myopathies patients Treg percentage did not alter (1.95±0.7% before IVIg therapy and 1.97±0.6% post-IVIg therapy) (Figure 1). Thus, these results indicate that anti-inflammatory effects IVIg therapy is associated with enhancement of Tregs in autoimmune patients and these Tregs might further help to restore immune tolerance.
Figure 1.
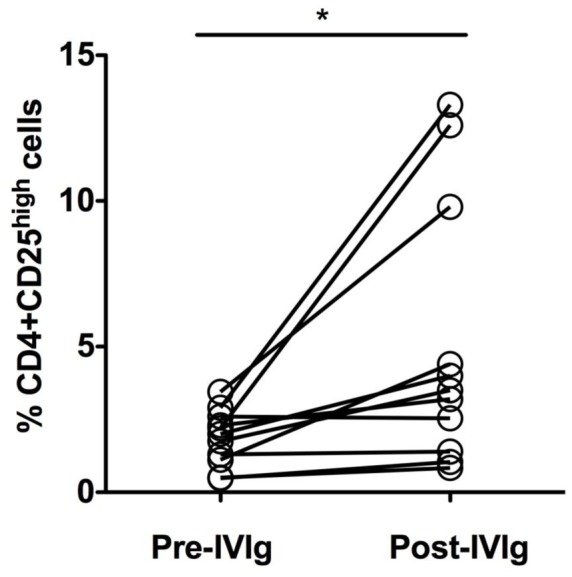
Changes in the percentage of regulatory T cells in autoimmune patients before (Pre-IVIg) and following IVIg (Post-IVIg) therapy. Peripheral blood mononuclear cells were isolated from heparinized blood samples and CD4+CD25high Tregs were analyzed by flow cytometry (LSR II, BD Biosciences) by using fluorescence-conjugated monoclonal antibodies (BD Biosciences) The patients are represented by open circles. *, P<0.05 by Student-t-test.
Although several immunosuppressive drugs including steroids can enhance Tregs [7], IVIg has an added advantage wherein this therapy is not an immunosuppressor rather an immunomodulator. Hence adverse effects associated with immunosuppressive therapies can be avoided by IVIg therapy. The enhancement of Tregs following IVIg therapy might implicate several mutual nonexclusive mechanisms[8–10]. It is known that inflammatory cytokines suppress Tregs[8] and by neutralizing these inflammatory mediators, IVIg might favor Treg expansion. In addition, IVIg is known to modulate the maturation and function of innate immune cells and these modulated innate cells may expand Tregs. Alternatively, IVIg can reciprocally regulate pathogenic Th17 and Tregs[10].
Acknowledgments
Financial support information: Supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Université Pierre et Marie Curie and Université Paris Descartes, European Community’s Seventh Framework Programme [FP7/2007–2013] under Grant Agreement No: 260338 ALLFUN.
References
- 1.Bayry J, Negi VS, Kaveri SV. Intravenous immunoglobulin therapy in rheumatic diseases. Nat Rev Rheumatol. 2011;7:349–59. doi: 10.1038/nrrheum.2011.61. [DOI] [PubMed] [Google Scholar]
- 2.Seite JF, Shoenfeld Y, Youinou P, Hillion S. What is the contents of the magic draft IVIg? Autoimmun Rev. 2008;7:435–39. doi: 10.1016/j.autrev.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Kessel A, Ammuri H, Peri R, Pavlotzky ER, Blank M, Shoenfeld Y, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–75. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- 4.Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–22. doi: 10.1182/blood-2007-03-079947. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Qian L, Wang G, Zhang H, Wang X, Chen K, et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjogren’s syndrome. J Rheumatol. 2007;34:2438–45. [PubMed] [Google Scholar]
- 7.de Paz B, Alperi-Lopez M, Ballina-Garcia FJ, Prado C, Gutierrez C, Suarez A. Cytokines and regulatory T cells in rheumatoid arthritis and their relationship with response to corticosteroids. J Rheumatol. 2010;37:2502–10. doi: 10.3899/jrheum.100324. [DOI] [PubMed] [Google Scholar]
- 8.Bayry J, Siberil S, Triebel F, Tough DF, Kaveri SV. Rescuing CD4+CD25+ regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy. Drug Discov Today. 2007;12:548–52. doi: 10.1016/j.drudis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 9.De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–11. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddur MS, Vani J, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibition of differentiation, amplification and function of human Th17 cells by intravenous immunoglobulin. J Allergy Clin Immunol. 2011;127:823–30.e827. doi: 10.1016/j.jaci.2010.12.1102. [DOI] [PubMed] [Google Scholar]


