Abstract
Rationale
Pericytes are key regulators of vascular maturation, but their value for cardiac repair remains unknown.
Objective
We investigated the therapeutic activity and mechanistic targets of saphenous vein-derived pericyte progenitor cells (SVPs) in a mouse myocardial infarction (MI) model.
Methods and Results
SVPs have a low immunogenic profile and are resistant to hypoxia/starvation (H/S). Transplantation of SVPs into the peri-infarct zone of immunodeficient CD1/Foxn-1nu/nu or immunocompetent CD1 mice attenuated left ventricular dilatation and improved ejection fraction compared to vehicle. Moreover, SVPs reduced myocardial scar, cardiomyocyte apoptosis and interstitial fibrosis, improved myocardial blood flow and neovascularization, and attenuated vascular permeability. SVPs secrete vascular endothelial growth factor A, angiopoietin-1, and chemokines and induce an endogenous angiocrine response by the host, through recruitment of vascular endothelial growth factor B expressing monocytes. The association of donor- and recipient-derived stimuli activates the proangiogenic and prosurvival Akt/eNOS/Bcl-2 signaling pathway. Moreover, microRNA-132 (miR-132) was constitutively expressed and secreted by SVPs and remarkably upregulated, together with its transcriptional activator cyclic AMP response element-binding protein, on stimulation by H/S or vascular endothelial growth factor B. We next investigated if SVP-secreted miR-132 acts as a paracrine activator of cardiac healing. In vitro studies showed that SVP conditioned medium stimulates endothelial tube formation and reduces myofibroblast differentiation, through inhibition of Ras-GTPase activating protein and methyl-CpG-binding protein 2, which are validated miR-132 targets. Furthermore, miR-132 inhibition by antimiR-132 decreased SVP capacity to improve contractility, reparative angiogenesis, and interstitial fibrosis in infarcted hearts.
Conclusion
SVP transplantation produces long-term improvement of cardiac function through a novel paracrine mechanism involving the secretion of miR-132 and inhibition of its target genes.
Keywords: pericytes-based cell therapy, myocardial infarction, angiogenesis, VEGF-B, microRNA-132
With myocardial infarction (MI) remaining a major cause of morbidity and mortality worldwide, cell therapy now aims to offer a novel option for cardiac repair.1 Clinical trials showed that administration of bone marrow-derived progenitor cells (PCs) improves left ventricular (LV) function in patients with coronary artery disease.2-4 However, more specialized cells are warranted to fulfill specific regenerative needs of the ischemic myocardium.
Pericytes provide the physical strength and nurturing signals that instruct neovessels to organize in a stable and efficient tubular network.5 On the other hand, ischemic disease and associated risk factors may impair pericyte recruitment.6-8 Therefore, a supply-side approach with fresh pericytes from exogenous sources could be helpful therapeutically. However, difficulties in isolating and expanding bona-fide pericytes from accessible human tissues have so far precluded clinical applications.
Two main mural cell populations, probably originating from a common embryonic ancestor, have been described in adult tissues based on stringent topographical, clonogenic, antigenic, and functional criteria.9,10 A population of CD34− cells, typically located around capillaries and microvessels in multiple human organs and hence acknowledged as pericytes, give rise to mesenchymal cells with multilineage differentiation capacity and promote reparative myogenesis on transplantation in models of muscular dystrophy.11,12 Another population of CD34+ cells, located around the vasa vasorum in the adventitia of arteries and veins, also express typical pericyte markers (NG2, PDGFRβ, and RGS5) together with mesenchymal (CD44, CD90, CD73, CD29) and stemness antigens (Oct-4 and Sox-2). This adventitial subset contains PCs that may contribute to angiogenesis as well as atherosclerosis and neointima formation.13-17
Recently, we have shown that CD34+ PCs from human fetal aorta adventitia possess a robust regenerative capacity in mouse models of peripheral limb ischemia and ischemic below-knee ulcers in diabetic mice.18,19 Aiming to find a similar regenerative population for autologous cell therapy, we succeeded in isolating and expanding a clonogenic population of CD34+/CD31− pericyte progenitors from saphenous vein leftovers of patients undergoing coronary artery bypass graft surgery.20 On transplantation into mouse ischemic limbs, saphenous vein-derived pericyte progenitors (SVPs) proved to be superior to an equal dosage of circulating proangiogenic cells (previously identified as EPCs) in supporting blood flow recovery. The benefit was attributed to potentiation of reparative angiogenesis by as yet incompletely understood interactions between SVPs and endothelial cells (ECs).20
The present study demonstrates that SVP transplantation induces long-term improvements in a mouse model of MI, through angiocrine activities of donor and recipient cells. Moreover, we show that SVPs produce and release the microRNA-132 (miR-132), which exerts proangiogenic, prosurvival, and antifibrotic activity via inhibition of its targets Ras-GTPase activating protein (RasGAP, also named RASA1) and methyl-CpG-binding protein 2 (MeCP2).
Methods
Expanded methods are provided as an online supplement, available at http://circres.ahajournals.org.
Induction of Infarct and Cell Therapy
MI was induced in 8-week-old male immunodeficient CD1-FOX-Onu/nu (Charles River) or immunocompetent CD1 mice (Harlan) by occlusion of the left anterior descending coronary artery,21 followed by injection of Dil-stained SVPs (3×105 or 1×106 cells per heart), human bone marrow mesenchymal stem cells (MSCs; 1×106 per heart) or PBS at 3 different sites along the infarct border zone. To investigate the importance of miR-132 in post-MI recovery, cells were transfected with anti-miR-132 or scrambled sequence (Applied Biosystems) before transplantation.
Hemodynamic Measurements
Cardiac function was monitored using a high-resolution echocardiograph (Vevo 770, Visual Sonics, n=14 per group).21 LV pressures were assessed using a high-fidelity 1.4F transducer tipped catheter inserted into the LV chamber.21
Myocardial blood flow was measured using intraventricularly-injected fluorescent microspheres.21 In vivo vascular permeability was determined by assessing the distribution of fluorescent-conjugated dextran (see Expanded Methods).
Analyses on Explanted Hearts
LV samples were collected at 5, 14 and 42 days post-MI for histological, immunohistochemical and molecular analyses. Cell apoptosis and scar size were determined using terminal deoxynucleotidyl transferase dUTP nick end labeling and Azan Mallory staining, respectively.21
Cell Culture for In Vitro Experiments
SVPs and human umbilical vein ECs (HUVECs, Lonza) were cultured on fibronectin and in endothelial growth medium containing 2% FBS (Lonza). MSCs were grown using mesenchymal cell medium (Lonza). In selected experiments, the effects of hypoxia (1% O2) and serum deprivation (H/S) on cell proliferation (BrdU Incorporation Assay, Roche), apoptosis (caspase-3/7, Promega), and expressional profile (Western blot and RT-PCR) were studied.
Adult rat cardiomyocytes were isolated using collagenase perfusion and used immediately for experiments. Mouse cardiac fibroblasts were isolated and cultured as described.22
Role of miR-132 on SVP-Associated Functions
SVPs were transfected with scrambled oligonucleotide or anti-miR-132 (50 nmol/L) and tested for their capacity to promote network formation by HUVECs on Matrigel (BD Biosciences).20 Similar assays were repeated using HUVECs exposed to conditioned medium from SVPs (SVP-CM) or MSCs (MSC-CM) or unconditioned medium (UCM).
To verify SVP paracrine influence on cardiac cells, rat cardiomyocytes were exposed to hypoxia for 18 hours in the presence of SVP-CM and then assayed for caspase-3/7 activity. Similarly, we determined the effect of SVP-CM on mouse cardiac fibroblast proliferation and differentiation into myofibroblasts, as induced by angiotensin II (AngII, 100 nmol/L). Myofibroblasts were distinguished by double immunostaining for α-smooth muscle actin and MeCP2.
Molecular Analyses
Total RNA was isolated from flash-frozen LV myocardium, SVPs, SVP-CM, and MSC-CM using TRIzol (Invitrogen) and reverse-transcribed using Qiagen reverse transcriptase kit, followed by cDNA amplification using Quantitect predesigned primers (Qiagen). miR-132 was measured by TaqMan PCR. Data were normalized to the small RNA RNU6B (Applied Biosystems).
Protein extracts from LV myocardium or SVPs were used for western blotting and ELISA. AngII and norepinephrine levels in plasma and LV myocardium were measured using commercial ELISA.
Statistical Analysis
Comparison of multiple groups was performed by ANOVA. Two-group analysis was performed by Student t test. Values were expressed as means±SEM. Probability values (P) less than 0.05 were considered significant.
Results
Verification of SVP Antigenic Profile
By flow cytometry and immunocytochemistry, we confirmed the typical SVP antigenic phenotype described earlier (Online Figure I, A and B)20 and verified that it is not modified by Dil-labeling prior to transplantation or culture under H/S. Following in vitro exposure to an enriched medium that supports cardiomyocyte differentiation,23 SVPs acquired the typical cardiomyocyte markers αSA, GATA4, and connexin-43, while retaining the NG2 marker (Online Figure I, C). Furthermore, we found that SVPs express intermediate levels of major histocompatibility complex class I human leukocyte antigens A, B, and C and are negative for class II human leukocyte antigen HLA-DR. SVPs were also negative for CD80 and Fas ligand as assessed by RT-PCR (Online Figure I, D and IE).
SVP Transplantation Benefits Early Post-MI Recovery in Both Immunodeficient and Immunocompetent Mice
As shown in Online Table I, transplantation of SVPs (1×106) in the heart of CD1-FOXOnu/nu-immunodeficient mice attenuated the LV anterior wall thinning (P<0.05 versus vehicle) and the decrease of LV ejection fraction (LVEF; P<0.05) and cardiac output (P<0.05) at 14 days post-MI. Furthermore, cell therapy improved pressure indexes (P<0.05).
Next, we tested SVPs (at 3×105 or 1×106) in the infarcted heart of immunocompetent mice and followed up the recovery for 2 weeks. Both cell doses improved volumetric and functional parameters (Online Figure II, A–H) and pressure indexes (Online Figure II, I–K) and induced a leftward shift of the LV pressure-volume curve compared to vehicle (Online Figure II, L).
SVPs Exert Long-Term Improvements in Immunocompetent Mice
Next, we tested the long-term effect of SVP transplantation in immunocompetent mice with MI. Human bone marrow-derived MSCs were used as a cellular control. As shown in Online Figure III, A, the SVP-treated group had an improved survival. The experiment was stopped at 42 days because the pre-established mortality end point (50%) was reached in vehicle-injected mice. HR increased following MI, with no difference between groups (data not shown).
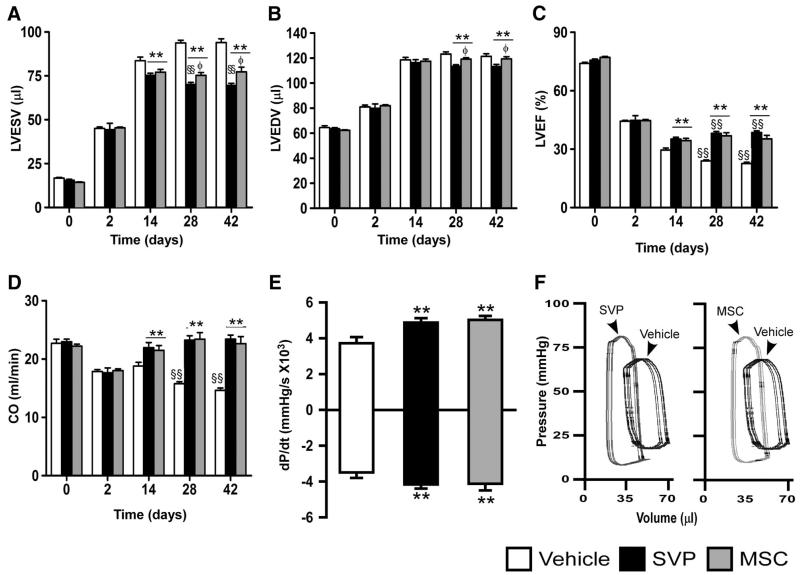
Echocardiographic parameters did not differ among groups at 2 days post-MI, thus confirming the consistence of procedure for MI induction but were remarkably improved by SVPs at subsequent measurements (Figure 1A–D and Online Figure III, B and C). Specifically, SVP transplantation blunts the progression of adverse cardiac remodeling (Figure 1A and 1B and Online Figure III, B and C) and decline of LVEF (Figure 1C). At 42 days, cardiac output was reduced by 36% in vehicle-injected mice, whereas it had completely recovered in SVP-transplanted mice (Figure 1D). The frequency of the composite end point, consisting of mortality plus an LVEF of less than 35%, was 40% in SVP-treated and 100% in vehicle-treated mice. Furthermore, LV pressure indexes and pressure/volume relationship were improved in SVP-transplanted mice at 42 days post-MI (Figure 1E&F). SVP-transplanted mice showed reduced neurohormonal activation especially at the myocardial level (P<0.05 versus vehicle-injected, Online Figure III, D and E).
Figure 1. SVP transplantation prevents late LV dysfunction in immunocompetent mice.
A–E, Bar graphs showing hemodynamic parameters. F, Representative pressure/volume loops. Data are means±SE (n=13 mice per group). **P<0.01 vs vehicle-treated; §§P<0.01 vs corresponding-treatment group at 14 days post-MI; ɸP<0.05 vs SVP-transplanted.
Human MSCs, used as a cellular control, showed similar improvements in survival (Online Figure III, A) and contractility (Figure 1C–1F) but were inferior to SVPs in blunting LV anterior wall thinning (P<0.05, Online Figure III, C) and LV chamber dilatation (P<0.05, Figure 1A and 1B).
SVPs Maintain Their Original Phenotype After Engraftment in the Infarcted Myocardium
Next, we checked the engraftment of transplanted Dil-labeled SVPs using confocal microscopy. At 5 days post-transplantation, Dil-labeled SVPs formed patches in the border zone (Figure 2Ai), while, from 14 days on, they were fewer and prevalently located in the infarct zone (Figure 2Aii). SVP engraftment was confirmed by staining for human mitochondrial antigen (data not shown).
Figure 2. Graft localization and promotion of angiogenesis.
A–C, Representative confocal images showing the localization of Dil-labeled SVPs (red) at 5 (Ai) and 42 days (Aii) post-MI. Dil-labeled SVPs, positive for NG2 (white, Bi) and PDGFRβ (white, Bii), are juxta-posed to ECs identified by intravenously-injected isolectin (green). Some SVPs are positive for PCNA (pale blue, arrowheads, C). Nuclei stained blue by DAPI. Scale bars are 50 μm. D, E, Bar graphs showing LV myocardial blood flow (D) and vascular permeability (E). F, Representative fluorescent image and bar graph showing the density of capillaries, assessed by counting small-size (<10 μm in diameter) isolectin-positive structures, and arterioles, assessed by counting α-smooth muscle actin-positive structures. Scale bars are 50 μm. Dotted lines delineate the infarct zone. Data are means±SE (n=6 in each group). ##P<0.01 and ###P<0.001 vs sham; *P<0.05 and **P<0.01 vs vehicle.
Multicolor fluorescence microscopy shows that Dil-labeled SVPs are juxtaposed to isolectin-positive capillary ECs and express typical pericyte markers NG2 and PDGFRβ (Figure 2B), with some being positive for the proliferation marker proliferating cell nuclear antigen (Figure 1C). Tridimensional confocal microscopy confirms the association of SVPs with perfused vessels, stained by intracardially injected Isolectin (Online Movie). Altogether, these data indicate that SVPs retain their original antigenic and peri-vascular phenotype on transplantation in the infarcted heart.
SVP Transplantation Improves Myocardial Perfusion and Reparative Neovascularization
Using fluorescent microspheres, we documented that SVP transplantation increases LV blood flow at 14 days post-MI (Figure 2D). Furthermore, SVPs attenuate vascular permeability (Figure 2E), which reportedly contributes to myocyte damage and interstitial fibrosis.5
Immunohistochemistry analysis of peri-infarct microvasculature from early to late phase of recovery showed persistently higher levels of capillaries and arterioles in SVP-transplanted hearts (P<0.01 versus vehicle, Figure 2F). This was associated with increased EC proliferation (P<0.05, Online Figure IV, A) and reduced EC apoptosis (P<0.05, Online Figure IV, B). MSC-transplanted hearts showed a similar improvement in capillarization but not in arteriologenesis (Online Figure V, A and B).
Angiocrine Response Activated by SVP Transplantation
SVPs secrete large amounts of vascular endothelial growth factor (VEGF)-A and angiopoietin-1 (Ang-1) and modest quantity of VEGF-B186, as assessed by ELISA of CM. Secretion of VEGF-A and Ang-1 is further augmented by H/S, which simulates in vitro the ischemic environment encountered by SVPs on transplantation (Online Figure VI). Moreover, SVP transplantation increased the myocardial levels of immunoreactive VEGF-A, VEGF-B186, and Ang-1 (Figure 3A).
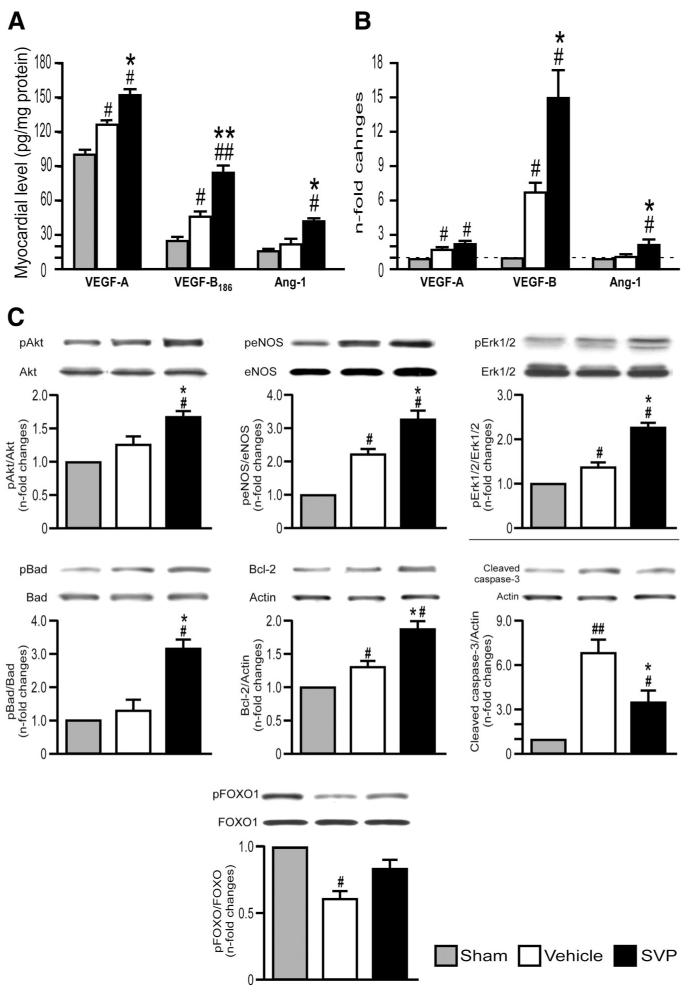
Figure 3. Activation of angiogenic factors and survival kinases.
A, B, Bar graphs showing the protein (A) and mRNA (B) levels of murine GFs in myocardium at 14 days post-MI. C, Representative western blotting and bar graphs showing myocardial activation of survival signaling pathway at 14 days post-MI. Data are means±SE (n=6 mice per group). #P<0.05 and ##P<0.01 vs sham; *P<0.05 and **P<0.01 vs vehicle.
As shown in Figure 3B, SVP transplantation also increased the mRNA levels of murine VEGF-B and Ang-1 in infarcted myocardium. Recruited CD45 and Mac-3 monocytes represent a rich source of angiogenic cytokines.24 They may contribute to the recipient’s angiocrine response because the abundance VEGF-B-expressing CD45+ monocytes was significantly increased in the peri-infarct zone of SVP-transplanted hearts at 5 days post-MI. (P<0.01 versus vehicle, Online Figure VII). In addition, SVP transplantation upregulated the expression of CX3C chemokine receptor 1 (data not shown), an antigenic marker shared by proangiogenic murine Ly-6Clow and human CD16+ monocytes.24 Consistent with its content of interleukin-8 (IL-8; 397±138 pg/mL/105 cells, n=8) and monocyte chemotactic protein-1 (1082±473 pg/mL/105 cells, n=6), SVP-CM stimulated by 2.0±0.3-fold the migration of human CD14+CD16+ cells in a transwell assay (P<0.05 versus UCM, n=5, data not shown).
VEGF and Ang-1 induce angiogenic and survival responses through Akt and extracellular signal regulated kinase (Erk).25,26 Consistently, Western blot analysis showed the activation of Akt/eNOS/Erk1/2/Bcl-2 signaling pathway and increased phosphorylation of Bad and FOXO1 in SVP-treated hearts, which was mirrored by the reduction of cleaved caspase 3 levels (Figure 3C). These data indicate that SVPs stimulate myocardial healing trough paracrine and host-intrinsic angiogenic and survival mechanisms.
SVPs Exert Paracrine Angiogenic Effects on Cultured ECs Through miR-132
Angiogenic GFs, like VEGF, and cellular kinases, like Akt, stimulate the expression of target genes by inducing the phosphorylation of the transcription factor cAMP response element binding protein (CREB) at Ser-133.27 By this mechanism VEGF induces the sustained upregulation of miR-132 in ECs, resulting in suppression of p120RasGAP and consequent Ras-dependent induction of EC proliferation and angiogenesis in vitro and in vivo.28 Hence, we next investigated if miR-132 is implicated in promotion of angiogenesis by SVPs.
Importantly, we found that miR-132 is expressed by SVPs under basal conditions and upregulated by 3.0±0.3-fold following H/S (P<0.01 versus normoxia, Figure 4Ai), this effect being associated with increased CREB phosphorylation (data not shown). Likewise, stimulation with VEGF-B increases miR-132 expression in SVPs by 1.30±0.02-fold (P<0.05 versus vehicle), while VEGF-A is ineffective (P=N.S., data not shown).
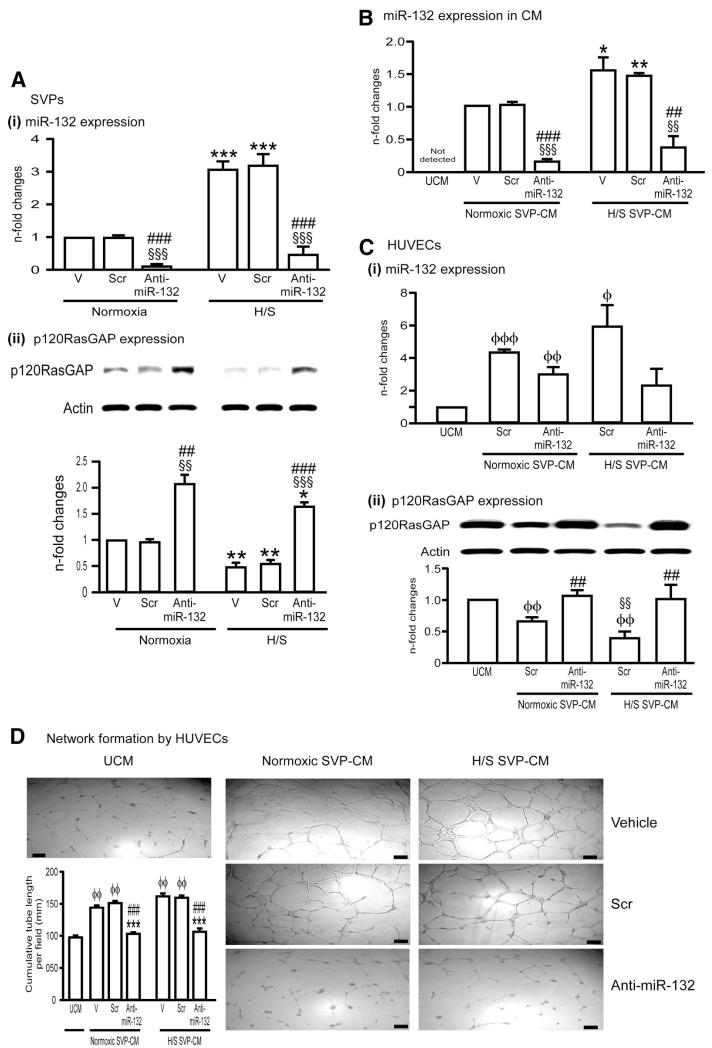
Figure 4. SVPs exert paracrine effects through miR-132.
A, Bar graphs showing induction of miR-132 (Ai) and inhibition of its target p120RasGAP (Aii) in SVPs exposed to H/S. AntimiR-132 transfection (50nmol/L) suppressed miR-132 expression while inducing p120RasGAP expression (Aii). Control SVPs were transfected with scrambled sequence (Scr, 50nmol/L), or exposed to transfectionvehicle (V). B. Levels of miR-132 in SVP-CM. Data are means±SE from experiments performed in quadruplicates. *P<0.05, **P<0.01, and ***P<0.001 vs corresponding group under normoxia; §§P<0.01 and §§§P<0.001 vs vehicle; ##P<0.01 and ###P<0.001 vs Scr. C, Bar graphs showing miR-132 levels (Ci) and p120RasGAP protein expression (Cii) in HUVECs exposed to SVP-CM collected under different conditions described above. D, Representative images and bar graphs showing the ability of SVP-CM to enhance HUVEC network formation and abrogation of this effect by CM of antimiR-132-transfected SVPs. HUVECs were cultured on matrigel with SVP-CM for 24 hours. Data are means±SE of experiments performed in quadruplicates. ɸP<0.05; ɸɸP<0.01 vs unconditioned medium (UCM); ɸɸɸP<0.001; §§P<0.01 vs corresponding group under normoxic SVP-CM; ##P<0.01 and ###P<0.001 vs Scr.
To investigate the functional relevance of miR-132, we transfected SVPs with anti-miR-132 and confirmed the inhibition of miR-132 (Figure 4Ai) and consequent upregulation of miR-132 target gene, p120RasGAP (Figure 4Aii). The anti-miR-132 slightly reduced SVP proliferation and survival in vitro (Online Figure VIII, A). We previously reported that SVPs remarkably improve the network-forming capacity of human ECs through physical and paracrine interactions.20 Here, we show that inhibition of miR-132 abrogates the capacity of SVPs to support the network formation by HUVECs in coculture (Online Figure VIII, B).
We measured miR-132 levels in SVP-CM and found that SVPs not only produce but also release miR-132 especially under H/S (Figure 4B). Anti-miR causes miRNA sequestration and/or degradation.29 Hence, we found miR-132 to be remarkably reduced in CM of anti-miR–transfected SVPs (Figure 4B). Furthermore, SVP-CM upregulates miR-132 expression in HUVECs (Figure 4Ci), resulting in concurrent inhibition of p120RasGAP (Figure 4Cii). These effects might be attributable to miR-132 transfer from SVP-CM to ECs and/or stimulation of de novo miRNA synthesis by paracrine factors contained in CM, ie, VEGF-A and Ang-1.
Next, we investigated the consequences of miR-132 inhibition on SVP-CM-induced promotion of angiogenesis. Consistent with our previous study,20 we found that SVP-CM stimulates HUVEC proliferation (Online Figure IX, A) and network formation capacity (Figure 4D), both effects being negated here by transfecting SVPs with anti-miR-132. Altogether, these data indicate that the miR-132, either taken up from CM or synthesized on CM stimulation, is crucial for EC growth and networking. VEGF and Akt act as CREB-dependent activators of miR-132; in turn, miR-132 can activate Akt unlocking Ras, a well acknowledged inductor of PI3K/Akt pathway30 In line, we found that pAkt levels are increased in HUVECs exposed to SVP-CM and reduced by miR-132 inhibition (Online Figure IX, B).
Comparison of CMs from SVPs and MSCs revealed that the former contains ≈3-fold higher levels of VEGF-A and Ang-1 (Online Figure X, Ai–iii). Likewise, miR-132 expression was superior in SVP-CM compared to MSC-CM (P<0.01, Online Figure XAiv). However, both SVP-CM and MSC-CM stimulate HUVEC network formation compared to their respective unconditioned media (P<0.01, Online Figure X, B).
Involvement of miR-132 in SVP-Induced Reparative Vascularization of Infarcted Heart
Immunohistochemical analyses of cardiac p120RasGAP revealed that the miR-132 target is mainly expressed in coronary ECs of sham-operated hearts. After MI, p120RasGAP was also expressed on cardiomyocytes and fibroblasts (Online Figure XI, A). SVP transplantation remarkably reduced p120RasGAP as assessed by confocal microscopy (Figure 5A) and Western blot (Online Figure XI, B). This effect was abrogated when SVPs were transfected with anti-miR-132 prior to transplantation (Figure 5A and Online Figure XI, B). We verified the sustained inhibition of miR-132 in SVPs transfected with anti-miR-132 (Online Figure XII).
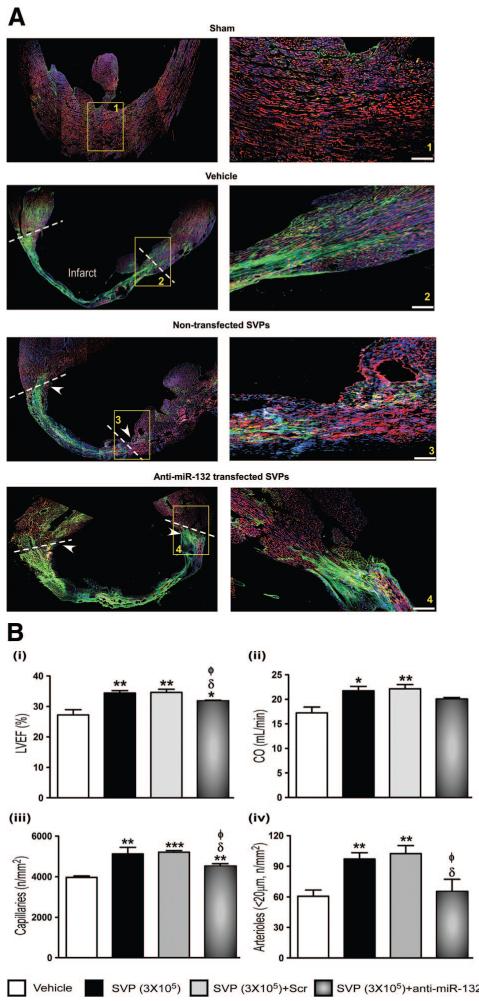
Figure 5. A, Immunofluorescence confocal microscopy images showing the levels of miR-132 target gene p120RasGAP in myocardium.
Arrowheads indicate the site of SVP injection, dotted lines delimitate the infarct area and magnification panel of boxed area is shown in right panel. B, Bar graphs showing hemodynamic data (i and ii) and vascular profile (iii and iv) at 14 days post-MI. Data are means±SE (n=5 mice per group except for hemodynamic measurements which consisted of 6 mice). *P<0.05, **P<0.01, and ***P<0.001 vs vehicle; δP<0.05 vs nontransfected SVPs; ɸP<0.05 vs Scrtransfected SVPs.
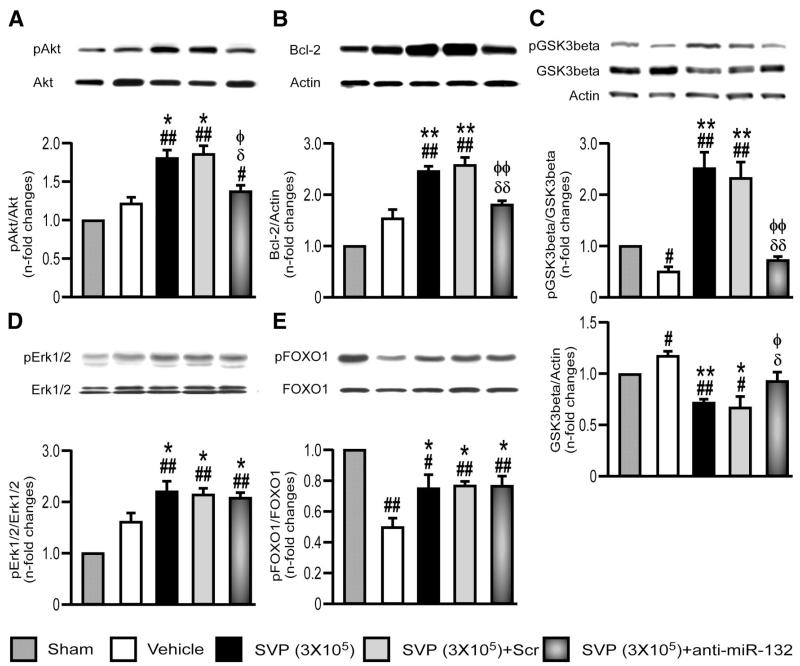
Importantly, anti-miR-132-transfected SVPs were inferior to naïve SVPs or scrambled-transfected SVPs in improving echocardiographic endpoints (Figure 5Bi and 5Bii and Online Figure XI, Ci), dP/dt (Online Figure XI, Ciii) angiogenesis (Figure 5Ciii and 5Civ) and EC survival and proliferation (Online Figure XI, Ciii and Online Figure XI, Civ). Nonetheless, the improvement afforded by SVPs was not completely abrogated, thus suggesting participation of miR-132-dependent and independent mechanisms. We transplanted equal numbers of naïve or anti-miR-132-transfected SVPs and verified that anti-miR-132 does not influence SVP engraftment (Online Figure XIII). Hence, reduction in SVP therapeutic activity by miR-132 inhibition has to be ascribed to functional rather than quantitative reduction. Looking at the impact of miR-132 inhibition on molecular endpoints, we found a reversal of Akt/Bcl-2 upregulation in hearts transplanted with anti-miR-132-transfected SVPs (Figure 6A&B). Akt inhibits GSK3β by phosphorylating it at Ser-9. Furthermore, GSK3β represents a predicted inhibitory target of miR-132. Consistently, we found that naïve SVPs remarkably increase Ser-9-phosphorylated GSK3β, while reducing total GSK3β; both the effects were inhibited by anti-miR-132 (Figure 6C). In contrast, Erk1/2 activation and FOXO1 phosphorylation (Figure 6D and 6E), as well as VEGF-B and Ang-1 upregulation (data not shown) were not affected following miR-132 inhibition. Altogether, these data indicate the crucial contribution of miR-132 in the proangiogenic and healing action of SVPs in the infarcted heart.
Figure 6. Inhibition of miR-132 attenuates SVP-induced activation of survival signaling.
A–E, Representative immunoblots and bar graphs showing the expression of pAkt (A), Bcl-2 (B), pGSK3-beta (C), pErk1/2 (D), and pFOXO1 (E) at 14 days post-MI in the myocardium of mice treated with naïve, scrambled-transfected or antimiR-132-transfected SVPs. Data are means±SE (n=5 mice per group). #P<0.05 and ##P<0.01 vs sham; *P<0.05 and **P<0.01 vs vehicle; δP<0.05 and δδP<0.01 vs nontransfected SVPs; ɸP<0.05 and ɸɸP<0.01 vs SCr-transfected SVPs.
Involvement of miR-132 in SVP-Induced Prevention of Cardiac Remodelling
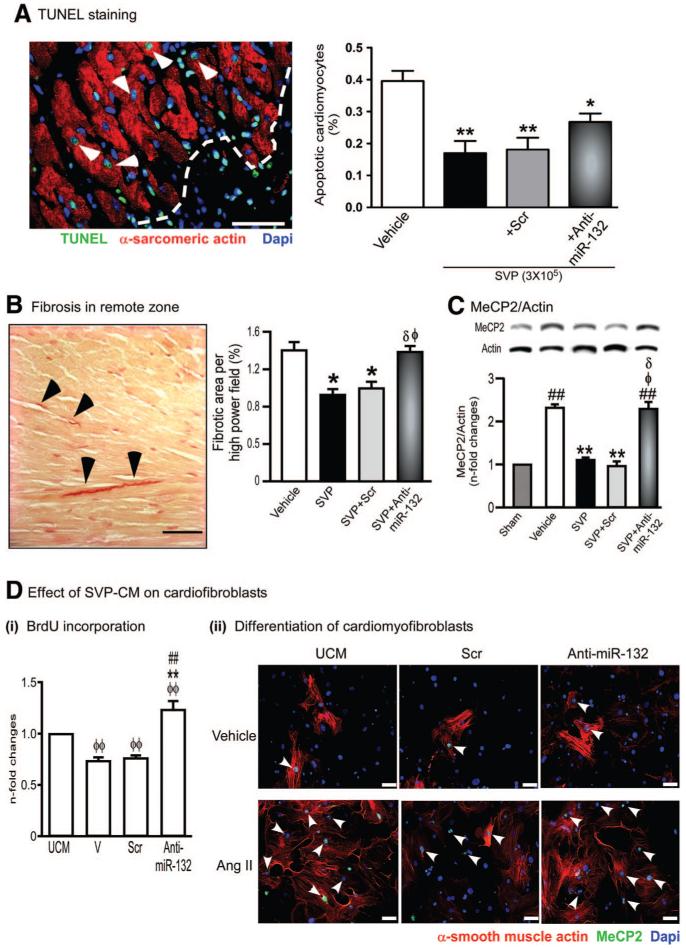
We next investigated the impact of SVPs on cardiomyocytes and cardiac fibroblasts. In SVP-treated mice, the infarct scar was reduced (25.4±1.7% in SVP versus 32.3±1.3% in vehicle, P<0.05). Furthermore, SVP-transplanted hearts showed reduced cardiomyocyte apoptosis, this effect being blunted by SVP transfection with anti-miR-132 (P<0.05, Figure 7A). To verify if cardiomyocytes are a direct target of SVP paracrine action, we exposed adult rat cardiomyocytes to hypoxia in the presence of SVP-CM or UCM and found that the former reduces the levels of caspase-3/7 activity. AntimiR-132-SVP-CM produced similar antiapoptotic effects (Online Figure XIV), thus suggesting that SVPs promote cardiomyocyte survival independently of miR-132 or through other in vivo actions of miR-132, eg, angiogenesis.
Figure 7. SVP transplantation attenuates cardiomyocyte apoptosis and interstitial fibrosis.
A, Representative immunofluorescence image and bar graph showing abundance of apoptotic cardiomyocytes at 14 days post-MI in hearts injected with vehicle or transplanted with naïve, scrambled-transfected or antimiR-132-transfected SVPs. Data are means±SE (n=5 mice per group). *P<0.05 and **P<0.01 vs vehicle. B, Representative image and bar graph showing fibrosis in the spared myocardium. C, Representative immunoblots and bar graph showing the expression of MeCP2 in myocardium (n=5 mice per group). ##P<0.01 vs Sham; **P<0.01 vs Vehicle; δP<0.05 vs nontransfected SVPs; ɸP<0.05 vs SCrtransfected SVPs. D, Bar graph (i) and immunocytochemical images (ii) showing the effect of SVP-CM on BrdU incorporation (i) and differentiation of murine cardiac fibroblasts into myofibroblasts (ii). Cardiac fibroblasts were treated with Angiotensin II (Ang II, 100 nmol/L) for 24 hours to induce differentiation into myofibroblasts in the presence or absence of SVP-CM. Differentiated myofibroblasts are identified by positive staining for α-smooth muscle actin and MeCP2 (arrow head). Data are means±SE of experiments performed in quadruplicate. ɸɸP<0.01 vs unconditioned medium (UCM); **P<0.01 vs vehicle (V); ##P<0.01 vs scrambled sequence (Scr). Scale bars are 50 μm.
Although SVPs acquire cardiomyocyte markers under inductive conditions in vitro (see above), we could not find any Dil-labeled SVP coexpressing cardiac antigens in vivo, thus discounting the possibility that improvement of cardiac function is due to in vivo transdifferentiation into cardiomyocytes. On the other hand, SVP transplantation increased the number of cardiac stem cells in the infarct border zone (Online Figure XV).
SVP transplantation remarkably reduces interstitial fibrosis in the spared myocardium (P<0.05 versus vehicle, Figure 7B). Furthermore, we found that MeCP2, another validated target for miR-132 and a key regulator of fibrogenesis and myofibroblast differentiation,31 is increased in infarcted hearts and reduced by SVP transplantation (Figure 7C). The inhibition of fibrosis and MeCP2 by SVPs was reverted by transfection of SVPs with antimiR-132 (Figure 7B and 7C). SVP-CM markedly reduced mouse cardiac fibroblasts proliferation (Figure 7Di) and differentiation into myofibroblasts as well as MeCP2 expression (Figure 7Dii). These effects were abrogated when exposing fibroblasts to CM of anti-miR-132-transfected SVPs (Figure 7D and Online Figure XVI).
Finally, extensive histological investigation excluded the presence of tumors or calcification in SVP-transplanted hearts, whereas calcification was frequently observed in MSC-transplanted hearts (Online Figure XVII). Altogether, these data indicate the positive action of SVPs in cardiac cell survival and fibrosis and are reassuring on a safety standpoint.
Discussion
The present study newly shows the prolonged therapeutic benefit of SVPs from coronary artery disease patients in a mouse model of MI. Furthermore, we report for the first time that miR-132 is constitutively expressed and released by human SVPs and implicated in SVP proangiogenic activity in vitro and in vivo. Hence, this is, to the best of our knowledge, the first report of human PCs being therapeutically beneficial through an miR-132-mediated mechanism.
Recent findings from our laboratory indicate that human SVPs can build new vessels in ischemic limbs more efficiently than endothelial progenitor cells.20 The present study extends the application to a mouse model of MI and, in line with recommendations of advisory boards (Somatic Cell Therapy for Cardiac Disease, http://www.fda.gov), establishes the optimal dosage and long-term therapeutic benefit of SVP transplantation on clinical, hemodynamic, and mechanistic endpoints. At variance with MSCs, which reportedly differentiate into multiple cell types on transplantation into the infarcted heart,32 SVPs maintain their original antigenic phenotype on engraftment. This represents an important difference, but certainly not a disadvantage, when considering that differentiation could trigger a switch in MSC antigen composition rendering them susceptible to both humoral and cell-mediated cytotoxicity, eventually resulting in loss of therapeutic efficacy at late stages of recovery.33 In the present study, MSCs were used as a cellular control and not for direct comparison; hence, further investigation is warranted to establish the relative therapeutic potency and immunologic properties of the 2 cell populations.
By confocal microscopy, we documented the peri-vascular localization of transplanted SVPs. This physical association suggests that transplanted cells improve myocardial recovery mainly by nurturing reparative angiogenesis. This possibility is further supported by the observation of increased myocardial blood flow and improved vascular barrier function. Moreover, long-term follow up of vascular remodeling documented a biphasic response, consisting of the early augmentation of both capillaries and arterioles and late potentiation of arteriole formation. Noteworthy, the founder population originates from PCs surrounding arterioles in the adventitia of the human saphenous vein. The capacity of SVPs to relocate around and support the growth of coronary arterioles opens new perspectives for predictable delivery of PCs to specific regenerative targets in the infarcted myocardium.
Our previous study indicates that SVPs stimulate EC proliferation by a paracrine mechanism involving Ang-1 and that ECs reciprocally promote SVP recruitment through PDGF-BB.20 SVPs also release large amount VEGF-A. Using mouse-specific primers, here we found that SVP transplantation upregulates VEGF-B and to a less extent Ang-1 at mRNA level. Both VEGF-B and Ang-1 are potent stimulators of arteriogenesis8,34,35 and VEGF-B also exerts antiapoptotic effects in the ischemic heart.35 Immunohistochemistry studies suggest that the source of endogenous VEGF-B were the infiltrating CD45 positive cells. Although not demonstrated, other angiogenic factors could have been released from same cell source, because the recruited monocytes abundantly express various angiogenic chemokines.36 In addition, we found that SVP-transplanted hearts express high levels of the CX3C chemokine receptor 1, a typical receptor of proangiogenic Ly-6Clow monocytes that are recruited during phase 2 of post-MI recovery.26 Furthermore, SVPs secrete interleukin-8, a potent chemoattractant for CXCR1- and CXCR2-positive leukocytes, and monocyte chemotactic protein-1, a cytokine that exerts chemotactic activity for CCR2- and CCR4-positive monocytes. Accordingly, the SVP-CM increases the migration of human CD14+CD16+ cells in a transwell assay.
Our study newly documents the implication of miR-132 in the paracrine activation of reparative vascularization and inhibition of fibrogenesis by SVPs. MicroRNAs are small noncoding RNAs that regulate a wide spectrum of processes including physiological and pathological angiogenesis.37 The highly conserved miR-132 is expressed in ECs following GF-induced activation of proliferation.28 On VEGF stimulation, miR-132 is rapidly transcribed by CREB to suppress endothelial p120RasGAP expression, leading to Ras activation and induction of neovascularization.28 Apart from the endothelium, miR-132 is expressed in neuronal cells, circulating angiogenic cells (Emanueli et al, unpublished observations, 2011), and as shown here SVPs and bone marrow-derived MSCs. We also found that SVPs secrete miR-132, especially under H/S, and that SVP-CM is more abundant in angiogenic GFs and miR-132 compared with MSC-CM. Inhibition of miR-132 by SVP transfection with complementary anti-miR results in mild inhibition of proliferation and survival, thus indicating an intracrine homeostatic mechanism involving the miR-132 and its target p120RasGAP in SVPs. Furthermore, miR-132 inhibition abrogated the SVP-CM induced stimulation of HUVEC proliferation and tube formation, thus showing for the first time the pivotal participation of miR-132 in the angiocrine action of SVPs. These data are in keeping with recent evidence indicating that miRNAs are released through a ceramide-dependent secretory machinery and that the secreted miRNAs are transferable and functional in recipient cells.38 The tight juxtaposition of SVPs with ECs, including the establishment of peg-socket contacts, makes them ideally positioned for such transfer of miRNA signals.20
Mutagenesis studies of the seed sequences of 2 predicted miR-132 binding sites showed that miR-132 exerts a synergistic repression of p120RasGAP.28 We newly document the upregulation of p120RasGAP in infarcted myocardium and identify the wide distribution of this miR-132 target on vascular cells, cardiomyocytes, and fibroblasts, suggesting that all of them could be potentially influenced by locally synthesized or exogenously produced miR-132. Our finding that miR-132 inhibition blunts the benefits of SVP transplantation on contractility indexes, angiogenesis and cell survival is in keeping with universal effects of miR-132 on a wide spectrum of cardiac cells. However, in vitro studies showed that the SVP-CM induces cardiomyocyte survival independently of miR-132, thus suggesting that other paracrine factors contained in CM may be relevant for cardiomyocyte protection. Paracrine factors released by transplanted cells have been acknowledged to play a role in preservation of mature cardiomyocytes39 and cardiac PCs,40 although the exact nature of this interaction remains incompletely understood. In addition, both cardiomyocytes and cardiac PCs may benefit from improved perfusion in the area at risk. Hence, miR-132 might support cardiomyocytes indirectly through potentiation of reparative vascularization.
Data from anti-miR-132 inhibition indicate that miR-132 is instrumental to SVP capacity to alleviate interstitial fibrosis in infarcted hearts. Experiments using SVP-CM on isolated murine fibroblasts under basal and stimulated conditions confirm a direct paracrine inhibition of fibroblast growth and differentiation into myofibroblasts through a miR-132-mediated mechanism. Previous studies described the translation repressive influence of miR-132 on profibrotic MeCP2 in neuronal cells and hepatic cells. Unlocking the miR-132 translational block on MeCP2 leads to a series of methylation events culminating in myofibroblast transdifferentiation and liver fibrogenesis.31 Intriguingly, elevated MeCP2 expression has been reported to result in cardiac and skeletal abnormalities during development.41 To the best of our knowledge, however, this is the first report of MeCP2 upregulation in infarcted hearts and of cell therapy resulting in concurrent inhibition of interstitial fibrosis, myofibroblast differentiation, and MeCP2 expression.
Importantly, in the perspective of clinical application, no adverse effect or tumorogenesis has been observed to date with SVPs in small animal preclinical models. Ectopic calcification of blood vessels and heart valves represents a concern of vascular regenerative medicine as many angiogenic factors, cytokines, and PCs might exert both direct and indirect effects on bone and cartilage formation. Furthermore, osteoprogenitor cells may be derived from the circulation, neovessels themselves, or adventitial myofibroblasts17; however, the relation of these cells with pericytes remains unclear. The present study and our previous one in a limb ischemia model are reassuring with respect to promotion of calcification.20 Additional studies in larger animals are however warranted.
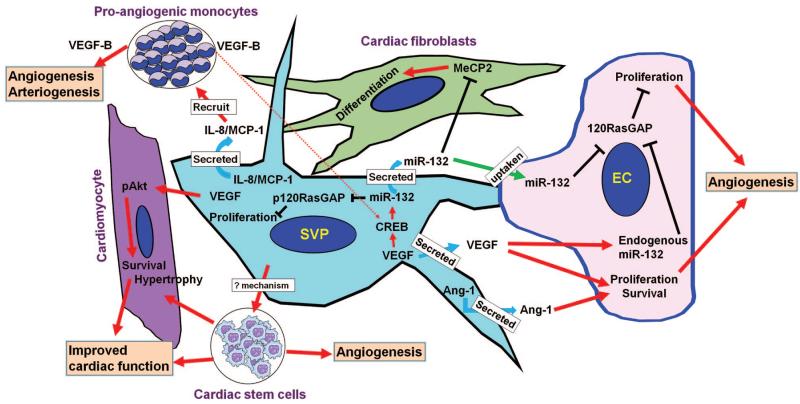
In conclusion, we found that human SVPs are potent inducers of reparative vascularization and cardiac healing through an integrated mechanism that involves reciprocal interactions between donor cells and the ischemic environment (summarized in Figure 8). Hence, this specialized PC population should be considered for future applications of cardiovascular regenerative medicine.
Figure 8. Cartoon image showing the mechanism of SVP induced protection in the myocardium post-MI.
Supplementary Material
Novelty and Significance.
What Is Known?
Arteries and veins contain progenitor cells with proangiogenic properties and clonogenic cells expressing mesenchymal and pericytic markers have been localized in the adventitia around the vasa vasorum.
Pericyte-like cells from human saphenous veins stimulate endothelial cell growth and reparative angiogenesis in a mouse model of peripheral ischemia through a paracrine mechanism involving angiopoietin-1 and platelet-derived growth factor.
Pericyte recruitment is fundamental for stabilization of neovascularization, but this process is dampened in cardiovascular disease; hence, new-formed vessels are fragile, unstable, and hyperpermeable to plasma protein.
What New Information Does This Article Contribute?
Pericytes expanded from leftovers of human saphenous veins stimulate the neovascularization and functional recovery of infarcted mouse hearts.
We demonstrate that this action is principally due to secretion of paracrine factors and release of microRNA-132 (miR-132), which activates angiogenic and antifibrotic mechanisms in the recipient heart by inhibiting Ras-GTPase activating protein and methyl-CpG-binding protein 2, respectively.
Although current cell therapy is dominated by bone marrow-derived cells, additional research on other types of stem cells is needed for optimal treatment of cardiovascular disease. This study focuses on human pericytes as potential candidates for vascular stabilization in the infarcted heart. We demonstrate that transplanted pericytes relocate around and support the growth of coronary arterioles, suggesting a peculiar tropism of these cells instrumental to therapeutic benefit. The physical contact between pericytes and resident endothelial cells may strengthen the nascent vascularization, thus reducing microvascular permeability and myocardial edema, which negatively impact cardiac function. Growing evidence indicates that miRNAs can be transferred between cells, thereby modulating functional activities in the target cell. Human pericytes express and secrete miR-132, which is taken up by endothelial cells to enhance their angiogenic activity. Moreover, transplantation of human pericytes promotes proangiogenic and antifibrotic effects in the infarcted heart through the release of miR-132, resulting in the inhibition of miR-132 target genes. Knocking-down miR-132 in pericytes abrogates these beneficial actions. Human pericytes could be a valuable source of angiogenic cells for future use in cardiovascular regenerative medicine.
Acknowledgments
We thank Andrea Caporali (Bristol University) for his expert advice in miRNA data interpretation.
Sources of Funding
This study was supported by the British Heart Foundation (BHF: PG/06/096/21325), the FP7 program of the European Community (Resolve Chronic Inflammation and Achieve Healthy Ageing, 202047), and the National Institute for Health Research, Bristol Biomedical Research Unit in Cardiovascular Medicine. Rajesh Katare is supported by a project grant from British Heart Foundation (PG/09/086). Costanza Emanueli is a BHF Senior Research Fellow.
Non-standard Abbreviations and Acronyms
- angiopoietin-1
Ang-1
- Anti-miR-132
microRNA-132 inhibitor
- AT1R
angiotensin II type 1 receptor
- CREB
cyclic AMP response element-binding protein
- EC
endothelial cell
- Erk
extracellular signal regulated kinase
- HUVEC
human umbilical vein endothelial cells
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MeCP2
methyl CpG binding protein 2
- MI
myocardial infarction
- miR-132
microRNA-132
- MNC
mononuclear cells
- MSC
mesenchymal stem cells
- MSC-CM
conditioned medium from mesenchymal stem cells
- PC
progenitor cells
- RasGAP
Ras-GTPase activating protein
- Scr
scrambled sequence
- SVP
saphenous vein-derived pericyte progenitor
- SVP-CM
conditioned medium from SVPs
- UCM
unconditioned medium
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
None.
References
- 1.Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart (British Cardiac Society) 2010;96:792–800. doi: 10.1136/hrt.2007.139394. [DOI] [PubMed] [Google Scholar]
- 2.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 3.Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, Assmus B, Dimmeler S, Zeiher AM, Hamm C. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. American Heart Journal. 2009;157:541–547. doi: 10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Kang S, Yang YJ, Li CJ, Gao RL. Effects of intracoronary autologous bone marrow cells on left ventricular function in acute myocardial infarction: a systematic review and meta-analysis for randomized controlled trials. Coronary Artery Disease. 2008;19:327–335. doi: 10.1097/MCA.0b013e328300dbd3. [DOI] [PubMed] [Google Scholar]
- 5.Dobaczewski M, Akrivakis S, Nasser K, Michael LH, Entman ML, Frangogiannis NG. Vascular mural cells in healing canine myocardial infarcts. J Histochem Cytochem. 2004;52:1019–1029. doi: 10.1369/jhc.3A6210.2004. [DOI] [PubMed] [Google Scholar]
- 6.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. 2002;50:71–79. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 7.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 8.Chen JX, Stinnett A. Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha (HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and normalizes immature vasculature in db/db mice. Diabetes. 2008;57:3335–3343. doi: 10.2337/db08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Xu Q. Adventitial biology: differentiation and function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:1523–1529. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- 11.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nature Cell Biology. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 12.Crisan M, Casteilla L, Lehr L, Carmona M, Paoloni-Giacobino A, Yap S, Sun B, Leger B, Logar A, Penicaud L, Schrauwen P, Cameron-Smith D, Russell AP, Peault B, Giacobino JP. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells (Dayton, Ohio) 2008;26:2425–2433. doi: 10.1634/stemcells.2008-0325. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. The Journal of Clinical Investigation. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. American Journal of Physiology. 2005;289:C1396–C1407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- 15.Klein D, Weisshardt P, Kleff V, Jastrow H, Jakob HG, Ergun S. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PloS One. 2011;6:e20540. doi: 10.1371/journal.pone.0020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasquinelli G, Tazzari PL, Vaselli C, Foroni L, Buzzi M, Storci G, Alviano F, Ricci F, Bonafe M, Orrico C, Bagnara GP, Stella A, Conte R. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem cells (Dayton, Ohio) 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 17.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circulation Research. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 18.Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, Ciusani E, Stassi G, Siragusa M, Nicosia R, Peschle C, Fascio U, Colombo A, Rizzuti T, Parati E, Alessandri G. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, Madeddu P. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circulation Research. 2009;104:1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katare R, Caporali A, Emanueli C, Madeddu P. Benfotiamine improves functional recovery of the infarcted heart via activation of pro-survival G6PD/Akt signaling pathway and modulation of neurohormonal response. J Mol Cell Cardiol. 2010;49:625–638. doi: 10.1016/j.yjmcc.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation. 1997;96:2656–2666. doi: 10.1161/01.cir.96.8.2656. [DOI] [PubMed] [Google Scholar]
- 23.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 24.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circulation Research. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 26.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes & development. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo LD, Kessler KM, Pincheira R, Warren RS, Donner DB. Vascular endothelial cell growth factor activates CRE-binding protein by signaling through the KDR receptor tyrosine kinase. The Journal of Biological Chemistry. 2001;276:25184–25189. doi: 10.1074/jbc.M102932200. [DOI] [PubMed] [Google Scholar]
- 28.Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, King PD, Weis SM, Cheresh DA. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nature Medicine. 2010;16(8):909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres AG, Fabani MM, Vigorito E, Gait MJ. MicroRNA fate upon targeting with anti-miRNA oligonucleotides as revealed by an improved Northern-blot-based method for miRNA detection. RNA. 2011;17:933–943. doi: 10.1261/rna.2533811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Current Topics in Microbiology and Immunology. 2010;346:143–169. doi: 10.1007/82_2010_56. [DOI] [PubMed] [Google Scholar]
- 31.Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, Mann DA. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705–714. e701–e704. doi: 10.1053/j.gastro.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 34.Ueda K, Takano H, Niitsuma Y, Hasegawa H, Uchiyama R, Oka T, Miyazaki M, Nakaya H, Komuro I. Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice. The Journal of Clinical Investigation. 2010;120:2016–2029. doi: 10.1172/JCI39896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahteenvuo JE, Lahteenvuo MT, Kivela A, Rosenlew C, Falkevall A, Klar J, Heikura T, Rissanen TT, Vahakangas E, Korpisalo P, Enholm B, Carmeliet P, Alitalo K, Eriksson U, Yla-Herttuala S. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms. Circulation. 2009;119:845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- 36.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. Journal of Molecular and Cellular Cardiology. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovascular Research. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 38.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Science. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature Medicine. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 40.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-Saavedra M, Carrasco L, Sura-Trueba S, Demarchi Aiello V, Walz K, Neto JX, Young JI. Elevated expression of MeCP2 in cardiac and skeletal tissues is detrimental for normal development. Human Molecular Genetics. 2010;19:2177–2190. doi: 10.1093/hmg/ddq096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.