Abstract
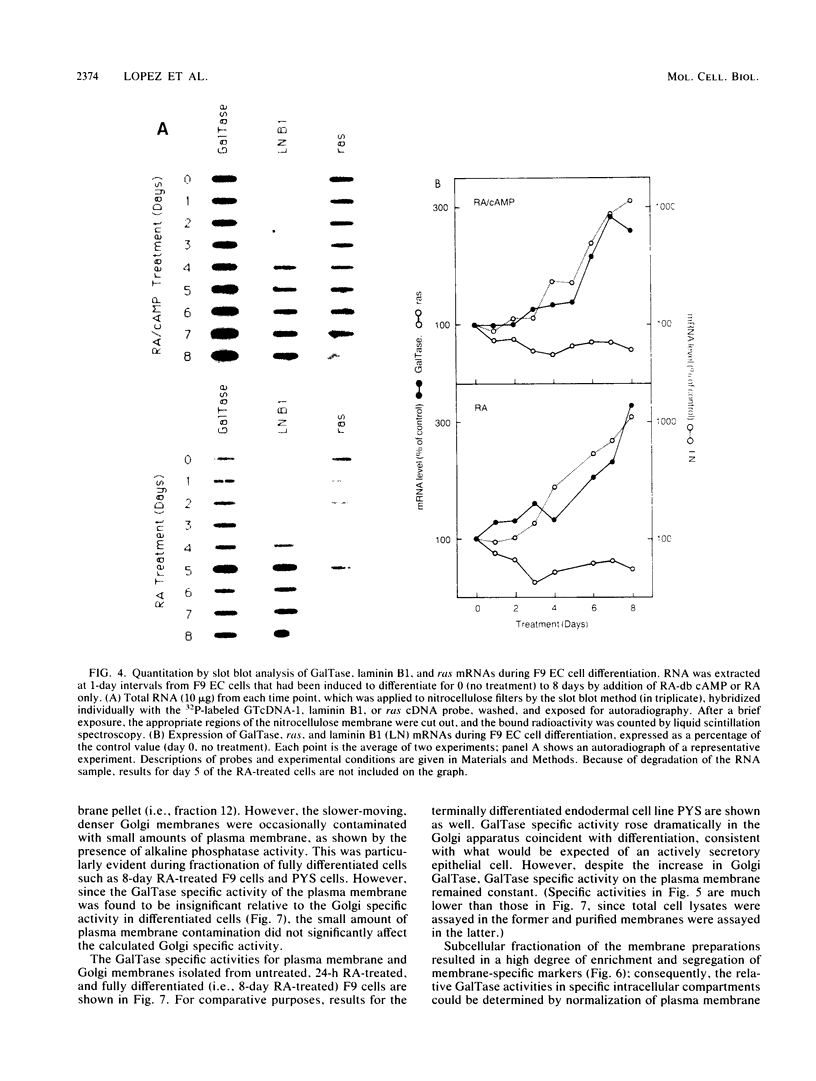
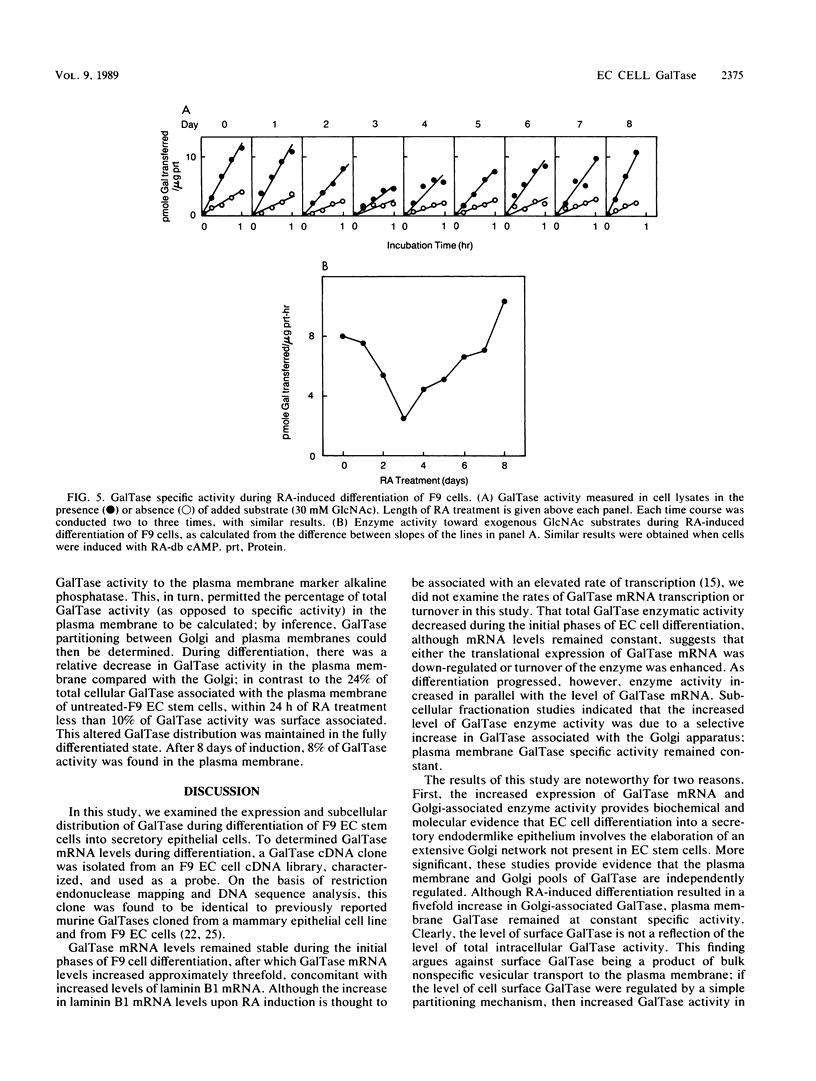
beta-1,4-Galactosyltransferase (GalTase) has two functionally distinct subcellular distributions. In the Golgi apparatus, GalTase participates in the glycosylation of secretory and membrane-bound glycoproteins, whereas on the cell surface it mediates specific aspects of intercellular adhesion. For this study, a murine GalTase clone was obtained by screening a lambda gt10 cDNA library made from F9 embryonal carcinoma cells with a heterologous bovine GalTase cDNA probe. The murine GalTase cDNA probe was used in conjunction with assays of GalTase activity to investigate the expression and distribution of GalTase during differentiation of F9 stem cells into secretory endodermal epithelium. During the initial phase of F9 cell differentiation, GalTase mRNA levels remained relatively constant; however, as differentiation progressed, as assayed by expression of the differentiation-specific marker laminin B1, GalTase mRNA levels and enzyme activity rose dramatically. Furthermore, subcellular fractionation of these cells showed that the increased GalTase levels were specifically associated with the Golgi apparatus, whereas GalTase specific activity on the plasma membrane remained constant. These results show that levels of cell surface and Golgi GalTase change relative to one another during F9 cell differentiation and suggest that these functionally distinct pools of GalTase are independently and differentially regulated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayna E. M., Shaper J. H., Shur B. D. Temporally specific involvement of cell surface beta-1,4 galactosyltransferase during mouse embryo morula compaction. Cell. 1988 Apr 8;53(1):145–157. doi: 10.1016/0092-8674(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Carlson D. M., David J., Rutter W. J. Galactosyltransferase activities in pancreas, liver and gut of the developing rat. Arch Biochem Biophys. 1973 Aug;157(2):605–612. doi: 10.1016/0003-9861(73)90680-2. [DOI] [PubMed] [Google Scholar]
- Colony P. C., Neutra M. R. Epithelial differentiation in the fetal rat colon. I. Plasma membrane phosphatase activities. Dev Biol. 1983 Jun;97(2):349–363. doi: 10.1016/0012-1606(83)90092-1. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Moog F. Inactive alkaline phosphatase in duodenum of nursling mouse: immunological evidence. Science. 1966 Nov 25;154(3752):1037–1038. doi: 10.1126/science.154.3752.1037. [DOI] [PubMed] [Google Scholar]
- Frazier M. L., Montagna R. A., Saunders G. F. Insulin gene expression during development of the fetal bovine pancreas. Biochemistry. 1981 Jan 20;20(2):367–371. doi: 10.1021/bi00505a022. [DOI] [PubMed] [Google Scholar]
- Gasser C. S., Simonsen C. C., Schilling J. W., Schimke R. T. Expression of abbreviated mouse dihydrofolate reductase genes in cultured hamster cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6522–6526. doi: 10.1073/pnas.79.21.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hyafil F., Babinet C., Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981 Nov;26(3 Pt 1):447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ebihara I., Killen P. D., Sasaki M., Cannon F. B., Yamada Y., Martin G. R. Genes for basement membrane proteins are coordinately expressed in differentiating F9 cells but not in normal adult murine tissues. Dev Biol. 1987 Aug;122(2):373–378. doi: 10.1016/0012-1606(87)90302-2. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchase R. B., Kidd V. J., Rivera A. A., Humphreys-Beher M. G. Cell surface expression of 4 beta-galactosyltransferase accompanies rat parotid gland acinar cell transition to growth. J Cell Biochem. 1988 Apr;36(4):453–465. doi: 10.1002/jcb.240360413. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mircheff A. K., Ahnen D. J., Islam A., Santiago N. A., Gray G. M. Complex subcellular distributions of enzymatic markers in intestinal epithelial cells. J Membr Biol. 1985;83(1-2):95–107. doi: 10.1007/BF01868742. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Ando T., Kimura T., Narimatsu H. Cloning and sequencing of a full-length cDNA of mouse N-acetylglucosamine (beta 1-4)galactosyltransferase. J Biochem. 1988 Aug;104(2):165–168. doi: 10.1093/oxfordjournals.jbchem.a122434. [DOI] [PubMed] [Google Scholar]
- Narimatsu H., Sinha S., Brew K., Okayama H., Qasba P. K. Cloning and sequencing of cDNA of bovine N-acetylglucosamine (beta 1-4)galactosyltransferase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4720–4724. doi: 10.1073/pnas.83.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper N. L., Hollis G. F., Douglas J. G., Kirsch I. R., Shaper J. H. Characterization of the full length cDNA for murine beta-1,4-galactosyltransferase. Novel features at the 5'-end predict two translational start sites at two in-frame AUGs. J Biol Chem. 1988 Jul 25;263(21):10420–10428. [PubMed] [Google Scholar]
- Shur B. D. Embryonal carcinoma cell adhesion: the role of surface galactosyltransferase and its 90K lactosaminoglycan substrate. Dev Biol. 1983 Oct;99(2):360–372. doi: 10.1016/0012-1606(83)90286-5. [DOI] [PubMed] [Google Scholar]
- Shur B. D. Evidence that galactosyltransferase is a surface receptor for poly(N)-acetyllactosamine glycoconjugates on embryonal carcinoma cells. J Biol Chem. 1982 Jun 25;257(12):6871–6878. [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Strous G. J. Golgi and secreted galactosyltransferase. CRC Crit Rev Biochem. 1986;21(2):119–151. doi: 10.3109/10409238609113610. [DOI] [PubMed] [Google Scholar]
- Tokumitsu S., Fishman W. H. Alkaline phosphatase biosynthesis in the endoplasmic reticulum and its transport through the Golgi apparatus to the plasma membrane: cytochemical evidence. J Histochem Cytochem. 1983 May;31(5):647–655. doi: 10.1177/31.5.6841969. [DOI] [PubMed] [Google Scholar]
- Tokumitsu S., Tokumitsu K., Fishman W. H. Intracellular alkaline phosphatase activity in cultured human cancer cells. Histochemistry. 1981;73(1):1–13. doi: 10.1007/BF00493127. [DOI] [PubMed] [Google Scholar]
- Wilcox C. A., Olson E. N. The majority of cellular fatty acid acylated proteins are localized to the cytoplasmic surface of the plasma membrane. Biochemistry. 1987 Feb 24;26(4):1029–1036. doi: 10.1021/bi00378a008. [DOI] [PubMed] [Google Scholar]





