Selected abstracts delivered at the 8th Annual AOSpine North America Fellows Forum
Consistent with EBSJ's commitment to fostering quality research, we are pleased to feature some of the most highly rated abstracts from the 8th Annual AOSpine North America Fellows Forum in Banff Canada. Enhancing the quality of evidence in spine care means acknowledging and supporting the efforts of young researchers within our AOSpine North America network. We look forward to seeing more from these promising researchers in the future.
Abstract
Study type: Basic science research report
Introduction: Spinal nerve-injury management and prevention constitute a substantial proportion of a spinal surgeon's practice. Functional recovery after peripheral nerve injuries is often unsatisfactory and to optimize the outcomes, an intimate understanding of these injuries is required. Sunderland classified peripheral nerve injuries into five grades.1 Grade 1 (neurapraxia) and grade 2 (axonal disruption) injuries usually recover with no or insignificant functional deficits within weeks to a few months, respectively. Injuries that are most difficult to manage clinically are the often mixed grade 3 (endoneurial disruption) and grade 4 (perineurial disruption) lesions where spontaneous functional recovery is limited or absent, resulting in neuroma in continuity (NIC). Traumatic NIC is characterized by aberrant intra- and extra- fascicular axonal regeneration and scar formation within an unsevered injured nerve, resulting in impaired and erroneous end-organ reinnervation.2,3 Animal models reproducing grade 1, 2, 3, and 5 lesions have been developed, but to our knowledge a clinically relevant rodent model of NIC has not been developed.4,5,6,7,8 The effective peripheral nerve regeneration and resilience of rodents make it challenging to recreate the NIC scenario.
Objective: Our goal was to develop a practical rodent model for focal traumatic NIC, demonstrating the characteristic histological features, supported by concordant functional deficits. Such a model may help us to identify this injury pattern earlier and allow development of intervention strategies to reduce neuronal misdirection, scar formation, and enhance regeneration for improved functional recovery.
Methods: Various injury techniques were tested on freshly harvested Lewis rat sciatic nerves ex vivo, and examined histologically before inflicting more refined injuries in vivo. The optimal experimental injuries combined a 50 g traction force applied with a spring scale hooked around the sciatic nerve, and focal three second maximal compression using a malleus nipper (Figure 1). Nerves were harvested at 0, 5, 13, 21, and 65 days, and processed for longitudinal 8 micron cryostat sectioning, H&E, laminin, neurofilament, and Masson's trichrome staining. Skilled locomotion (tapered beam, ladder rung) and flat plane locomotion for ground reaction force (GRF) analysis were performed serially up to 9 weeks with the experimental (n = 4) and simple (control) crush (n = 1) injuries by blinded animal behavior experts, using methods as recently described.9
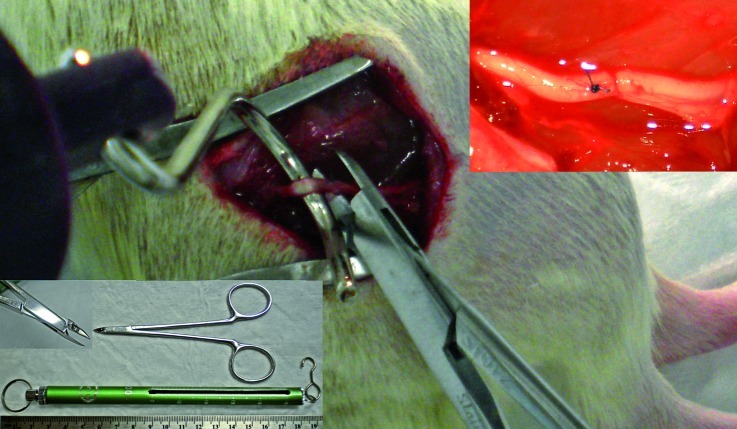
Figure 1.
Photograph illustrating the experimental injury. Fifty grams of traction is applied in a direction orthogonal to the native nerve course after external neurolysis, simultaneously, three second maximal compression is applied at the sciatic trifurcation, just distal to a mesoneurial suture. Malleus nipper with tip detail and 100 g spring scale in bottom left. In situ sciatic nerve immediately after injury (top right).
Results: Disruption of the endoneurium and perineurium with aberrant intra- and extrafascicular axonal regeneration and progressive fibrosis was consistently demonstrated histologically in ten out of ten nerves with experimental injuries. In contrast, crush injuries showed only signs of Wallerian degeneration (Figure 2). At 8 weeks, experimental animals made more errors during skilled locomotion as compared to nerve crush animals. GRFs revealed impaired vertical and fore-aft force generation by the injured limbs at week 9 in the experimental group, whereas GRFs from the simple crush animal revealed recovery at the same time point (Figure 3).
Figure 2.
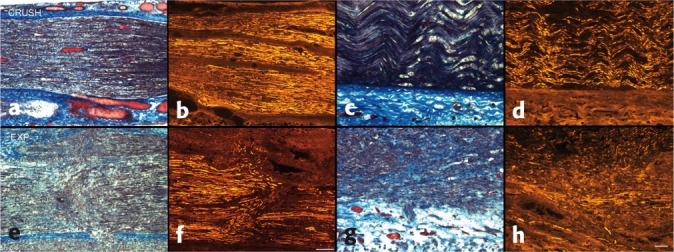
Injury zones at five days (a–d, bar = 200 µm) and 65 days (e–h, bar = 50 µm), comparing crush (top) to experimental (bottom) injuries; Masson's trichrome and neurofilament. Note the aberrant axonal sprouting and regeneration in the experimental injury group, associated with increased intrafascicular collagen, in contrast to orderly regeneration and lack of scar in the simple crush group.
Figure 3.
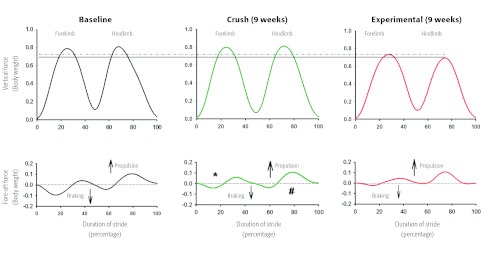
Mean vertical and fore-aft ground reaction forces at both baseline and 9 weeks from representative animals. Compared to baseline and crush-injured animal at 9 weeks, animals in the experimental group bear less weight on both their right (surgical) hind limb (solid line), and fore limb (dotted line) at 9 weeks. Comparable with historical data, the crush animal have improved braking (*) and propulsive (#) forces in fore and hind limbs (injured side) compared to the experimental group, though these have not returned to baseline values.
Conclusions: We have demonstrated histological features and poor functional recovery consistent with NIC formation in a rodent model. The injury mechanism employed combines traction and compression forces akin to the physical forces at play in clinical nerve injuries. Additional validating experiments are in progress.
Keywords: Locomotion, nerve regeneration, Sunderland grade 4 nerve injury
Footnotes
The Midha lab receives grant support from the Canadian Institute for Health Research and Integra Life Sciences Foundation. Protocols for animal experiments conducted by the Midha lab are approved by the University of Calgary Animal Care Committee and adheres strictly to the Canadian Council on Animal Care guidelines.
References
- 1.Sunderland S. Classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 2.Burger P C Scheithauer E W Vogel F S Surgical pathology of the nervous system and its coverings 4th ed. New York, NY: Churchill, Livingstone; 2002585–586. [Google Scholar]
- 3.Chen L, Gao S, Gu Y. et al. Histopathologic Study of the Neuroma-in-Continuity in Obstetric Brachial Plexus Palsy. Plast Reconstr Surg. 2008;121(6):2046–2054. doi: 10.1097/PRS.0b013e3181706e7e. [DOI] [PubMed] [Google Scholar]
- 4.Bridge P M, Ball D J, McKinnon S E. et al. Nerve crush injuries—A model for axonotmesis. Exp Neurol. 1994;127(2):284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 5.Tos P, Ronchi G, Papalia I. et al. Methods and Protocols in Peripheral Nerve Regeneration Experimental Research: Part 1—Experimental Models. Int Rev Neurobiol. 2009;87:47–79. doi: 10.1016/S0074-7742(09)87004-9. [DOI] [PubMed] [Google Scholar]
- 6.Mavrogenis A F, Pavlakis K, Stamatoukou A. et al. Current treatment concepts for neuromas-in-continuity. Injury. 2008;39S:S43–48. doi: 10.1016/j.injury.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Moradzadeh A, Brenner M J, Whitlock E L. et al. Bipolar Electrocautery. A Rodent Model of Sunderland Third-degree Nerve Injury. Arch Facial Plast Surg. 2010;12(1):40–47. doi: 10.1001/archfacial.2009.104. [DOI] [PubMed] [Google Scholar]
- 8.Song C, Zhang F, Zhang J. et al. Neuroma-in-Continuity Model in Rabbits. Ann Plast Surg. 2006;57(3):317–322. doi: 10.1097/01.sap.0000221512.06129.d3. [DOI] [PubMed] [Google Scholar]
- 9.Kemp S WP, Alant J, Walsh S K. et al. Behavioural and anatomical analysis of selective tibial nerve branch transfer to the deep peroneal nerve in the rat. Eur J Neurosci. 2010;31(6):1074–1090. doi: 10.1111/j.1460-9568.2010.07130.x. [DOI] [PubMed] [Google Scholar]
- 10.Kemp S W Webb A A Midha R Behavioural analysis of peripheral nerve regeneration through nerve growth factor (NGF) loaded T-tube chambers Can J Neurol Sci 200936301S31 (abstract I-07) [Google Scholar]