Abstract
Objective: The pathophysiology of arachnoiditis ossificans (AO) and its association with syringomyelia remains a rare and poorly understood phenomenon. Here, we present a case of AO associated with syringomyelia, a review of literature, and a discussion of current understanding of disease pathophysiology.
Methods: A literature review was performed using MEDLINE (January 1900–May 2010) and Embase (January 1900–May 2010) to identify all English-language studies that described AO with syringomyelia. The current report was added to published cases.
Results: Over 50 cases of AO are reported in literature, of which only eight are associated with syringomyelia. The various presumptive etiologies of syrinx formation include abnormalities in blood circulation, ischemia, hydrodynamic alternations in cerebrospinal fluid (CSF) flow, tissue damage, or incidental coexistence. Changing CSF dynamics related to decreased compliance of the subarachnoid space and subsequent paracentral dissection of the spinal cord may be implicated in the disease process. magnetic resonance imaging (MRI) scanning may identify the syrinx but fail to diagnose the calcified arachnoid. Five patients, including the current case, improved clinically following laminectomy and decompression.
Conclusions: Syringomyelia in association in AO is a rare phenomenon. A high index of suspicion is required and both MRI and computed tomography (CT) are recommended for diagnosis. The pathophysiology of syringomyelia in AO remains an area of ongoing research.
Abbreviations List
- AO
Arachnoiditis ossificans
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- MRI
Magnetic resonance imaging
Introduction
Calcification of the arachnoid membranes in the intrathecal space is a common finding on autopsy.1 However, arachnoiditis ossificans (AO) is a unique and uncommon phenomenon characterized by intrathecal bony metaplasia of the arachnoid membrane.1 This pathology develops following numerous predisposing processes including surgery, trauma, infection, myelography and subarachnoid hemorrhage.2,3,4 As of yet, the pathophysiology of AO is unknown; however, it may be a result of chronic inflammation of the arachnoid.5
Over 50 reports of AO are documented in literature; however, only eight of these are associated with syringomyelia.2,3,4,5,6,7,8,9 The pathophysiology of syrinx development in this disease state is controversial. Here, we present a case of AO-associated syringomyelia and provide a review of literature and discussion of the current understanding of disease pathophysiology.
Case Report
History
A 36-year-old male truckdriver was referred to our neurosurgical spine service with a 4-year history of progressive spastic paraparesis and gait imbalance. His past medical history was significant for cerebellar ganglioglioma requiring craniotomy and resection 17 years prior to presentation. This was complicated by CSF leakage and subsequent surgical repair. He had no upper extremity symtomatology and his bowel and bladder function was intact. Based on his history, his neurological symptoms were initially attributed to his cerebellar pathology. His symptoms however progressed to debilitating lower extremity spasticity such that he underwent several Botox injection procedures and further investigations were carried out.
On examination, the patient was myelopathic and grossly spastic in his lower extremities with increased tone bilaterally. He was unable to perform tandem walking and Romberg's test was positive. His pain and temperature sensation was intact; although proprioception in the lower extremities was impaired.
Imaging
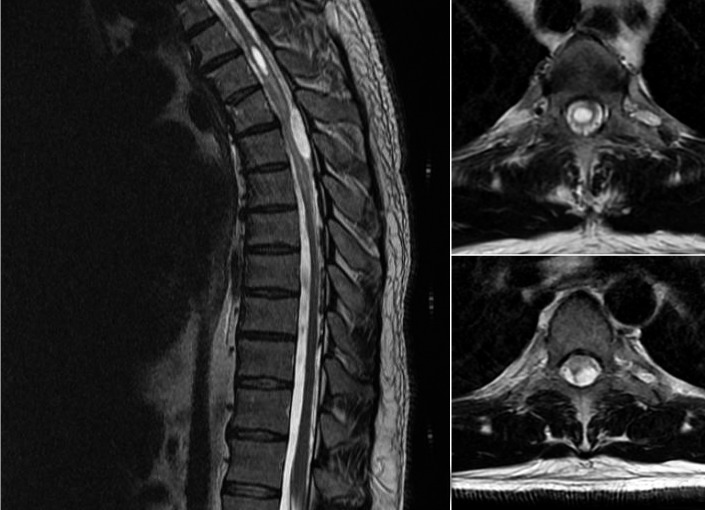
A plain film of the thoracic spine revealed no abnormalities. An MRI scan revealed a thoracic syrinx at the T3 level measuring 2 cm × 5 mm. There was no evidence of enhancing tumor associated with the syrinx. An intradural extramedullary mass along the left posterolateral aspect of the thecal sac was noted at T4–5. This lesion displaced the spinal cord anteriorly and was associated with abnormal signal within the spinal cord suggestive of myelomalacia or edema. Hypointense nodularity with the appearance of intradural calcification was seen posteriorly from T5–10 (Figure 1). Our initial differential diagnoses for this intradural extramedullary lesion included: meningioma, schwannoma, a drop metastasis from the previous cerebellar gangioglioma or a primary exophytic tumor. At this point, we proceeded with a T4–7 laminectomy for spinal cord decompression.
Figure 1.
Preoperative T2-weighted MRI scan a mid-sagittal view demonstrating a thoracic syrinx at the T3 level as well as an intradural extramedullary lesion displacing the spinal cord at the T4–5 levels b axial view at the level of T3 showing the large syrinx c axial view at the level of T4 demonstrating cord compression by the intradural extramedullary lesion. Abnormal cord signal is evident
Operative procedure
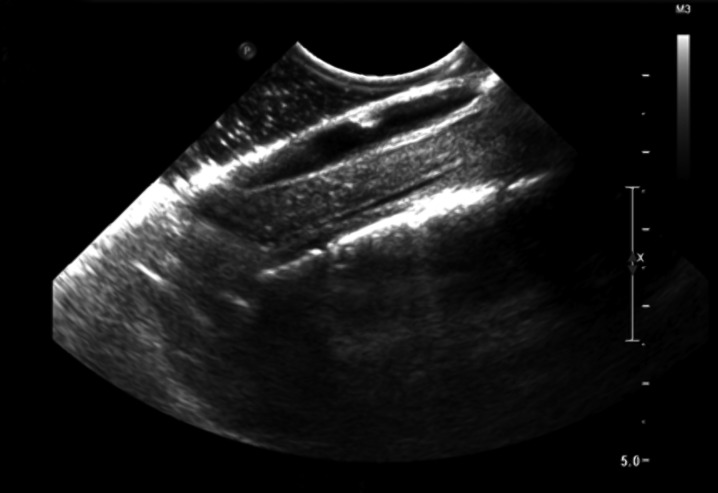
With monitoring of motor and somatosensory evoked potentials, a laminectomy was undertaken from T4–T7 and the thecal sac was exposed. Intraoperative ultrasound was used to identify and characterize the extent of the syrinx and calcified lesion (Figure 2). As the durotomy was performed, it was apparent that there was marked arachnoiditis with arachnoid calcification extending as low as T8–9. The lesion was visualized to be a large calcified segment of arachnoid densely adherent to the dorsal surface of the cord. Meticulous and careful dissection allowed the lesion to be elevated away from the cord surface. The lesion itself was approximately 38 × 18 mm (Figure 3). The cord was noted to be pulsatile following the decompression. A duroplasty was fashioned to reconstruct the subarachnoid space. Evoked potentials were unchanged throughout the procedure.
Figure 2.
Intraoperative ultrasonography demonstrating the syrinx as well as the hyerechoic calcified arachnoid
Figure 3.
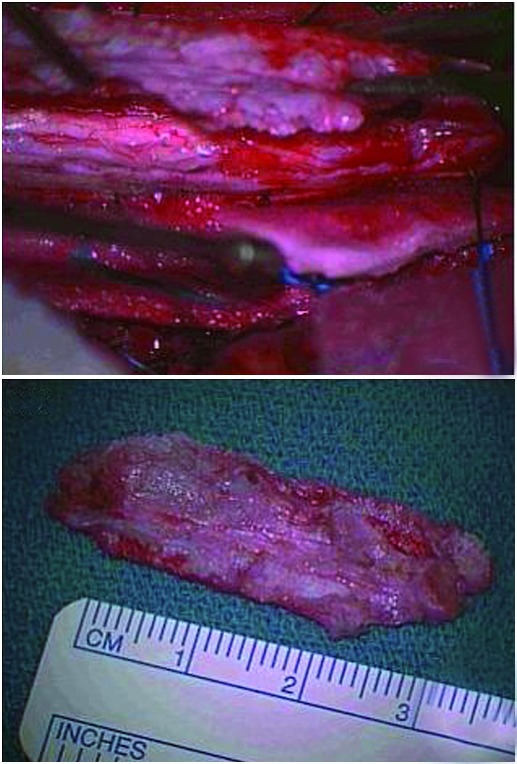
a Intraoperative image showing dissection and elevation of the calcified arachnoid plaque from the spinal cord. The calcified arachnoid is firmly adhered to the dorsal surface of the spinal cord b pathological specimen of calcified arachnoid plaque measuring 38 × 18 mm
Outcome
At 9-month follow up, the patient noted marked improvement in gait. He was able to ambulate without walking aids. His spasticity also improved although he continued to receive botox injections to his lower extremities.
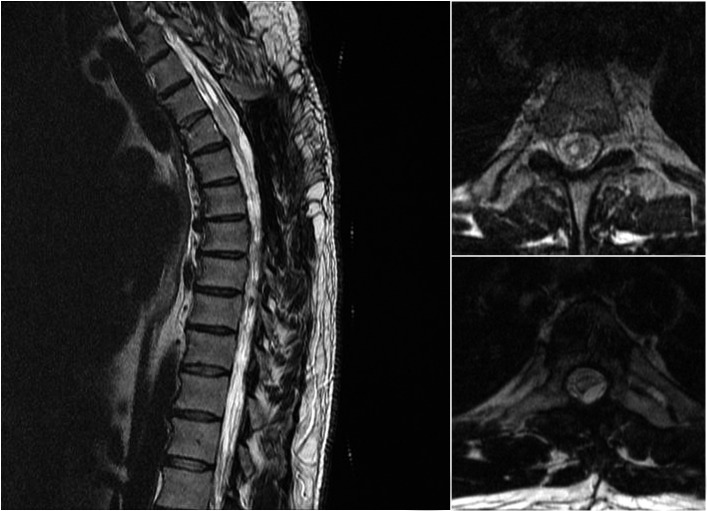
Repeat MRI revealed a significant decrease in the size of the lesion (Figure 4). The syrinx at T3 was significantly smaller compared to preoperatively and there was near complete resolution of the edema within the spinal cord. There was also a marked reduction in mass effect and compression of the spinal cord at the decompressed levels. Persistent adhesions of the arachnoid at other levels were noted.
Figure 4.
Postoperative T2-weighted MRI scan a mid-sagittal view demonstrates interval decompression of the spinal cord at the T4–5 level. The persistent syrinx has decreased substantially in size b axial view at the level of T3 showing interval decrease in syrinx size c axial view at the T4 level showing re-expansion of the thoracic cord following laminectomy and resection of calcified arachnoid
Discussion
The earliest clinicopathologic association of arachnoid calcification with syringomyelia dates to an autopsy study by Schwarz in 1897.10 Eight cases of arachnoiditis ossificans with associated syringomyelia have been published in literature (Table 1). In all of these cases, the patients presented with spastic paresis. Due to the authors' clinical suspicions, the syrinx was readily identified in the majority of cases. In one case, the diagnosis of AO was made intraoperatively as the preoperative MRI failed to identify arachnoid calcifications and a preoperative CT scan was not obtained.8
Table 1. Summary of conditions and weights used to calculate Charlson comorbidity score*.
| Clinical conditions | Weights |
|---|---|
| Myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, chronic lung disease, connective tissue disease, ulcer, chronic liver disease | 1 |
| Hemiplegia, moderate or severe renal disease, diabetes with complication, tumor, leukemia, lymphoma | 2 |
| Moderate or severe liver disease | 3 |
| Malignant tumor, metastasis, AIDS | 6 |
Weights are assigned to 19 predetermined clinical conditions with the final score being the sum of the weights assigned to the conditions with scores ranging from 0–37. One point for each additional decade of life over 50-years-of-age can be added to initial score to create a single index. The higher the weight total, the greater the likelihood of mortality.
In all reported cases, the syrinx localized to the thoracic cord within close proximity to the areas of AO. A thoracic laminectomy was consistently performed and durotomy and duroplasty usually achieved. The calcified arachnoid plaques were resected to variable extents due to their firm adherence to the spinal cord. In several cases, the syrinx was aspirated and a syringoperitoneal or syringopleural shunt was inserted.5,6,8 Collapse of the syrinx was noted with some postoperative clinical improvement in two of these reports.5,8 Clinical improvement was also noted by several authors who only performed decompression and removal of ossified plaque without myelotomy or shunting of the syrinx.7,9 This was also observed in the current study. Our study is the first to document a decrease in the size of the syrinx on postoperative imaging following spinal cord decompression in patients with AO without myelotomy or shunt insertion.
Domenicucci and colleagues7 classify AO into three categories based on the calcified arachnoid morphology. Type I ossifications are semicircular in appearance, type II are circumferential and type III are a honeycomb-like ossifications, which affect the cauda equina. In their reports and review, one-third of patients with type I or II were associated with syringomyelia and/or spinal cord cysts.
Syringomyelia in the setting of AO has been hypothesized to result from vessel ischemia, CSF flow alterations, or even as a coexisting incidental finding.2,5,6 In a large study of 105 patients, Milhorat and colleagues identified 23 cases of focal intramedullary cavitation separated from the fourth ventricle by a syrinx-free segment of spinal cord.11 These occurred in association with Chiari I malformations, cervical spinal stenosis, spinal arachnoiditis, basilar impression, occipital encephalocele and congenital cyst of the terminal ventricle. Syringomyelia was associated with arachnoiditis in 13% of these cases. The histological features of these syrinxes differed from those that communicate with the fourth ventricle or posttraumatic extracanalicular syrinxes in that these were associated extensive ependymal denudation, paracentral dissection and intracanalicular septations. Dissections occurred at the poles or thinnest segment of syrinx and occasionally ruptured through the pia to communicate with the subarachnoid space. The authors hypothesize alterations in CSF dynamics is the impetus for disease progression.
Studies of syringomyelia associated with Chiari I malformations have hypothesized that syrinx progression is a result of cerebellar tonsils, which occlude the subarachnoid space at the foramen magnum and act as a piston on the partially enclosed spinal subarachnoid space.12 This may exaggerate cervical subarachnoid pressure waves that compress the spinal cord externally and propate the syrinx caudally with each heartbeat. Similar CSF flow changes in arachnoiditis may explain the unique histological phenomenon of paracentral dissection and account for reported cases of rapid progression of the syrinx in AO.2
Another compelling theory relates to decreased compliance of the subarachnoid space. In animal models of syringomyelia where arachnoiditis was induced by injection of kaolin in the subarachnoid space of the upper thoracic spinal cord, it was found that subarachnoid space pressures were higher rostral to the arachnoiditis compared to caudal.13 Furthermore, when CSF flow was studied with injection of the CSF tracer horseradish peroxidase, it was found that perivascular spaces were enlarged and the tracer stained these spaces and the central canal within 10 minutes. A reduction in the compliance of the subarachnoid space in this setting may increase the perivascular flow of CSF contributing to the formation and enlargement of syringomyelia.
Controversy in literature remains regarding the need for drainage and shunting of the syrinx. The current study is the first to report that decompression alone results in a decrease in the syrinx size on follow-up imaging with clinical improvement of the myelopathy and spasticity. Given current understanding of syrinx pathophysiology, it is the authors' opinion that reconstruction of the subarachnoid space in an attempt to normalize CSF fluid circulation may be adequate to decrease the syrinx size. This may avoid the added risks of myelotomy and shunting. Further research to identify the pathophysiology and natural history of syringomyelia in the setting of arachnoiditis is necessary before recommendations are made to change current practice.
Conclusion
Syringomyelia associated with arachnoiditis ossificans is a rare condition, which may be due to altered CSF dynamics secondary to the ossified arachnoid. CT scans are recommended for diagnosis as MRI may lack the sensitivity to identify intrathecal ossification and calcifications. Laminectomy and decompression with or without syrinx drainage and shunting remain the standard of care. A broader understanding of the pathophysiology of syrinx formation and progression is necessary and such research is currently underway.
Acknowledgements
The authors thank Steve Lunny for his help in formatting the figures included in the manuscript.
Footnotes
This report has received no financial support.
Point of View: Evidence-based Care Approach
Author: Osmar Moraes
Institution: Neurosurgical Department, Saint's Marceline University Hospital, Sao Paulo, Brazil
Arachnoiditis calcificans (or “ossificans”) remains poorly understood and is a rare condition.1,2 With the advent of MRI, chances for its diagnosis have improved and the development of microsurgical treatment increases prospects for safe surgical intervention. There is still not enough data in the literature to determine if we can define a ‘best therapy’ at this pointing time. Further cases likely stand to influence our understanding of this condition and its recommended treatment substantially.
The association of calcifying or ossifying arachnoiditis with syringomyelia is even more rare, as shown with the eight cases described in this article, in addition to two cases described by Mello et al.
Operative treatment does not interrupt the ossification process, which continues over time, and frequently seems to result in an unfortunate progressive deterioration of an affected patient. Spinal cord cavitations can occur due to spinal cord tethering, stretching, and central cord edema formation, accompanied by cerebrospinal fluid flow impairment and pulse pressure changes. The results of surgical interventions are generally described as poor, offering short-term recovery with subsequent later deterioration. Multiple pathogenic factors are involved in this clinical syndrome including complex metabolic changes.
Laminectomy or laminotomy and decompression with or without syrinx drainage and shunting remain the standard of care as the authors stated. Little or nothing is known as to adjuvant therapies to limit recurrence of the calcification process.
Dominicucci and colleagues suggested a classification into three categories based on three cases and their review of the literature. They used the morphology of the calcification as the foundation of their classification, and found this to help determine the best surgical tactic.
As we analyze the current accepted pathophysiology of arachnoid calcification and its possible influence on spinal cord function, in addition to my personal anecdotal (unpublished) experience in two personal cases, the best available data we have to date suggests the following:
The case presented is a Domenicucci type 1, representing a case with one or more surgically resectable compressions caused by a calcified arachnoid. The recommended treatment consists of resection of the calcification for decompression and duraplasty as necessary. In the absence of improvement or with progression of symptoms associated with a concurrent syringomyelia, myelotomy for cerebrospinal fluid drainage and shunting had been recommended.
The case described in the preceding text is the first one in the literature treated with decompression alone, and is very well documented. The demonstrated decrease in the syrinx size—in addition to a good clinical outcome—supports the recommendation of drainage surgery to be performed only in presence of progression of symptoms.
As a final observation this case has only 9-months of follow up, which is a short time from which to draw conclusions as to eventual surgical success.
Editorial Staff Perspective
Case reports have been frequently touted as representing the ‘bottom of the food chain’ in the evidence pyramid. The preceding case report explicitly presents a classic indication for a meaningful case report even in this day and age of insistence on ‘higher levels of evidence’. This exceedingly rare condition was luckily identified and after thorough review treated by expert surgeons armed with skill set and expertise to handle even the rarest and most unusual of cases.
We all continue to gain by case reports in these settings, especially if they go through the extra effort to summarize the past publication history in the topic in a mini metanalysis—as was done in this case. Caution should, however, be exercised in the over application of greater inferences for larger patient populations based on such case reports.
References
- 1.Kaufman A B, Dunsmore R H. Clinicopathological considerations in spinal meningeal calcification and ossification. Neurology. 1971;21(12):1243–1248. doi: 10.1212/wnl.21.12.1243. [DOI] [PubMed] [Google Scholar]
- 2.Papavlasopoulos F, Stranjalis G, Kouyialis A T. et al. Arachnoiditis ossificans with progressive syringomyelia and spinal arachnoid cyst. J Clin Neurosci. 2007;14(6):572–576. doi: 10.1016/j.jocn.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Nelson J. Intramedullary cavitation resulting from adhesive spinal arachnoiditis. Arch Neurol Psychiatry. 1943;50(1):1–7. [Google Scholar]
- 4.Van Paesschen W, Van den Kerchove M, Appel B. et al. Arachnoiditis ossificans with arachnoid cyst after cranial tuberculous meningitis. Neurology. 1990;40(4):714–716. doi: 10.1212/wnl.40.4.714. [DOI] [PubMed] [Google Scholar]
- 5.Kahler R J, Knuckey N W, Davis S. Arachnoiditis ossificans and syringomyelia: a unique case report. J Clin Neurosci. 2000;7(1):66–68. doi: 10.1054/jocn.1998.0144. [DOI] [PubMed] [Google Scholar]
- 6.Nagpal R D, Gokhale S D, Parikh V R. Ossification of spinal arachnoid with unrelated syringomyelia. Case report. J Neurosurg. 1975;42(2):222–225. doi: 10.3171/jns.1975.42.2.0222. [DOI] [PubMed] [Google Scholar]
- 7.Domenicucci M, Ramieri A, Passacantilli E. et al. Spinal arachnoiditis ossificans: report of three cases. Neurosurgery. 2004;55(4):985. doi: 10.1227/01.neu.0000137281.65551.54. [DOI] [PubMed] [Google Scholar]
- 8.Slavin K V, Nixon R R, Nesbit G M. et al. Extensive arachnoid ossification with associated syringomyelia presenting as thoracic myelopathy. Case report and review of the literature. J Neurosurg. 1999;91(S2):223–229. doi: 10.3171/spi.1999.91.2.0223. [DOI] [PubMed] [Google Scholar]
- 9.Revilla T Y, Ramos A, Gonzalez P. et al. Arachnoiditis ossificans. Diagnosis with helical computed tomography. Clin Imaging. 1999;23(1):1–4. doi: 10.1016/s0899-7071(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz E. Präparate von einem Falle syphilitischer Meningomyelitis mit Höhlenbildung im Rükenmarke und besonderen degenerativen Veränderungen der Neuroglia. Wien. klin. Wchnschr. 1897;110:17. [Google Scholar]
- 11.Milhorat T H, Capocelli A L, Anzil A P. et al. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82(5):802–812. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- 12.Heiss J D, Patronas N, Devroom H L. et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91(4):553–562. doi: 10.3171/jns.1999.91.4.0553. [DOI] [PubMed] [Google Scholar]
- 13.Abou-Hamden A, Jones N R, Stoodley A. et al. Investigations of cerebrospinal fluid dynamics in a sheep model of traumatic syringomyelia. The proceedings of the spine society meeting of Australasia, April 2003 (Abstract) 2003;9 [Google Scholar]
Additional references
- 1.Mello L R, Bernardes C I, Feltrin Y. et al. Thoracic spine arachnoid ossification with and without cord cavitation: report of three cases. J Neurosurg. 2001;94 01:115–120. doi: 10.3171/spi.2001.94.1.0115. [DOI] [PubMed] [Google Scholar]
- 2.Faure A, Khalfallah M, Perrouin-Verbe B. et al. Arachnoiditis ossificans of the cauda equina Case report and review of the literature. J Neurosurg. 2002;97 02:239–243. doi: 10.3171/spi.2002.97.2.0239. [DOI] [PubMed] [Google Scholar]