Abstract
Objective. To evaluate the efficacy and safety of oral Huangqi formulae for the treatment of stable COPD. Methods. The major databases were searched until September 2010 and supplemented with a manual search. Randomized controlled trials (RCTs) of oral Huangqi formulae that reported on lung function, St. George's Respiratory Questionnaire, symptom improvement and/or frequency of exacerbations were extracted by two reviewers. The Cochrane tool was used for the assessment of risk of bias in the included trials. Data were analyzed with RevMan 5.1.2 software. Results. 25 RCTs (1,661 participants) were included. Compared with conventional therapy (CT) alone, oral Huangqi formulae plus CT increased FEV1, and a similar result was found comparing Huangqi formulae with no treatment. Improvements in SGRQ total score, COPD-related symptoms and reduction of frequency of exacerbations were found in patients receiving Huangqi formulae plus CT compared to those receiving CT alone or CT plus placebo. No serious adverse events were reported. However, there were some methodological inadequacies in the included studies. Conclusions. The benefits of Huangqi formulae for stable COPD were promising, but its efficacy and safety have not been established due to methodological weakness and possible bias in the reported results. Further rigorously designed studies are warranted.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation and manifests as progressive dyspnea accompanied by deterioration of lung function [1]. It is a major cause of morbidity, disability, and mortality. The World Health Organization (WHO) estimated that COPD ranked fifth in terms of burden of disease worldwide [2]. In China, COPD affects 8.2% of people aged 40 years or older [3]. It was the fourth leading cause of death in cities and the third in rural areas [4].
Studies have shown that pharmacotherapy cannot modify the trend of decline in lung function [5, 6]. Chinese herbal medicine is commonly used for COPD, especially for the stable stage, in China and other Asian countries. Clinical studies suggest that herbal formulae that include Huangqi (Radix Astragalus membranaceus) were effective for stable COPD [7–31]. It often serves as a principal medicine (i.e., the ingredient provides the principal curative action on the main syndrome or primary symptom [32]) in formulae for COPD. However, the quality of these studies had not been evaluated systematically, and some of the reports for the effects of Huangqi were conflicting. Therefore, this systematic review was conducted to evaluate the evidence for the efficacy and safety of oral Huangqi for treating stable COPD.
2. Methods
2.1. Search Strategy
Comprehensive searches were performed for three English language databases and five Chinese databases, which included PubMed (from 1966), Embase (from 1985), Cochrane Central Register of Controlled Trials, Chinese Biomedical Database (CBM, from 1979), China National Knowledge Infrastructure (CNKI, from 1994), VIP medicine information system (VMIS, from 1989), Wanfang (from 1998), and TCM-Online (from 1949), from the inceptions of the databases to September 2010, without language restriction. A manual search was conducted of evidence-based medicine (EBM) reports on Chinese prescriptions by Japan Society for Oriental Medicine, EBM special committee [31]. The focus of the search was randomized controlled trials (RCTs) of oral Huangqi formula for stable COPD.
Search terms included chronic obstructive pulmonary disease, chronic bronchitis, emphysema, COPD, chronic obstructive lung disease, chronic obstructive airway disease, chronic airflow obstruction, traditional Chinese medicine, Chinese herbal drugs, complementary and alternative medicine, phytotherapy, herbs, herbal Medicine, Astragalus, Huangqi, Beiqi, Milkvetch Root, controlled clinical trial, and their synonyms. The literature was screened based on title, abstract, and full text as needed. Full details on the search strategy are described in the appendix.
2.2. Study Selection
The inclusion criteria were as follows: (1) RCTs with patients diagnosed with COPD in the stable stage [1, 33], which manifests as dyspnea, cough, and phlegm which remain stable or are rather mild; (2) Huangqi formula (taken orally as decoction, pill, powder, or capsule) alone or in combination with conventional therapy compared with placebo, no treatment, or conventional therapy as controls. Huangqi serves as a principal medicine, defined as follows: the properties of Huangqi are consistent with the main aims of the formula, or the dosage of Huangqi is relatively large (more than 15 g). Conventional therapy includes bronchodilators (beta2-agonists, anticholinergics, methylxanthine), corticosteroids, exercise training, smoking cessation, etc [1]. (3) Outcome measurements include spirometric parameters (forced expiratory volume in one second, FEV1), St. George's Respiratory Questionnaire (SGRQ) total score, symptom improvement, and/or frequency of exacerbations. The exclusion criteria were (1) trials that included patients with asthma or other non-COPD disorders; (2) test interventions that were combined with other TCM therapies such as acupuncture, acupoint injection; (3) Chinese herbs or other TCM therapies were used in the control group.
2.3. Data Extraction and Risk of Bias Assessment
Two authors (Lei Wu and Yuanbin Chen) independently assessed studies based on the inclusion and exclusion criteria. If needed, two other reviewers (Lin Lin and Zehuai Wen) were consulted. Data on the details of study design, participants, interventions, control medicine, outcome measures, numbers of dropouts, and number and nature of any adverse events reported were extracted to a predefined form.
Assessment of symptom improvement was based on the chronic bronchitis section on the Guidance for Clinical Research on New Drugs of TCM [34], where responses were categorized into four levels (symptom control, very good, good, and no effect). The proportion of patient responses for the following symptoms (cough, sputum, dyspnea, and rale) was assessed according to the previous levels.
Two authors (Lei Wu and Yuanbin Chen) independently assessed the risk of bias of the included studies using the Cochrane tool [35]. Any discrepancies in assessment were decided by discussion. Other authors (Zehuai Wen and Xinfeng Guo) were consulted to make the final decision when needed. To verify unclear information on methodology and therapy, attempts were made to contact the authors of the original papers via phone, email, or mail. If the authors were not contactable after 3 times by phone or email, they were sent mail and given one month to reply.
2.4. Data Analysis
Data were analyzed by RevMan 5.1.2 (Cochrane Collaboration), and Stata 12.0 software (StataCorp LP, College Station, TX, USA). Dichotomous data were presented as risk ratio (RR) and continuous outcomes as mean difference (MD), with 95% confidence intervals (95% CI). Statistical heterogeneity was assessed by Cochrane's Q test. If the analysis showed low heterogeneity (P ≥ 0.10 and I 2 ≤ 50%), data were synthesized using a fixed-effects model. Otherwise, a random-effects model was applied. Subgroup analyses were performed if sufficient numbers of RCTs were available and sensitivity analyses were undertaken as required. Publication bias was assessed by funnel plot analysis if the group included more than five trials [36]. Egger's test was conducted if it was difficult to determine whether the funnel plots were symmetrical.
3. Results
3.1. Description of Studies
Of 13,254 potentially relevant citations, 8,952 were excluded as duplications, 4,012 were excluded for not meeting the inclusion criteria after reading the titles and abstracts, and further 265 studies were excluded after reading the full articles and/or contacting the authors. Finally, 25 studies (including 1,661 participants) that met all the selection criteria were included (Figure 1).
Figure 1.
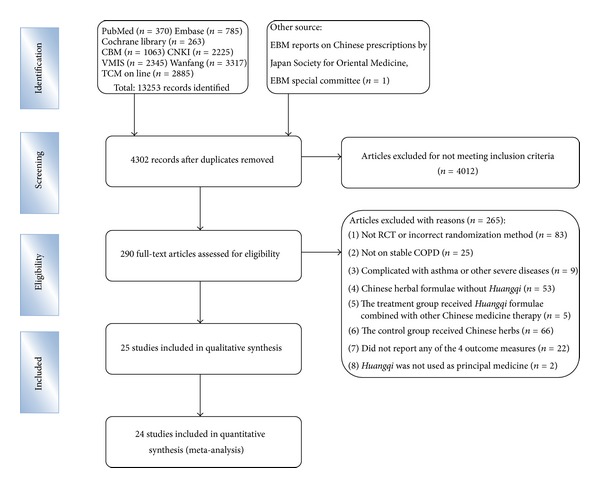
Flow diagram showing the trial selection process for the systematic review.
Of these 25 studies, 24 were conducted in China [7–30] and 1 in Japan [31]. Twenty-two were published and 3 were dissertations [20, 22, 26]. All patients were diagnosed as stable COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) or guidelines issued by Committee of Respiratory Disease, Chinese Medical Association. Disease severity in the included trials ranged from mild to very severe COPD. The duration of patients' COPD ranged from 4 to 30 years.
Nineteen studies compared Huangqi formulae plus conventional therapy with conventional therapy [7, 9–11, 14–19, 22, 24–31]. Two studies compared Huangqi formulae plus conventional therapy with placebo plus conventional therapy [12, 21]. Three studies compared Huangqi formulae with no treatment [8, 13, 23]. One study compared a Huangqi formula with conventional therapy [20]. The duration of treatment varied from 2 weeks to 6 months. One trial reported a 1-year follow-up period [16].
Five studies indicated that Huangqi was used as the principal medicine [12, 17, 18, 20, 26]. In the twenty remaining studies, we considered Huangqi to be the principal medicinal when its properties were consistent with the main aims of the formulae or its dosage was more than 15 g, even when the authors did not indicate that it was used as a principal medicinal. The characteristics of the included studies are summarized in Tables 1 and 2.
Table 1.
Characteristics of included studies.
| First author, year [Ref] | Location | No. of participants (R/A) | Age Mean ± SD (years) |
Severity of COPD | COPD History Mean ± SD (years) |
|---|---|---|---|---|---|
| Xu 2007 [7] |
China | T: 30/30 C: 30/30 |
T: 67 ± 19.31 C: 64 ± 20.97 |
T: II: 12, III: 18 C: II: 13, III: 17 |
NR |
| Hong 2004 [8] |
China | T: 20/20 C: 18/18 |
T: 67.70 ± 5.68 C: 66.89 ± 5.57 |
T: II: 5, III: 15 C: II: 4, III: 14 |
T: 15.05 ± 5.54 C: 14.33 ± 7.28 |
| Jia 2007 [9] |
China | T: 30/30 C: 25/25 |
T: 61.6 ± 6.1 C: 63.2 ± 5.3 |
T: I: 5, II: 18, III: 7 C: I: 3, II: 17, III: 5 |
T: 15.25 ± 4.01 C: 13.12 ± 3.38 |
| Wang 2005 [10] |
China | T: 20/20 C: 20/20 |
T: 59.4 ± 7.5 C: 60.9 ± 7.9 |
T: II A: 8, II B: 9, III: 3 C: II A: 9, II B: 9, III: 2 |
T: 11.2 ± 4.1 C: 11.8 ± 4.5 |
| Cui 2004 [11] |
China | T: 20/20 C: 20/20 |
T: 62.5 C: 61.5 |
NR | NR |
| Lin 2003 [12] |
China | T: 30/30 C: 30/30 |
T: 62 C: 60.5 |
NR | T: 16 ± NR C: 15.4 ± NR |
| Zhang 2006 [13] |
China | T: 24/24 C: 22/22 |
T: 66.70 ± 6.60 C: 67.89 ± 4.57 |
T: II: 5, III: 19 C: II: 4, III: 18 |
T: 14.05 ± 6.54 C: 15.33 ± 5.0 |
| Feng 2006 [14] |
China | T: 36/36 C: 33/33 |
48–73 | NR | NR |
| Zhao 2003 [15] |
China | T: 15/15 C: 15/15 |
T: 51.71 ± 8.10 C: 48.87 ± 7.73 |
NR | NR |
| Li 2006 [16] |
China | T: 85/85 C: 40/40 |
T: 53 C: 49 |
NR | T: 22 ± NR C: 25 ± NR |
| Xu 2004 [17] |
China | T: 25/25 C: 24/24 |
T: 63 ± 6.4 C: 62.9 ± 7.0 |
NR | T: 17.7 ± 9.5 C: 16.9 ± 12.3 |
| Luo 2002 [18] |
China | T: 36/36 C: 20/20 |
T: 63.86 ± 12.56 C: 63.90 ± 12.56 |
NR | T: 20.36 ± 11.21 C: 19.70 ± 10.00 |
| Wang 2007 [19] |
China | T: 30/30 C: 30/30 |
T: 62.8 C: 60.5 |
NR | T: 15 ± NR C: 18 ± NR |
| Huang 2008 [20] |
China | T: 16/16 C: 16/16 |
T: 74.75 ± 6.17 C: 73.81 ± 3.71 |
T: II: 8, III: 8 C: II: 8, III: 8 |
NR |
| He 2010 [21] |
China | T: 49/49 C: 49/49 |
75 ± 5.8 | NR | 11.25 ± 5.86 |
| Guan 2006 [22] |
China | T: 37/30 C: 37/30 |
T: 65.5 ± 8.21 C: 65.10 ± 12.42 |
T: 0:7, I: 4, II: 4, III: 15 C: 0:4, I: 5, II: 11, III: 10 |
NR |
| Wei 2007 [23] |
China | T: 60/60 C: 60/60 |
Not reported | NR | NR |
| Ma 2010 [24] |
China | T: 30/30 C: 30/30 |
Not reported | I to II | NR |
| Ding 2009 [25] |
China | T: 53/53 C: 53/53 |
T: 61.23 ± 3.65 C: 62.16 ± 3.03 |
T: I: 35, II: 18 C: I: 31, II: 22 |
T: 17.21 ± 3.02 C: 16.79 ± 2.41 |
| Zhou 2005 [26] |
China | T: 30/30 C: 30/30 |
T: 63.63 ± 7.77 C: 64.83 ± 7.37 |
NR | NR |
| Hu, 2009 [27] | China | T: 35/35 C: 32/32 |
T: 64.7 ± 7.5 C: 63.2 ± 5.4 |
NR | NR |
| Chen 2009 [28] |
China | T: 30/30 C: 30/30 |
70.1 | NR | NR |
| Liang 2009 [29] |
China | T: 32/32 C: 33/33 |
T: 69.73 C: 70.69 |
T: I: 0, II: 13, III: 12, IV: 7 C: I: 0, II: 15, III: 12, IV: 6 |
T: 17.32 ± NR C: 17.02 ± NR |
| Zhang 2009 [30] |
China | T: 60/56 C: 60/48 |
68.4 | NR | NR |
| Tatsumi 2002 [31] |
Japan | T: 34/34 C: 37/37 |
Not reported | II to III | NR |
T: treatment; C: control; NR: not reported; R: number of subjects randomized; A: number of subjects analysed; SD: standard deviation.
Table 2.
Characteristics of included studies.
| First author, year [Ref] | Intervention (ingredients of Huangqi formulae) | Control | Duration /followup | Adverse event | Outcome measures | |||
|---|---|---|---|---|---|---|---|---|
| Lung function | PESI | SGRQ | FCOPDE | |||||
| Xu, 2007 [7] | Manzufei decoction (Ginseng, Huangqi, Prepared Rehmannia Root, Tatarian Aster Root, White Mulberry Root-Bark, Chinese Caterpillar Fungus, Chinese Magnoliavine Fruit, Liquorice Root, Salvia Root, Peach Seed, Earthworm, Dwarf Lilyturf Tuber, English Walnut Seed) + Bronchodilators | Bronchodilators | 2 weeks/NR | No | Yes (no FEV1) | No | No | No |
|
| ||||||||
| Hong, 2004 [8] | Yufeining pills (Ginseng, Huangqi, Polygonatum sibiricum, White Atractylodes Rhizome, Human Placenta, English Walnut Seed, Dodder Seed, Asiatic Cornelian Cherry Fruit, Bitter Apricot Seed, Snakegourd Fruit, Thunberg Fritillary Bulb, Salvia Root, Peach seed) | No treatment | 6 mths/NR | No | Yes | No | No | No |
|
| ||||||||
| Jia, 2007 [9] | Yiqihuoxue decoction (Huangqi, Earthworm, Figwort Root, Salvia Root, Heterophylly Falsestarwort Root, Chinese Angelica) + Ipratropium bromide | Ipratropium bromide | 6 mths/NR | NR | Yes (no FEV1) | No | Yes | Yes (noncontinuous data) |
|
| ||||||||
| Wang, 2005 [10] | Yifeijianpi decoction (Huangqi, Tangshen, White Atractylodes Rhizome, Poria, Divaricate Saposhnikovia Root, Pinellia Tuber, Dried Tangerine Peel, Earthworm, Common Coltsfoot Flower, Liquorice Root) + Hydrochloric acid ammonia bromine tablet | Salbutamol + Hydrochloric acid ammonia bromine tablet | 8 weeks/NR | NR | Yes (no FEV1) | No | No | No |
|
| ||||||||
| Cui, 2004 [11] | Tongfei decoction (Unprocessed Rehmannia Root, Chinese Angelica, Huangqi, Salvia Root, Lily Bulb, Dwarf Lilyturf Tuber, Ginseng, Pinellia Tuber, Thunberg Fritillary Bulb, Snakegourd Fruit, Poria, Liquorice Root, Fructus Perillae, Citrus Red) + Ipratropium bromide+ Oxygen therapy | Ipratropium bromide + Oxygen therapy | 4 weeks/NR | No | Yes | No | No | No |
|
| ||||||||
| Lin, 2003 [12] | Jianpiyifei granule (Ginseng, White Atractylodes Rhizome, Poria, Dwarf Lilyturf Tuber, White Mulberry Root-Bark, Huangqi) + Conventional treatment | Placebo + Conventional treatment | 2 mths/NR | NR | Yes (no FEV1) | Yes | No | No |
|
| ||||||||
| Zhang, 2006 [13] | Jianpiyifeibushen decoction (Huangqi, Tangshen, White Atractylodes Rhizome, Poria, Dwarf Lilyturf Tuber, Coastal Glehnia Root, Malaytea Scurfpea Fruit, Dodder Seed, Glossy Privet Fruit, Tokay Gecko, Bitter Apricot Seed, Snakegourd Fruit, Thunberg Fritillary Bulb, Salvia Root, Sichuan Lovage Rhizome) | No treatment | 6 mths/NR | NR | Yes (no FEV1) | No | No | Yes (noncontinuous data) |
|
| ||||||||
| Feng, 2006 [14] | Jianpibufei decoction (Heterophylly Falsestarwort Root, Huangqi, Tokay Gecko, White Atractylodes Rhizome, Largetrifoliolious Bugbane Rhizome, Chinese Thorowax Root, Chinese Angelica, Common Coltsfoot Flower, Dodder Seed, Leech) + Doxofylline tablet + Contracting lip breathing + Oxygen therapy | Doxofylline table + Contracting lip breath + Oxygen therapy | 30 days/NR | NR | Yes (no FEV1) | Yes | No | No |
|
| ||||||||
| Zhao, 2003 [15] | Herbal decoction (Huangqi, Cassia Bark, Radix Aconiti Praeparata, Prepared Rehmannia Root, Common Yam Rhizome, Asiatic Cornelian Cherry Fruit, Poria, Oriental Waterplantain Rhizome, Tree Peony Root Bark, Dwarf Lilyturf Tuber, Chinese Magnoliavine Fruit) + Pulmonary rehabilitation | Pulmonary rehabilitation | 4 weeks/NR | NR | Yes (no FEV1) | No | No | No |
|
| ||||||||
| Li, 2006 [16] | Bufeihuoxue Decoction (Huangqi, White Atractylodes Rhizome, Common Yam Rhizome, Peach Seed, Salvia Root, Sanqi, Red Peony Root, Safflower, Dried Tangerine Peel, Bitter Apricot Seed, Dwarf Lilyturf Tuber) + Bronchodilators + Expectorants | Bronchodilators + Expectorants | 8 weeks/1 year | NR | Yes | Yes | No | Yes |
|
| ||||||||
| Xu, 2004 [17] | Fufangqiqi decoction (Huangqi, Polygonatum Sibiricum, Epimedium Herb, Sanqi) + Ipratropium bromide | Nucleotide and casein oral solution + Ipratropium bromide | 6 mths/NR | NR | Yes | No | No | Yes |
|
| ||||||||
| Luo, 2002 [18] | Baofeidingchuan granule (Tangshen, Huangqi, Salvia Root, Chinese Angelica, Unprocessed Rehmannia Root, Dwarf Lilyturf Tuber, Platycodon Root, Liquorice Root, Earthworm, Epimedium Herb, etc) Theophylline controlled release capsules + Carbocisteine + Contracting lip breathing | Theophylline controlled release capsules + Carbocisteine + Contracting lip breathing | 3 mths/NR | NR | Yes | No | No | No |
|
| ||||||||
| Wang, 2007 [19] | Bufeiyishen capsule (Tangshen, Huangqi, White Atractylodes Rhizome, Dwarf Lilyturf Tuber, Salvia Root, Chinese Caterpillar Fungus, Prepared Rehmannia Root, etc) + Bronchodilators + Mucus lytic agent + Antioxidants + Oxygen therapy | Bronchodilators + Mucus lytic agent+ Antioxidants + Oxygen therapy | 6 mths/NR | NR | Yes | No | No | No |
|
| ||||||||
| Huang, 2008 [20] | Butihuatan decoction (Huangqi, Heterophylly Falsestarwort Root, Cassia Bark, Chinese Angelica, Salvia Root, Fructus Perillae, Radish Seed, Pepperweed Seed, Leaf of Leatherleaf Mahonia) | Salbutamol + Theophylline + Hydrochloric acid ammonia bromine tablet + α-Chymo- trypsin | 2 mths/NR | NR | Yes (no FEV1) | Yes | No | No |
|
| ||||||||
| He, 2010 [21] | Buzhongyiqi granule (Ginseng, Huangqi, White Atractylodes Rhizome, Chinese Angelica, Dried Tangerine Peel, Divaricate Saposhnikovia Root, Largetrifoliolious Bugbane Rhizome, Poria, Fragrant Solomonseal Rhizome, Coastal Glehnia Root) + Theophylline | Placebo + Theophylline | 6 mths/NR | No | Yes (no FEV1) | No | No | Yes |
|
| ||||||||
| Guan, 2006 [22] | Feikang granule (Huangqi, Leech,Pinellia Tuber, Earthworm,) + Conventional therapy | Conventional therapy | 3 mths/NR | NR | Yes (no FEV1) | No | No | No |
|
| ||||||||
| Wei, 2007 [23] | Yiqihuayu decoction (Huangqi, Herba Gynostemmatis, Cairo Morningglory Root or Leaf, Largetrifoliolious Bugbane Rhizome, Platycodon Root, Common Anemarrhena Rhizome, Zedoary Rhizome, Peach Seed) | No treatment | 3 mths/NR | NR | Yes | No | No | No |
|
| ||||||||
| Ma,2010 [24] | Bufei decoction (Tangshen, Huangqi, Prepared Rehmannia Root, White Mulberry Root-Bark, Malaytea Scurfpea Fruit, Tokay Gecko, Tatarian Aster Root, Chinese Magnoliavine Fruit, Liquorice Root) + Pulmonary rehabilitation | Pulmonary rehabilitation | 12 weeks/NR | NR | Yes (no FEV1) | No | Yes | Yes |
|
| ||||||||
| Ding, 2009 [25] | Herbal decoction (Tangshen, Huangqi, Magnetite, English Walnut Seed, Chinese Magnoliavine Fruit, Tatarian Aster Root, Common Coltsfoot Flower, Fructus Perillae, Citrus Red, Aloeswood, Liquorice Root, Human Placenta, Tokay Gecko) + Theophylline tablet | Theophylline tablet | 2 mths/NR | NR | Yes | Yes | No | No |
|
| ||||||||
| Zhou, 2005 [26] | Feisaitong mixture (Huangqi, Coix Seed, Platycodon Root, Radish Seed, Salvia Root, Earthworm, Common Anemarrhena Rhizome) + Salbutamol | Salbutamol | 1 mth/NR | No | Yes (no FEV1) | Yes | No | No |
|
| ||||||||
| Hu, 2009 [27] | Jiajianbufei decoction (Tangshen, Huangqi, Figwort Root, Dwarf Lilyturf Tuber, Malaytea Scurfpea Fruit, Morinda Root, Dodder Seed, Stemona Japonica, White Mulberry Root-Bark, Dried Tangerine Peel, Platycodon Root, Salvia Root) + Oxygen therapy + Hydrochloric acid ammonia bromine tablet + Theophylline + Salbutamol | Oxygen therapy + Hydrochloric acid ammonia bromine tablet + Theophylline + Salbutamol | 2 mths/NR | NR | Yes | Yes | No | No |
|
| ||||||||
| Chen, 2009 [28] | Jiaweiqiweiduqi decoction (Asiatic Cornelian Cherry Fruit, Common Yam Rhizome, Prepared Rehmannia Root, Tree Peony Root Bark, Oriental Waterplantain Rhizome, Poria, Chinese Magnoliavine Fruit, Huangqi, Tangshen, White Atractylodes Rhizome) + salmeterol/fluticasone | Salmeterol/fluticasone | 12 weeks/NR | Yes | Yes | No | Yes | No |
|
| ||||||||
| Liang, 2009 [29] | Dongping decoction (Chinese Caterpillar Fungus, Huangqi, White Atractylodes Rhizome, Divaricate Saposhnikovia Root) + Bronchodilators | Bronchodilators | 12 mths/NR | NR | Yes (no FEV1) | Yes | No | No |
|
| ||||||||
| Zhang, 2009 [30] | Yifeiyangyin decoction (Lily Bulb, Unprocessed Rehmannia Root, Prepared Rehmannia Root, Thunberg Fritillary Bulb, Platycodon Root, Fructus Auranti, Dwarf Lilyturf Tuber, Radix Paeoniae Alba, Chinese Angelica, Coastal Glehnia Root, Common Yam Rhizome, Poria, Huangqi, Liquorice Root ) + Conventional treatment | Conventional treatment | 6 mths/NR | NR | Yes | No | Yes | No |
|
| ||||||||
| Tatsumi, 2002 [31] | Buzhongyiqi decoction (Huangqi, Ginseng, White Atractylodes Rhizome, Liquorice Root, Chinese Angelica, Dried Tangerine Peel, Largetrifoliolious Bugbane Rhizome, Chinese Thorowax Root, Ginger) + Inhaled bronchodilators, inhaled corticosteroids, or both | Inhaled bronchodilators, ICS, or both | 6 mths/NR | No | No | No | Yes | Yes |
PESI: percentage of effectiveness of symptom improvement; SGRQ: St. George's Respiratory Questionnaire; FCOPDE: frequency of COPD exacerbation; NR: not reported.
3.2. Risk of Bias of the Included Studies
After attempts at verification by contacting the authors of the original papers through phone, e-mail, or mail, 14 of the included trials still lacked a detailed description of the method of randomization, and 19 trials did not indicate whether blinding was applied. Only two trials described the method of generation of randomization sequence, allocation concealment, double blinding, and placebo manufacturing method. Two trials reported dropouts; one of them explained the reason was loss of contact with the participants, and the other did not provide reasons. No trials reported whether they had used intention-to-treat analysis. Selective outcome reporting was judged as low risk of bias in 2 trials, uncertain risk in 19 trials, and high risk in 4 trials. No study had a registered or published protocol; so, judgment was based on the outcome measures specified in the method section of the study and was informed by discussion with the author when possible. Most of the included trials lacked a sample size calculation (Figure 2).
Figure 2.
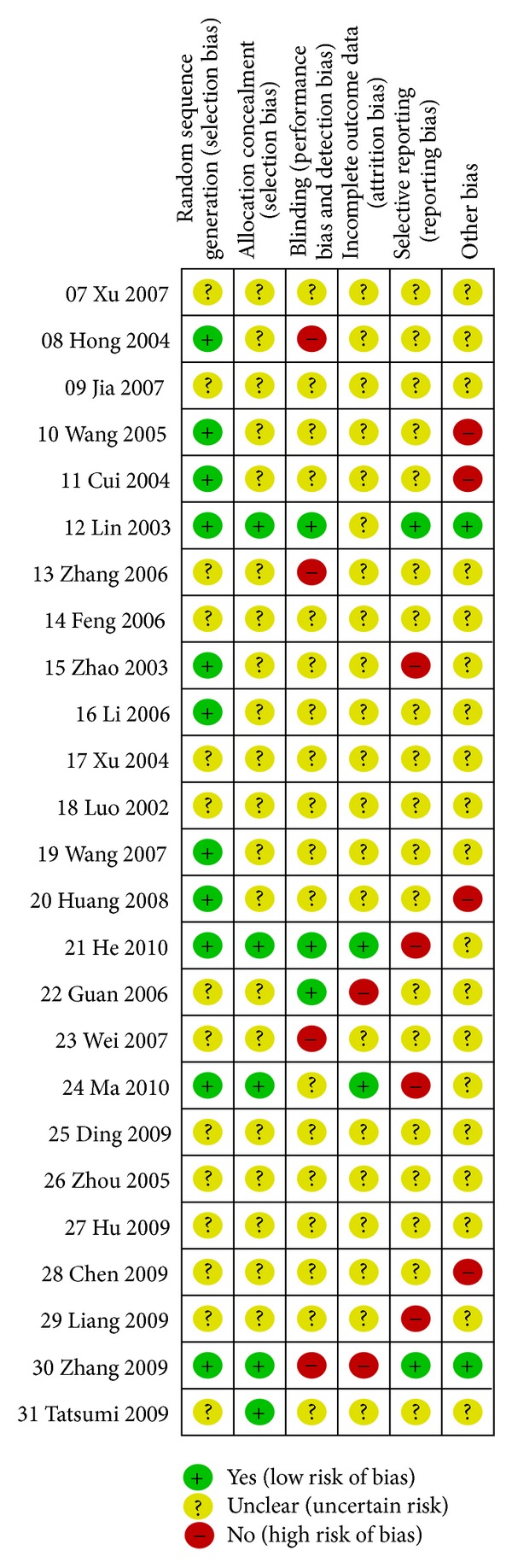
Summary of assessment of risk of bias for each included study.
3.3. Publication Bias
The number of studies reporting SGRQ scores and the frequency of exacerbations was less than five; so, a funnel plot was not applicable. The characteristics of studies reporting FEV1 were different, and the number of trials in each subgroup was less than five; so, funnel plots were also not applicable. For symptom improvement, the symmetry of the funnel plot was not clear; so, Egger's test was conducted. It indicated that the effect of publication bias was not significant (t = 1.39, 95% CI, −0.69 to 2.48, P = 0.215) (Figure 3).
Figure 3.
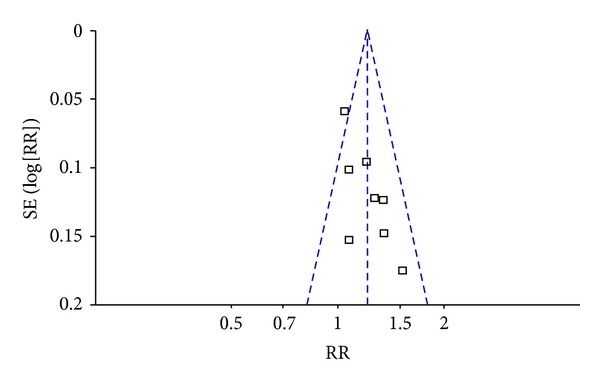
Funnel plot of publication bias using symptom improvement.
3.4. Outcome Measures
3.4.1. Spirometric Parameters
Subgroup analyses were performed according the type of comparison (Figure 4). Subgroup 1 compared Huangqi formulae plus conventional therapy with conventional therapy alone [11, 16–19, 25, 27, 28, 30]. FEV1 increased 0.27 L (95% CI: 0.11 to 0.43) based on 9 trials, but there was high heterogeneity in this subgroup. So, further analyses were performed according to duration of treatment as follows: more than, equal to, or less than 3 months. The former 2 categories had low heterogeneity. There was a significant difference in FEV1 in the trials which had durations of more than 3 months (MD 0.33, 95% CI: 0.20 to 0.46) [17, 19, 30] and no significant difference in those of 3 months duration (MD 0.07, 95% CI: −0.07 to 0.20) [18, 28]. For trials of less than 3 months [11, 16, 25, 27], FEV1 increased (MD 0.36, 95% CI: 0.29 to 0.43), but there was heterogeneity. So, sensitivity analysis was conducted. It produced low heterogeneity with a significant difference (MD 0.49, 95% CI: 0.41 to 0.57) when one trial [16] was removed because the age distribution of participants differed from the other trials.
Figure 4.
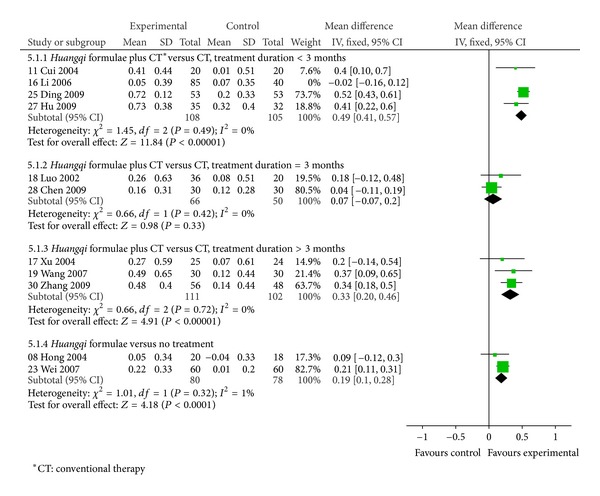
Forest plot of Huangqi formulae plus conventional therapy versus conventional therapy, or Huangqi formulae versus no treatment in patients with stable COPD: change in FEV1 (L).
Subgroup 2 compared Huangqi formulae with no treatment [8, 23]. FEV1 increased significantly by 0.19 L based on 2 trails (95% CI: 0.10 to 0.28).
3.4.2. Quality of Life
Patients receiving Huangqi formulae plus conventional therapy showed a significantly greater reduction in SGRQ total score (MD −5.04, 95% CI: −7.48 to −2.61) than those receiving conventional therapy alone, based on 3 trials [24, 28, 30] (Figure 5).
Figure 5.
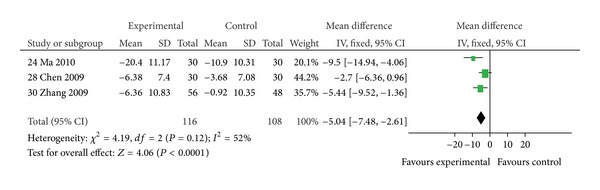
Comparison of Huangqi formulae plus conventional therapy versus conventional therapy alone in patients with stable COPD: change in SGRQ total scores.
3.4.3. Symptom Improvement
Patients receiving Huangqi formulae plus conventional therapy were more likely to show improvements in COPD-related symptoms (RR: 1.21, 95% CI: 1.12 to 1.31) when compared with those receiving conventional therapy alone or conventional therapy plus placebo, based on 8 trials [12, 14, 16, 20, 25–27, 29] (Figure 6).
Figure 6.
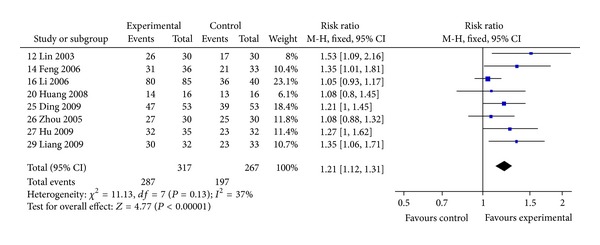
Comparison of Huangqi formulae plus conventional therapy versus conventional therapy alone in patients with stable COPD: overall symptom improvement (defined as “good” or above).
3.4.4. Frequency of Exacerbations
Four trials reported frequency of exacerbations at pre- and posttreatment [16, 17, 21, 24]. Compared with placebo plus conventional therapy or conventional therapy alone, the frequency of exacerbations in patients receiving Huangqi formulae plus conventional therapy was significantly reduced (MD −0.97, 95% CI: −1.57 to −0.37), but the heterogeneity was high (Figure 7). This appeared due to variation between trials in terms of the control interventions used, duration, and trial quality. Overall, the better result appeared to be of He et al., 2010 [21].
Figure 7.
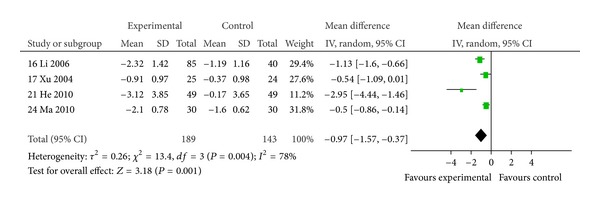
Comparison of Huangqi formulae plus conventional therapy versus placebo plus conventional therapy or conventional alone in patients with stable COPD: change in frequency of exacerbations.
3.5. Adverse Events
Six trials reported that no adverse events occurred [7, 8, 11, 21, 26, 31]. One trial (60 participants) reported hoarseness in the control group treated by salmeterol/fluticasone [28], but there was no causality assessment for this adverse event. The other trials did not report adverse events; so, there was insufficient data to assess whether the combination of Huangqi formulae plus conventional therapy affected the adverse event rate.
4. Discussion
This systematic review includes 24 RCTs conducted in China and 1 RCT in Japan. All RCTs employed the herb Huangqi as a principal herb in the herbal formulae used in the test arms, and all studies only included patients assessed as suffering stable COPD. The comparators were mostly conventional therapy, but this was variable and was not clearly specified in some studies. This is likely to have been a source of heterogeneity in the meta-analyses, but it also reflects usual care since patients with stable COPD would typically receive a variety of conventional therapies which may be varied according to response and individual need. Eight studies were of six months, while the others were of shorter treatment duration and only one had a followup at one year; so, the results can only be considered relevant to the relatively short-term management of COPD.
The meta-analysis results indicate that the use of Huangqi formulae could significantly improve lung function measured as FEV1 when compared with no treatment based on two studies, and it produced an additional improvement when combined with conventional therapies based on 9 studies, four of which were of over three months duration. The incidence of exacerbations also appeared to decline when Huangqi formulae were combined with conventional therapies. Improvements in quality of life based on SGRQ were evident, but this was based on only three studies. Also, these Huangqi-containing formulae appeared to be well tolerated, even when combined with conventional medications, since seven studies reported that no adverse events were noted.
All the included trials demonstrated at least some methodological deficiencies leading to potential risks of bias. Only eleven provided evidence of adequate randomization procedures, and only three were effectively blinded to participants and investigators. Consequently, the results should be interpreted with caution. Therefore, the potential benefits of oral Huangqi formulae for stable COPD need to be further appraised through trials that employ rigorous methodology and include adequate assessment of the safety profiles of the interventions. In addition, we found that the reporting of trial methods and procedures was frequently unclear and insufficient. Therefore, we suggest that all reports of RCTs published in China should be required to comply with the CONSORT statement [37] and the publication of protocols should be encouraged.
There has been increasing interest in complementary and alternative medicine (CAM) for the treatment of COPD, especially the use of Chinese herbal medicines [38, 39]. A recent cross-sectional study in Australia suggested that nearly one in five (17.3%) individuals with moderate to severe COPD had used some form of herbal preparation [39]. Therefore, reviews of the state of the evidence base are essential.
From viewpoint of TCM, patients with stable COPD usually manifest with Qi-deficiency syndrome [40]. One of the characteristics of Qi-deficiency is that the patient easily suffers from colds which commonly lead to acute exacerbations of COPD. Huangqi is one of the principal herbs used for reinforcing Qi. It has been widely used for preventing and alleviating common colds; so, its clinical use in COPD is predicated on a putative benefit in preventing colds and reducing COPD exacerbations.
From the experimental perspective, one line of research into Huangqi has focused on its effects on inflammation. The main pathological characteristic of COPD is chronic airway inflammation involving a number of proinflammatory mediators and cytokines. Also, oxidative stress is increased in COPD, which amplifies inflammation and may result in corticosteroid resistance. An invivo study suggests that Huangqi may reduce inflammatory infiltration and inhibit the inflammatory response in the airway through downregulating the expression of TNF-α and IL-8 [41]. Flavonoids in Huangqi may protect the erythrocyte membrane from attack by free radicals and appear to eliminate free radicals [42]. One study has shown that airway inflammation induced by cigarette smoke was reversed by astragaloside IV (AST IV), another active constituent of Huangqi, in a dose-dependent fashion. This effect appeared due to its anti-inflammatory and antioxidant properties, including NF-κB inactivation [43]. Other studies suggested that AST IV possesses anti-inflammatory and immune regulation activity and can be used for preventing asthma attacks [44]. The previous pharmacological properties of Huangqi may at least partially explain the clinical benefits reported by the studies included in this paper.
An earlier paper [45] evaluated the effects of a diverse range of herbal medicines in COPD, and a subsequent paper narrowed the focus to ginseng and ginseng-containing formulae [46]. The strengths of this paper are it focuses only on formulae that share the same principal ingredient and there is experimental evidence that supports the application of this herb as a modulator of inflammation.
The main limitations to this paper are the potential sources of bias due to methodological defects and inadequacies in reporting. Although publication bias was not a major issue, the previous issues potentially lead to overreporting of positive results, selective reporting of outcome measures, and underreporting of adverse events. Also, the use of a diversity of conventional therapies as comparators makes it difficult to assess the magnitude of any effects and to interpret their clinical significance.
Therefore, the potential benefits of oral Huangqi formulae for stable COPD evident in this paper need to be further appraised through suitably powered clinical trials that employ standardized conventional therapies as comparators over a sufficient period to determine whether any effects are of sufficient clinical relevance to warrant modification to current best practice for the management of stable COPD.
5. Conclusions
Oral Huangqi formulae appear beneficial in terms of improving lung function, quality of life, and symptoms and in reducing the incidence of exacerbations for patients with stable COPD, but these apparent benefits require further appraisal through higher quality trials that strictly adhere to methodological principles and procedures.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgments
This work was supported by the Joint Sino-Australia Project of the International Research Network for Traditional and Complementary Medicine (IRN-TCM), titled “Evaluation of Traditional and Scientific Evidence of Chinese Medicine (ETSE-CM),” and the Program of International S&T Cooperation from the Ministry of Science and Technology, China (no. 2012DFA31760).
Abbreviations
- AST IV:
Astragaloside IV
- CAM:
Complementary and alternative medicine
- CBM:
Chinese biomedical
- CI:
Confidence interval
- CNKI:
China national knowledge infrastructure
- COPD:
Chronic obstructive pulmonary disease
- EBM:
Evidence-based medicine
- ETSE-CM:
Evaluation of Traditional and Scientific Evidence of Chinese Medicine
- FCOPDE:
Frequency of COPD exacerbationdatabase
- FEV1:
Forced expiratory volume in 1 second
- ICS:
Inhaled corticosteroids
- IL-8:
Interleukin-8
- IRN-TCM:
International Research Network for Traditional and Complementary Medicine
- ITT:
Intention-to-treat
- IV:
Inverse variance
- MD:
Mean difference
- NF-κB:
Nuclear factor-κB
- PESI:
Percentage of effectiveness of symptom improvement
- RR:
Risk ratio
- RCTs:
Randomized controlled trials
- SGRQ:
St. George's Respiratory Questionnaire
- TCM:
Traditional Chinese medicine
- TNF-α:
Tumor Necrosis Factor-α
- VMIS:
VIP medicine information system
- WHO:
World Health Organization.
Appendix
Search Strategies
English Databases
-
#1:
Chronic obstructive pulmonary disease OR COPD OR emphysema OR Chronic obstructive lung disease OR Chronic obstructive airway disease OR emphysema, pulmonary disease OR Airflow obstruction, Chronic OR Chronic Airflow Obstruction OR Pulmonary Emphysema
-
#2:
Traditional Chinese Medicine OR Chinese Traditional Medicine OR Chinese Herbal Drugs OR Chinese Drugs, Plant OR Medicine, Traditional OR Ethnomedicine OR Ethnobotany OR Medicine, Kampo OR TCM OR Alternative Medicine OR Complementary Medicine OR Phytotherapy OR Herbology OR Plants, Medicinal OR Plant Preparations OR Plant Extracts OR Plants, Medicine OR Materia Medica OR Single Prescription OR Herbs OR Chinese Medicine Herb OR Herbal Medicine
-
#3:
Clinical Trial OR clinical study OR biomedical research OR Controlled Trial OR Controlled study OR random control Trial OR random control study OR Multicenter Study OR random allocation OR double-blind OR single-blind OR comparative study OR evaluation study OR follow-up study OR prospective study OR research design OR control group OR placebo control OR dummy control OR blinding OR clinical research OR medical trial OR in vivo study OR case control study OR intervention study
-
#4:
Astragalus OR Milkvetch OR Huangqi OR Beiqi OR Milkvetch Root
-
#5:
#1 AND #2 AND #3
-
#6:
#1 AND #3 AND #4
-
#7:
#5 OR #6.
Chinese Databases (search by using simplified Chinese character)
-
#1:
Man Xing Zu Sai Xing Fei Bing (Chronic obstructive pulmonary disease) OR Man Xing Zu Sai Xing Fei Ji Bing (Chronic obstructive pulmonary disease) OR Man Zu Fei (Chronic obstructive pulmonary disease) OR Man Xing Zu Sai Xing Fei Bu Ji Bing (Chronic obstructive lung disease) OR COPD OR Zu Sai Xing Fei Ji Bing (Obstructive pulmonary disease) OR Zu Sai Xing Fei Bing (Obstructive pulmonary disease) OR Man Xing Zu Sai Xing Fei Qi Zhong (Chronic obstructive pulmonary emphysema) OR Zu Sai Xing Fei Qi Zhong (Obstructive pulmonary emphysema)
-
#2:
Zhong Yi (Traditional Chinese Medicine) OR Guo Yi (Chinese Medicine) OR Chuan Tong Yi Xue (Traditional Medicine) OR Han Fang (Kampo) OR Han Yi (Kampo Medicine) OR Dong Yi (Vietnamese traditional medicine) OR Min Zu Yi Yao (Ethnomedicine) OR Bian Zheng (Syndrome Differentiation) OR Zhi Fa (Therapeutic Method) OR Zhi Ze (Therapeutic Principle) OR Zhong Xi Yi (Chinese and Western medicine) OR Zhong Xi Yi Jie He (Integration of Chinese and Western medicine) OR Zhong Xi Yao (Chinese and Western medicine) OR Zhong Yao (Chinese Medicine) OR Cao Yao (Herbal Medicine) OR Zhong Cheng Yao (Chinese patent medicine) OR Zhi Wu Yao (Phytomedicine) OR Zhen Ci (acupuncture) OR Jiu (moxibustion) OR Jing Luo (meridian/channel) OR Xue Wei (acupoint) OR Shui Zhen (Aqueous acupuncture) OR Er Xue (ear point) OR Er Zhen (ear acupuncture) OR Fang Xue (Bloodletting) OR Ci Xue (pricking blood) OR Ci Luo (collateral pricking) OR Dian Zhen (electronic acupuncture) OR Ba Guan (cupping) OR Tui Na (tuina) OR An Mo (massage) OR Mei Hua Zhen (plum-blossom needle) OR San Leng Zhen (three-edged needling) OR Mai Xian (catgut-embedding) OR Yao Yun (hot medicinal compress) OR Guan Chang (enema)
-
#3:
Huang Qi (Milkvetch) OR Bei Qi (Milkvetch) OR Huang Qi (variant of Chinese character, Milkvetch)
-
#4:
#1 AND #2
-
#5:
#1 AND #3
-
#6:
#4 OR #5.
References
- 1.GOLD. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2010, http://www.goldcopd.
- 2.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nature Medicine. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 3.Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. American Journal of Respiratory and Critical Care Medicine. 2007;176(8):753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 4.Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest. 2011;139(4):920–929. doi: 10.1378/chest.10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the lung health study. Journal of the American Medical Association. 1994;272(19):1497–1505. [PubMed] [Google Scholar]
- 6.Vestbo J, Sørensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353(9167):1819–1823. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 7.Xu LS. The influence of combined treatment of traditional Chinese medicine and western medicine on pulmonary function with the patients of moderate and severe COPD. China and Foreign Medical Journal. 2007;5(2):55–56. [Google Scholar]
- 8.Hong ML, Gao LY, Dai SZ, et al. The effects of Yufeining on respiratory muscle strength and central respiratory drive in patients with COPD. Chinese Journal of Information on Traditional Chinese Medicine. 2004;11(11):961–963. [Google Scholar]
- 9.Jia GL, Tong FJ, Yu DZ, et al. The clinical study of Yiqihuoxue combined ipratropium bromide inhalation in the treatment of chronic obstructive pulmonary disease. Zhejiang Journal of Traditional Chinese Medicine. 2007;42(9):510–511. [Google Scholar]
- 10.Wang S, Ji HY, Zhang NZ, et al. Effect of yifei jianpi recipe on inflammatory cells, levels of interleukin-8 and tumor necrosis factor-alpha in sputum from patients with chronic obstructive pulmonary disease. Chinese Journal of Integrated Traditional and Western Medicine. 2005;25(2):111–113. [PubMed] [Google Scholar]
- 11.Cui ZB, Yuan YD, Liu SH, et al. Intervention effect of tongfei mixture on nocturnal hypoxia in patients with chronic obstructive pulmonary disease. Chinese Journal of Integrated Traditional and Western Medicine. 2004;24(10):885–888. [PubMed] [Google Scholar]
- 12.Lin L, Tang CY, Xu YJ. Clinical observation of spleen-lung nourishing granule in treating respiratory muscle fatigue of stable chronic obstructive pulmonary disease. Shanghai Journal of Traditional Chinese Medicine. 2003;37(11):10–12. [Google Scholar]
- 13.Zhang MX. Clinical research on stable COPD treated by Jianpi Yifei Bushen method. Chinese Journal of Information on Traditional Chinese Medicine. 2006;13(7):64–65. [Google Scholar]
- 14.Feng XZ, Yao HQ. Clinical research on Jianpi Bufei method combined with western medicine in treating stable chronic obstructive pulmonary disease in 36 cases. Qinghai Medical Journal. 2006;36(9):82–83. [Google Scholar]
- 15.Zhao W, Luo FM, He CQ. Effects of lung rehabilitation and Bushen Chinese herbs in treating stable chronic obstructive pulmonary disease with lung function and quality of life. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2003;12(24):2643–2644. [Google Scholar]
- 16.Li YQ, Yang CX. Clinical research on stable COPD treated by Bufei Huoxue method. Beijing Journal of Traditional Chinese Medicine. 2006;25(11):643–645. [Google Scholar]
- 17.Xu DS, He CY. Effect of Fufang Qiqi Decoction on prevention and treatment of chronic obstructive pulmonary diseases. Chinese Archives of Traditional Chinese Medicine. 2004;22(6):991–992. [Google Scholar]
- 18.Luo XF, Chai XJ, Chen YM, et al. Clinical research on Bufei Dingchuan Decoction in treating 36 cases of chronic obstructive pulmonary disease. Journal of Traditional Chinese Medicine. 2002;43(4):268–270. [Google Scholar]
- 19.Wang YF. Clinical research of Bufei Yishen capsule in treating COPD. Shandong Journal of Traditional Chinese Medicine. 2007;47(13):30–31. [Google Scholar]
- 20.Huang G. Clinical validation of Butihuatan decoction in treating lung spleen kidney qi deficiency with the patients of COPD [M.S. Dissertation] Guiyang University of TCM; 2008. [Google Scholar]
- 21.He YC, Chen HL, Zhang RF. Clinical research on quality of life of oral Buzhongyiqi in chronic obstructive pulmonary disease. Chinese Archives of Traditional Chinese Medicine. 2010;28(3):506–507. [Google Scholar]
- 22.Guan QH. Therapy of Chinese medicine of Qixu Xueyu Tanzu syndrome of COPD and research of molecular biology index on airway remodeling [Doctor's Dissertation] Beijing University of Chinese Medicine; 2006. [Google Scholar]
- 23.Wei B, Cui SF, Zheng YX. Yiqi Huayu Decoction treatment of 60 cases of respiratory muscle fatigue syndrome. Shanxi Journal of Traditional Chinese Medicine. 2007;28(12):1590–1591. [Google Scholar]
- 24.Ma JM, Meng ZR, Yin JC. Role of Bufei Tang in the recovery treatment of stable chronic obstructive pulmonary disease. Chinese Journal of Hospital Pharmacy. 2010;30(5):404–406. [Google Scholar]
- 25.Ding YH, Yuan CY. Buyi Feishen Decoction treatment of stable chronic obstructive pulmonary disease. Heilongjiang Journal of Traditional Chinese Medicine. 2009;(5):24–25. [Google Scholar]
- 26.Zhou YF, Jing YX. Clinical research on the effects of treating chronic obstructive pulmonary disease with Feisaitong Heji [Master's Dissertation] Shandong University of Traditional Chinese Medicine; 2005. [Google Scholar]
- 27.Hu CQ. Jiajian Bufei Decoction combined with western medicine treatment of 35 cases of stable chronic obstructive pulmonary disease. Journal of TCM Research. 2009;22(11):24–26. [Google Scholar]
- 28.Chen Q, Xu C, Cai Y, Ye L. Clinical research of combined treatment of traditional Chinese medicine and western medicine on chronic obstructive pulmonary disease. Journal of Fujian University of TCM. 2009;19(4):12–14. [Google Scholar]
- 29.Liang AW, Huang MX, Gu LX, et al. Effect of Dongpingtang with TCM theory “winter disease being cured in summer” on pulmonary function and quality of life in patients with chronic obstructive pulmonary disease in stable stage. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2009;19(6):333–336. [Google Scholar]
- 30.Zhang BM, Shen ZX, Peng SF. Yifei Yangyin decoction on the treatment of chronic obstruction pulmonary disease. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2009;18(24):159–160. [Google Scholar]
- 31.Japan Society for Oriental Medicine EBM special committee. 2002 median reports: evidence-based medicine (EBM) reports on Chinese prescriptions. Japanese Journal of Oriental Medicine. 2002;53(5):1–80. [Google Scholar]
- 32.WHO Regional Office for the Western Pacific. WHO international standard terminologies on traditional medicine in the western pacific region. WPRO Nonserial Publication. 2007 [Google Scholar]
- 33.Group of Chronic Obstructive Pulmonary Diseases. Committee of Respiratory Disease. Chinese Medical Association. Guideline for diagnosis and treatment of chronic obstructive pulmonary disease (2007) Chinese Journal of Tuberculosis and Respiratory Disease. 2007;30(1):8–17. [Google Scholar]
- 34.Zheng XY. Guidance for Clinical Research on New Drugs of TCM. China Medical Science Press; 2002. [Google Scholar]
- 35.Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, editors; Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2008. http://handbook.cochrane.org/ [Google Scholar]
- 36.Wang Z, Zhang YH, Xu QQ. Several methods of publication bias evaluation. Chinese Journal of Health Statistics. 2009;(26):539–541. [Google Scholar]
- 37.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 38.Currie GP, Douglas JG. ABC of chronic obstructive pulmonary disease: Non-pharmacological management. British Medical Journal. 2006;332(7554):1379–1381. doi: 10.1136/bmj.332.7554.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George J, Ioannides-Demos LL, Santamaria NM, et al. Use of complementary and alternative medicines by patients with chronic obstructive pulmonary disease. Medical Journal of Australia. 2004;181(5):248–251. doi: 10.5694/j.1326-5377.2004.tb06262.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu ZG, Li ZG, Peng B. Plasma metabonomic studies on the stable phase chronic obstructive pulmonary disease patients of Fei-qi deficiency syndrome and the Chinese materia medica intervention. Chinese Journal of Integrated Traditional and Western Medicine. 2011;31(12):1619–1626. [PubMed] [Google Scholar]
- 41.Yang Y, Hou JS, Chi HJ, et al. Huangqi chronic obstructive pulmonary disease rat airway inflammation intervention function experimental research. Shandong Medicine. 2010;50(20):49–51. [Google Scholar]
- 42.Li Q. Research progress of pharmacological effects of Huangqi. Medicine Industry Information. 2006;3(11):128–129. [Google Scholar]
- 43.Chen L, Sun BB, Wang T, et al. Cigarette smoke enhances β-defensin 2 expression in rat airways via nuclear factork-κB activation. European Respiratory Journal. 2010;36(3):638–645. doi: 10.1183/09031936.00029409. [DOI] [PubMed] [Google Scholar]
- 44.Yuan X, Sun S, Wang S, Sun Y. Effects of astragaloside IV on IFN-gamma level and prolonged airway dysfunction in a murine model of chronic asthma. Planta Medica. 2011;77(4):328–333. doi: 10.1055/s-0030-1250408. [DOI] [PubMed] [Google Scholar]
- 45.Guo R, Pittler MH, Ernst E. Herbal medicines for the treatmet of COPD: a systematic review. European Respiratory Journal. 2006;28(2):330–338. doi: 10.1183/09031936.06.00119905. [DOI] [PubMed] [Google Scholar]
- 46.An X, Zhang AL, Yang AW, et al. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respiratory Medicine. 2011;105(2):165–176. doi: 10.1016/j.rmed.2010.11.007. [DOI] [PubMed] [Google Scholar]


