Abstract
Novel large, rod-shaped magnetotactic bacteria (MTB) were discovered in intertidal sediments of the Yellow Sea, China. They biomineralized more than 300 rectangular magnetite magnetosomes per cell. Phylogenetic analysis based on the 16S rRNA gene sequence revealed that they are affiliated with the Alphaproteobacteria and may represent a new genus of MTB.
TEXT
Magnetotactic bacteria (MTB) are ubiquitous in the water column and in sediments of freshwater and marine habitats (1, 2). MTB can form intracellular crystals termed magnetosomes, usually consisting of magnetite or greigite (3–5). MTB benefit from these magnetosomes, which confer an ability to orient and navigate along geomagnetic field lines, a unique form of motility referred as magnetotaxis (1, 3, 6, 7). MTB have been identified in Proteobacteria, Nitrospirae, and candidate division OP3 (5, 8–12), and a variety of morphological types have been found (6), of which coccoid is the dominant morphology (13–19). Here, we report a novel group of large, marine, rod-shaped MTB collected from Huiquan Bay (36°03′N, 120°21′E), China.
Sediments and water were collected and stored in 500-ml plastic bottles, and MTB collected using the capillary racetrack method were observed by optical microscopy (BX51; Olympus) using the hanging-drop method in an applied magnetic field (20, 21). Freshly collected MTB exhibited various cell shapes, including rods, vibrios, spirilla, and the dominant coccoid morphotype. After incubation in the dark at room temperature for 6 months (15), the MTB community varied greatly, and a group of large, rod-shaped MTB increased in numbers and became the dominant morphotype. Previous reports have described temporal variations in MTB communities in microcosms under laboratory conditions (2, 22).
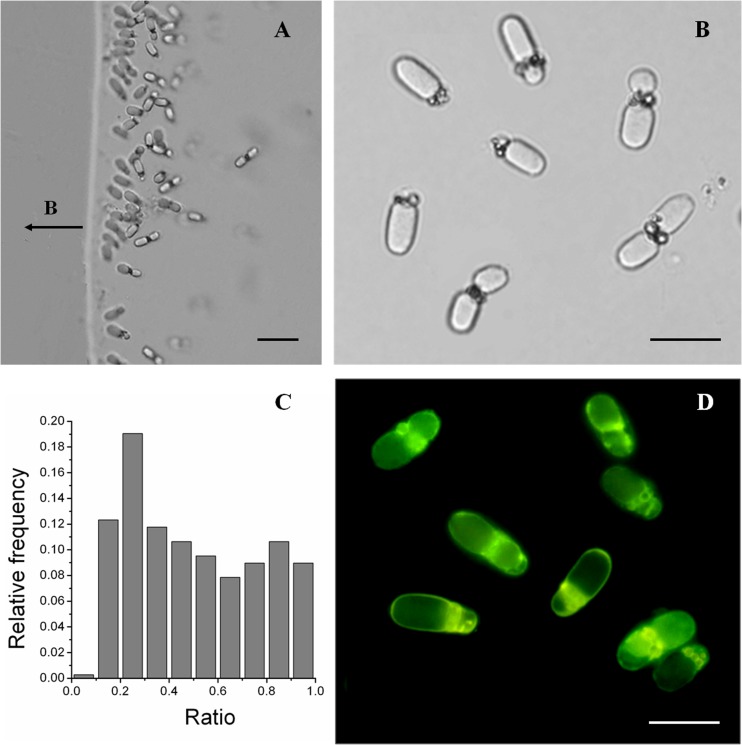
The laboratory-enriched rod-shaped MTB cells from sediments were homogeneous in morphology, with an average length of 10.07 ± 1.87 μm and an average width of 3.51 ± 0.49 μm. Differential interference contrast (DIC) microscopy revealed that these cells usually possessed an interstice dividing the cell into two parts (Fig. 1B). An analysis of the ratio distribution of the lengths of the two parts showed that the lengths were usually unequal (n = 357; Fig. 1C). When exposed to an applied magnetic field, the rod-shaped MTB display north-seeking polarity (Fig. 1A).
Fig 1.
Morphology of large, rod-shaped MTB based on optical microscopy. DIC images of large, rod-shaped MTB are shown in panels A and B. The ratio distribution of the lengths of the two parts is shown in panel C. Panel D shows the fluorescence of large, rod-shaped MTB exposed to blue light (wavelength, 450 to 480 nm). Scale bars, 20 μm in panel A and 10 μm in panels B and D.
Fluorescence microscopy of cells revealed membrane-like septa between the two parts (Fig. 1D), consistent with the morphology observed using DIC microscopy. When observed by transmission electron microscope (TEM; Hitachi H8100), two electron-dense structures were found around the center (Fig. 2A1). However, no obvious interstice was observed on the outer membrane of cells (Fig. 2A1), and energy-dispersive X-ray spectroscopy (EDXS) analysis showed no obvious differences in elemental composition. It may be that the membrane layer is not readily detected, possibly accounting for the observation of an interstice between the two parts when using DIC microscopy.
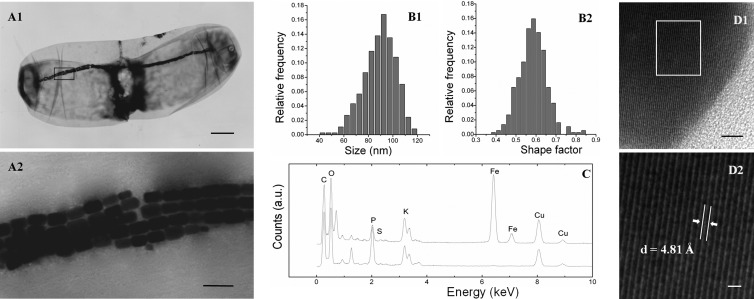
Fig 2.
Characteristics of large, rod-shaped MTB cells and intracellular features determined by TEM. (A1 and A2) Morphology of large, rod-shaped MTB cells (A1) and magnetosome chains (A2). (B1 and B2) Characteristics of magnetosomes: size histograms (B1) and shape factor distribution (B2). (C) EDXS analysis of magnetosomes. Top trace, magnetosomes (note the peaks of iron and oxygen); bottom trace, cell. a.u., arbitrary units. (D) High-resolution TEM images of a magnetsome (D1) and magnification of a selected area (D2). Scale bars, 2 μm in panel A1, 200 nm in panel A2, 5 nm in panel D1, and 1 nm in panel D2.
The large, rod-shaped MTB contained 320 to 567 magnetosomes per cell, arranged in a rope-like bundle formed by four to six parallel chains across the interface between the two parts of the cell (Fig. 2A2). A similar bundle of magnetosomes has been observed in large, freshwater, rod-shaped Nitrospirae strains (23) and large, watermelon-shaped MTB (12). Each magnetosome had a rectangular projected shape, with a length of 113 ± 16 nm and a width of 66 ± 11 nm (n = 370). This produced a shape factor of approximately 0.58 ± 0.07 (Fig. 2B). EDXS analysis indicated that the magnetosome crystals were composed of iron and oxygen (Fig. 2C). Consistently, the analysis by high-resolution TEM (HRTEM) identified magnetosome crystals as magnetite (Fig. 2D).
To determine the 16S rRNA gene sequence of the large, rod-shaped MTB, we extracted the DNA from purified samples, performed amplification by PCR using a pair of universal primers, 27f and 1492r (18, 24), and constructed a 16S rRNA gene library of 92 clones as previously reported (18). One dominant operational taxonomic unit (OTU) (86 clones, 93%) was identified by restriction fragment length polymorphism (RFLP) analysis and sequenced (13, 18).
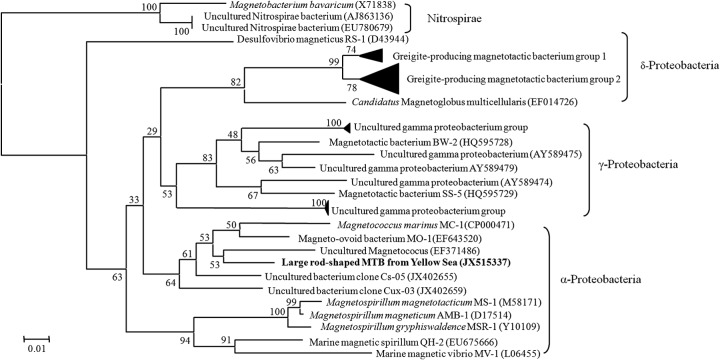
The sequence obtained was analyzed by BLAST and CLUSTAL W multiple alignment (25), and a phylogenetic tree was constructed using MEGA 4.0 (26, 27), applying the neighbor-joining method. Analysis revealed that the 16S rRNA gene sequence showed maximum sequence identity (91.7%) with uncultured magnetococci collected from intertidal sediments of the China Sea (EF371486) and affiliated with the class Alphaproteobacteria (Fig. 3). This sequence exhibited a divergence of >8% from those of all previously reported bacteria. Therefore, the rod-shaped MTB described here may represent a new genus of MTB.
Fig 3.
Phylogenetic tree showing the relationship between the novel, large, rod-shaped MTB and related magnetotactic bacteria. The tree was constructed based on neighbor-joining analysis using the sequence region from position 27 to position 1492 (E. coli numbering), and bootstrap values were calculated using 1,000 replicates. The description and accession number of the MTB whose sequence was determined in this study are shown in bold. GenBank accession numbers of the sequences used are indicated in parentheses. Scale bar, 0.01 substitution per nucleotide position.
We further corroborated the authenticity of the novel sequence as being that of rod-shaped MTB by fluorescence in situ hybridization (FISH). The specific oligonucleotide probe p-774 (5′-CCA ACA ACC AGC ACT CAT CG −3′; positions 774 to 793) was designed using Primer Premier 5.0 software and labeled with Cy3. The general probe EUB 338 (5′-GCT GCC TCC CRT AGG AGT-3′) labeled with 6-carboxyfluorescein (FAM) was used as a positive control. Escherichia coli cells were used as a negative control in hybridizations with specific probes. FISH was carried out in hybridization buffer containing 30% formamide according to a previously published protocol (28, 29). As expected, the general probe EUB338 hybridized with all bacteria in the samples (Fig. 4A). However, the specific probe p774 designed in this study recognized the rod-shaped MTB (Fig. 4B, white arrows) but not other bacteria (Fig. 4B, red arrow).
Fig 4.
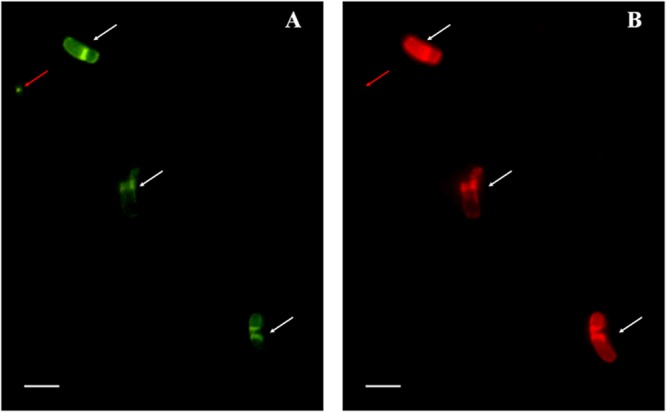
FISH analyses of magnetically enriched, rod-shaped MTB cells. The same microscopic field is shown following hybridization with the 5′-FAM-labeled universal bacterial probe EUB338 (A) and with the 5′-Cy3-labeled probe (B). Scale bars, 5 μm.
Although the 16S rRNA gene sequence of this large, rod-shaped MTB is most closely related to that of uncultured magnetococci, they are morphologically distinct. In fact, the relatively poor sequence identity (91.7%) between the marine rods and magnetococci clearly suggests that these organisms belong to different genera. Genome amplification from cells of low-abundance MTB, an approach pioneered by Schüler and colleagues (9, 30), has revealed the existence of novel and extended phylogenetic diversity that may escape detection by conventional cloning strategies. This approach, which uses a micromanipulator to separate single cells in combination with whole-genome amplification to determine the phylogenetics of MTB in environmental samples, represents a future trend for evaluating the phylogenetic diversity of environmental bacteria.
Nucleotide sequence accession numbers.
The sequence obtained in this study was deposited in GenBank under accession number JX515337.
ACKNOWLEDGMENTS
We thank Jianhong Xu for assistance in sampling and Ming Jiang for TEM observations.
This work was supported by the National Natural Science Foundation of China (NSFC 41206150, 41276170, 41106135, and 40776094) and the Special Construction Engineering Foundation for “Taishan scholar.”
Footnotes
Published ahead of print 1 March 2013
REFERENCES
- 1. Faivre D, Schüler D. 2008. Magnetotactic bacteria and magnetosomes. Chem. Rev. 108:4875–4898 [DOI] [PubMed] [Google Scholar]
- 2. Flies CB, Jonkers HM, de Beer D, Bosselmann K, Bottcher ME, Schüler D. 2005. Diversity and vertical distribution of magnetotactic bacteria along chemical gradients in freshwater microcosms. FEMS Microbiol. Ecol. 52:185–195 [DOI] [PubMed] [Google Scholar]
- 3. Bazylinski DA, Frankel RB. 2004. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2:217–230 [DOI] [PubMed] [Google Scholar]
- 4. Jogler C, Schüler D. 2009. Genomics, genetics, and cell biology of magnetosome formation. Annu. Rev. Microbiol. 63:501–521 [DOI] [PubMed] [Google Scholar]
- 5. Lefèvre CT, Menguy N, Abreu F, Lins U, Pósfai M, Prozorov T, Pignol D, Frankel RB, Bazylinski DA. 2011. A cultured greigite-producing magnetotactic bacterium in a novel group of sulfate-reducing bacteria. Science 334:1720–1723 [DOI] [PubMed] [Google Scholar]
- 6. Schüler D. 1999. Formation of magnetosomes in magnetotactic bacteria. J. Mol. Microbiol. Biotechnol. 1:79–86 [PubMed] [Google Scholar]
- 7. Frankel RB, Bazylinski DA, Johnson MS, Taylor BL. 1997. Magneto-aerotaxis in marine coccoid bacteria. Biophys. J. 73:994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amann R, Peplies J, Schüler D. 2006. Diversity and taxonomy of magnetotactic bacteria, p 25–36 In Schüler D. (ed), Microbiology monographs: magnetoreception and magnetosomes in bacteria. Springer Press, Heidelberg, Germany [Google Scholar]
- 9. Kolinko S, Jogler C, Katzmann E, Wanner G, Peplies J, Schüler D. 2012. Single-cell analysis reveals a novel uncultivated magnetotactic bacterium within the candidate division OP3. Environ. Microbiol. 14:1709–1721 [DOI] [PubMed] [Google Scholar]
- 10. Lefèvre CT, Frankel RB, Abreu F, Lins U, Bazylinski DA. 2011. Culture-independent characterization of a novel, uncultivated magnetotactic member of the Nitrospirae phylum. Environ. Microbiol. 13:538–549 [DOI] [PubMed] [Google Scholar]
- 11. Lefèvre CT, Viloria N, Schmidt ML, Pósfai M, Frankel RB, Bazylinski DA. 2012. Novel magnetite-producing magnetotactic bacteria belonging to the Gammaproteobacteria. ISME J. 6:440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin W, Li JH, Pan YX. 2012. Newly isolated but uncultivated magnetotactic bacterium of the phylum Nitrospirae from Beijing, China. Appl. Environ. Microbiol. 78:668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin W, Li JH, Schüler D, Jogler C, Pan YX. 2009. Diversity analysis of magnetotactic bacteria in Lake Miyun, northern China, by restriction fragment length polymorphism. Syst. Appl. Microbiol. 32:342–350 [DOI] [PubMed] [Google Scholar]
- 14. Lin W, Pan YX. 2009. Uncultivated magnetotactic cocci from Yuandadu Park in Beijing, China. Appl. Environ. Microbiol. 75:4046–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan HM, Zhu KL, Song T, Yu-Zhang K, Lefèvre CT, Xing SE, Liu M, Zhao SJ, Xiao T, Wu LF. 2008. Characterization of a homogeneous taxonomic group of marine magnetotactic cocci within a low tide zone in the China Sea. Environ. Microbiol. 10:1158–1164 [DOI] [PubMed] [Google Scholar]
- 16. Mann S, Sparks NH, Board RG. 1990. Magnetotactic bacteria: microbiology, biomineralization, palaeomagnetism and biotechnology. Adv. Microb. Physiol. 31:125–181 [DOI] [PubMed] [Google Scholar]
- 17. Spring S, Lins U, Amann R, Schleifer K, Ferreira L, Esquivel D, Farina M. 1998. Phylogenetic affiliation and ultrastructure of uncultured magnetic bacteria with unusually large magnetosomes. Arch. Microbiol. 169:136–147 [DOI] [PubMed] [Google Scholar]
- 18. Zhang WY, Zhou K, Pan HM, Yue HD, Jiang M, Xiao T, Wu LF. 2012. Two genera of magnetococci with bean-like morphology from intertidal sediments of the Yellow Sea, China. Appl. Environ. Microbiol. 78:5606–5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spring S, Amann R, Ludwig W, Schleifer KH, Schüler D, Poralla K, Petersen N. 1995. Phylogenetic analysis of uncultured magnetotactic bacteria from the alpha-subclass of Proteobacteria. Syst. Appl. Microbiol. 17:501–508 [Google Scholar]
- 20. Schüler D. 2002. The biomineralization of magnetosomes in Magnetospirillum gryphiswaldense. Int. Microbiol. 5:209–214 [DOI] [PubMed] [Google Scholar]
- 21. Wolfe RS, Thauer RK, Pfennig N. 1987. A ‘capillary racetrack’ method for isolation of magnetotactic bacteria. FEMS Microbiol. Ecol. 45:31–35 [Google Scholar]
- 22. Lin W, Pan YX. 2010. Temporal variation of magnetotactic bacterial communities in two freshwater sediment microcosms. FEMS Microbiol. Lett. 302:85–92 [DOI] [PubMed] [Google Scholar]
- 23. Vali H, Förster O, Amarantidis G, Petersen N. 1987. Magnetotactic bacteria and their magnetofossils in sediments. Earth Planet. Sci. Lett. 86:389–400 [Google Scholar]
- 24. Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175 In Stackebrandt E, Goodfellow M. (ed), Nucleic acid techniques in bacterial systematics. Wiley & Sons Press, Chichester, United Kingdom [Google Scholar]
- 25. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 27. Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simmons SL, Edwards KJ. 2007. Unexpected diversity in populations of the many-celled magnetotactic prokaryote. Environ. Microbiol. 9:206–215 [DOI] [PubMed] [Google Scholar]
- 29. Pernthaler J, Glöckner FO, Schönhuber W, Amann R. 2001. Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Methods Microbiol. 30:207–226 [Google Scholar]
- 30. Jogler C, Wanner G, Kolinko S, Niebler M, Amann R, Petersen N, Kube M, Reinhardt R, Schüler D. 2011. Conservation of proteobacterial magnetosome genes and structures in an uncultivated member of the deep-branching Nitrospira phylum. Proc. Natl. Acad. Sci. U. S. A. 108:1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]