Abstract
One of the currently most relevant resistance mechanisms in Enterobacteriaceae is the production of enzymes that lead to modern expanded-spectrum cephalosporin and even carbapenem resistance, mainly extended-spectrum β-lactamases (ESBLs) and carbapenemases. A worrisome aspect is the spread of ESBL and carbapenemase producers into the environment. The aim of the present study was to assess the occurrence of ESBL- and carbapenemase-producing Enterobacteriaceae and to further characterize ESBL- and carbapenemase-producing Enterobacteriaceae in rivers and lakes in Switzerland. ESBL-producing Enterobacteriaceae were detected in 21 (36.2%) of the 58 bodies of water sampled. One river sample tested positive for a carbapenemase-producing Klebsiella pneumoniae subsp. pneumoniae strain. Seventy-four individual strains expressing an ESBL phenotype were isolated. Species identification revealed 60 Escherichia coli strains, seven Klebsiella pneumoniae subsp. pneumoniae strains, five Raoultella planticola strains, one Enterobacter cloacae strain, and one Enterobacter amnigenus strain. Three strains were identified as SHV-12 ESBL producers, and 71 strains carried genes encoding CTX-M ESBLs. Of the 71 strains with CTX-M ESBL genes, 8 isolates expressed CTX-M-1, three produced CTX-M-3, 46 produced CTX-M-15, three produced CTX-M-55, one produced CTX-M-79, six produced CTX-M-14, and four produced CTX-M-27. Three of the four CTX-M-27 producers belonged to the multiresistant pandemic sequence type E. coli B2:ST131 that is strongly associated with potentially severe infections in humans and animals.
INTRODUCTION
One of the currently most important resistance mechanisms in members of the family Enterobacteriaceae that reduces the efficacy of modern expanded-spectrum cephalosporins is based on the plasmid-mediated production of enzymes that inactivate these compounds by hydrolyzing their β-lactam ring. Such resistance is caused by an increasing number of different point mutation variants of classical broad-spectrum β-lactamases (BSBLs), the so-called extended-spectrum β-lactamases (ESBLs). Many ESBLs are members of TEM and SHV β-lactamase families, whereas other groups, such as CTX-M, OXA, and PER β-lactamases have been described more recently (1, 2). Since the first description of ESBL-producing Enterobacteriaceae isolated from hospitalized humans, many nosocomial outbreaks and later, community-associated infections have been reported worldwide (3). Recently, ESBL-producing strains have also emerged in healthy human carriers (4), in healthy food-producing animals (5, 6), and household pets (7) as well as on food products like meat, fish, and raw milk (6).
Carbapenemases are a diverse group of β-lactamases belonging to the Ambler classes A, B, and D or Bush groups 2f, 3, and 2d, respectively (2), that even inactivate carbapenems. Class A carbapenemases (Bush group 2f) include the serine β-lactamases NmcA, Sme, IMI-1, and SFC-1 which are chromosomally encoded, as well as the clinically common plasmid gene-encoded KPC enzymes. Carbapenemases of this class are inhibited by clavulanic acid. Class B carbapenemases (Bush group 3) comprise the integron-encoded VIM types, the IMP, GIM-1, SPM-1,and SIM types of enzymes, and the plasmid gene-encoded NDM-1 carbapenemase. These metallo-β-lactamases are inhibited by EDTA but not by clavulanic acid. Class D (Bush group 2d) consists of OXA-48-type carbapenemases, which are encoded by plasmid genes, and not inhibited by EDTA and not inhibited or only weakly inhibited by clavulanic acid. In the last 5 years, carbapenemase-producing Enterobacteriaceae have been increasingly reported in humans worldwide (8). Moreover, recent data prove that in some countries, pigs (9) and cattle (10) also constitute a possible reservoir of carbapenemase producers.
A worrisome aspect is the spread of ESBL and carbapenemase producers into the environment, especially bodies of water. Rivers are considered to be of special importance as a reservoir of resistance genes since they are recipients of bacteria from different sources. The aim of the present study was (i) to assess the occurrence of ESBL- and carbapenemase-producing Enterobacteriaceae in rivers and lakes in Switzerland and (ii) to further characterize isolated strains.
MATERIALS AND METHODS
Sampling.
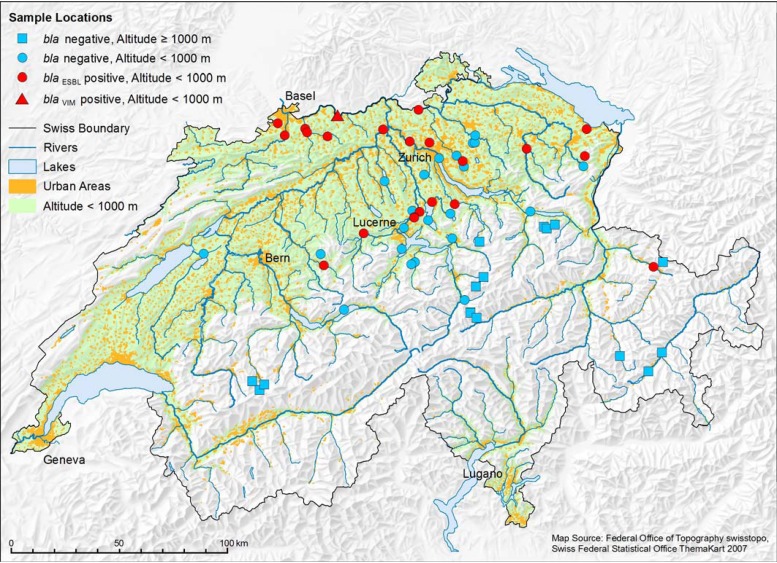
Between May and September 2012, a total of 58 different rivers (n = 40) and lakes (n = 18) in the German-speaking part of Switzerland were sampled, 14 of which were at altitudes higher than 1,000 m above sea level (Fig. 1).
Fig 1.
Map showing bodies of water, urban areas, and altitude discrimination as well as sample locations and ESBL/carbapenemase status. (Map source, Federal Office of Topography [swisstopo].)
Microbiological analysis.
From each water sample, 500 ml was concentrated by filtration through sterile 0.45-μm membrane filters (Millipore, Billerica, MA, USA). The filters were incubated for 24 h at 37°C in 10 ml of EE broth (Becton, Dickinson, Heidelberg, Germany) for enrichment. One loopful of each of the enrichment cultures was inoculated onto chromogenic Brilliance ESBL agar and Brilliance CRE agar (Oxoid, Hampshire, United Kingdom) to select for ESBL and carbapenemase producers, respectively, and incubated at 37°C for 24 h under aerobic conditions. All colonies with different coloration and morphology were picked from the selective plates and subcultured on sheep blood agar (Difco Laboratories) (5% sheep blood [SB055; Oxoid]) at 37°C for 24 h. Oxidase-negative isolates were thereafter subjected to identification by API ID 32 E (bioMérieux, Marcy l'Etoile, France). Some strains, yielding doubtful results, were subjected to genetic identification based on sequencing of rpoB gene fragments (11).
Antimicrobial susceptibility testing and ESBL confirmation.
All isolated strains were subjected to susceptibility testing against 13 antimicrobial agents by the disc diffusion method according to CLSI protocols and evaluated according to CLSI criteria (12). The antibiotics tested were ampicillin (AM), amoxicillin-clavulanic acid (AMC), cephalothin (CF), cefotaxime (CTX), ciprofloxacin (CIP), gentamicin (GM), tetracycline (TE), streptomycin (S), chloramphenicol (C), kanamycin (K), nalidixic acid (NA), sulfamethoxazole (SMZ), and trimethoprim (TMP) (Becton, Dickinson, Heidelberg, Germany). The AMC disc was placed next to the disc containing CTX, and thus, the resulting double-disc synergy effects were documented. ESBL production was confirmed by Etest-ESBL strips containing cefotaxime, ceftazidime, and cefepime alone and in combination with clavulanic acid (bioMérieux, Marcy l'Etoile, France) following the manufacturer's protocols. Additionally, MICs of imipenem were determined for all strains using Etest strips (bioMérieux, Marcy l'Etoile, France). Strains exhibiting resistance to three or more antibiotic classes were classified as multidrug resistant.
Characterization of β-lactamases.
Bacterial strains confirmed for producing ESBLs were further analyzed by PCR. DNA was extracted by a standard heat lysis protocol. Thereafter, five specific primer sets (custom synthesized by Microsynth, Balgach, Switzerland) were used to screen for β-lactamase-encoding genes belonging to the blaTEM, blaSHV, and blaCTX-M families (13, 14, 15). Carbapenem-resistant isolates were screened for blaVIM, blaKPC, blaNDM-1, and blaOXA-48 using previously published primer pairs (16, 17).
The resulting amplicons were either purified directly using the PCR purification kit (Qiagen, Courtaboeuf, France) according to the manufacturer's recommendations or replaced by longer ones covering the entire bla open reading frame (ORF) (15) in the case of blaCTX-M. Custom sequencing was performed by Microsynth (Balgach, Switzerland), and the nucleotide and translated protein sequences were analyzed with CLC Main Workbench 6.6.1. For database searches, the BLASTN program of NCBI (http://www.ncbi.nlm.nih.gov/blast/) was used.
Determination of Escherichia coli phylogenetic groups.
Phylogenetic analyses have shown that E. coli strains fall into four main phylogenetic groups (groups A, B1, B2, and D). Groups A and B1 typically contain commensal isolates, while members of groups B2 and D are considered to be opportunistic extraintestinal pathogens which often carry virulence-associated genes (18). Standard heat lysis DNA extracts from E. coli isolates were again used, and the phylogenetic groups were determined as described previously (18).
Multilocus sequence typing (MLST) of ESBL producers either expressing CTX-M-27 or belonging to the E. coli phylogenetic group B2.
Internal fragments of 7 housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) were sequenced (19) and alleles and sequence types (ST) were assigned in accordance with the E. coli MLST website (http://mlst.ucc.ie/mlst/dbs/Ecoli).
RESULTS
ESBL-producing Enterobacteriaceae were detected in 21 (36.2%) of the 58 rivers and lakes sampled. They were strictly confined to urban areas (Fig. 1). No ESBL producers were found in bodies of water more than 1,000 m above sea level. One river sample (1.7%) tested positive for a carbapenemase-producing Klebsiella pneumoniae subsp. pneumoniae strain harboring blaVIM. From the bodies of water where ESBL-producing Enterobacteriaceae were detected, 74 different isolates showing the ESBL phenotype could be isolated. Species identification revealed 60 E. coli strains, seven Klebsiella pneumoniae subsp. pneumoniae strains, five Raoultella planticola strains, one Enterobacter cloacae strain, and one Enterobacter amnigenus strain (Fig. 2). These isolates were further screened for bla genes encoding SHV, TEM, CTX-M group 1, CTX-M group 2, or CTX-M group 9 enzymes. Three strains (4.1%) (OW55E1, OW3E1, and OW96E1) were identified as SHV-type ESBL producers, and 71 strains carried genes coding for CTX-M ESBLs. Sixty-one of the blaCTX-M genes detected belonged to CTX-M group 1 (82.4% of the total blaESBL genes), 10 belonged to CTX-M group 9 (13.5%), and none belonged to CTX-M group 2. Further PCR analyses and sequencing revealed that eight isolates expressed CTX-M-1 (10.8%), three expressed CTX-M-3 (4.1%), 46 expressed CTX-M-15 (62.2%), three expressed CTX-M-55 (4.1%), one expressed CTX-M-79 (1.4%), six expressed CTX-M-14 (8.1%), and four expressed CTX-M-27 (5.4%). The blaSHV genes were sequenced only if an isolate showed an ESBL phenotype and did not have a blaCTX-M gene. Strains OW55E1, OW3E1, and OW96E1, which did not carry a blaCTX-M gene, all expressed SHV-12 (4.1%). Besides their blaCTX-M-ESBL, 24 isolates harbored an additional blaTEM gene, 3 isolates harbored a blaSHV gene, and 4 isolates harbored a blaTEM gene plus a blaSHV gene which, however, were all not responsible for the ESBL phenotype but coded for either TEM-1 or SHV-11 BSBLs.
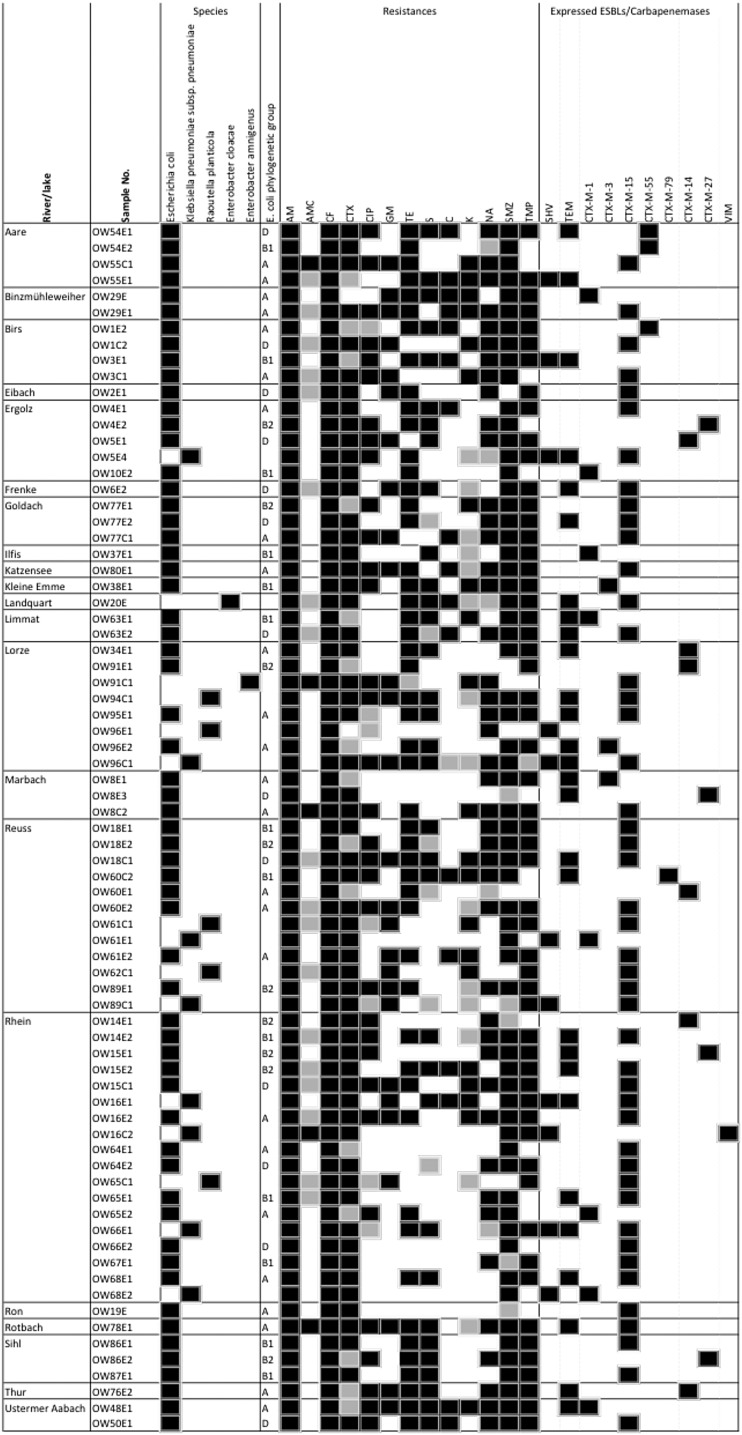
Fig 2.
Characteristics and antimicrobial susceptibility profiles of ESBL- and carbapenemase-producing Enterobacteriaceae isolated from Swiss rivers and lakes. Symbols: black square, positive result or resistant to a specific antimicrobial agent; white square, negative result or susceptible to a specific antimicrobial; gray square, intermediate toward a specific antimicrobial. Abbreviations: AM, ampicillin; AMC, amoxicillin-clavulanic acid; CF, cephalothin; CTX, cefotaxime; CIP, ciprofloxacin; GM, gentamicin; TE, tetracycline; S, streptomycin; C, chloramphenicol; K, kanamycin; NA, nalidixic acid; SMZ, sulfamethoxazole; TMP, trimethoprim.
In order to detect clusters as possible indicators for common origin, the blaCTX-M genes of the isolates were searched for silent mutations. Interestingly, 70 of the 71 open reading frames (ORFs) showed 100% DNA homology compared to their standard sequence as published by Jacoby and Bush (http://www.lahey.org/Studies/), while only one blaCTX-M-14 ORF, from isolate OW34E1, carried 3 silent mutations (data not shown). This indicated strong conservation of blaCTX-M genes and provided insufficient discriminatory power of silent mutations for epidemiological purposes.
All 74 isolates were resistant to ampicillin and the narrow-spectrum cephalosporin cephalothin. Only 57 (77%) of the isolates were found to be resistant to the expanded-spectrum cephalosporin cefotaxime in vitro, according to CLSI criteria, but of course, all 74 would have to be reported as resistant in a clinical context because of their blaESBL genotype. Four strains (5.4%) showed resistance to amoxicillin-clavulanic acid. Since no evidence for mechanistic resistance, such as the expression of an inhibitor-resistant TEM or SHV enzyme of the Bush-Jacoby group 2be or 2ber (2) was detected (data not shown), these phenotypes were most likely due to (i) hyperexpression of accompanying TEM-1 (strain OW78E1), (ii) hyperexpression of the ESBL itself (OW8C2 and OW55C1), or (iii) the inducible chromosomally encoded AmpC in strain OW91C1. Besides β-lactam resistance, susceptibility to other classes of antibiotics was tested. Hereby, 27 (36.5%) isolates were resistant to gentamicin, 21 (28.4%) were resistant to kanamycin, 34 (44.6%) were resistant to streptomycin, 44 (59.5%) were resistant to nalidixic acid, 34 (44.6%) were resistant to ciprofloxacin, 50 (67.6%) were resistant to tetracycline, 17 (23%) were resistant to chloramphenicol, 63 (85.1%) were resistant to sulfamethoxazole, and 55 (74.3%) were resistant to trimethoprim.
Phylogenetic typing of the E. coli isolates showed that 33.8% belonged to group A and 17.6% belonged to group B1, the two groups that typically contain commensal isolates. In contrast, 17.6% and 12.2% of the isolates belonged to the pathogenicity-associated extraintestinal E. coli groups D and B2, respectively. MLST was performed on (i) strains belonging to phylogenetic group B2, and (ii) E. coli expressing CTX-M-27 (Fig. 2). Eight of the 10 genotyped strains belonged to the epidemiologically important sequence type ST131 (strains OW4E2, OW14E1, OW15E1, OW15E2, OW18E2, OW77E1, OW86E2, and OW89E1). One isolate showed the sequence type ST1722 (OW8E3), and OW91E1 exhibited a not yet assigned sequence type (the different housekeeping genes and their alleles were adk-40, fumC52, gyrB156, icd-14, mdh-245, purA25, and recA17).
DISCUSSION
The number of studies investigating rivers as potential reservoirs of ESBL producers is still very limited (19, 20, 21, 22, 23), and data from only one river (Seine River, Paris, France) in central Europe are available (22). Rivers and lakes are considered especially relevant as putative reservoirs of multiresistant bacteria, since they collect surface waters containing materials from different origins, e.g., wastewater plants, water of urban or industrial effluents, agricultural activities, or rain. Thus, they provide an immense resistome, including pathogenic and nonpathogenic antibiotic-resistant bacteria (24).
In this study, 58 rivers and lakes were sampled and screened for the presence of ESBL- and carbapenemase-producing Enterobacteriaceae. The high occurrence of ESBL producers (36.2%) is worrisome, and even more so since Switzerland is a country with a strict policy of antibiotic use (25). Since ESBL-producing Enterobacteriaceae were not found in bodies of water above an altitude of 1,000 m despite the fact that sampling took place during the alpine farming season, one must consequently assume that the occurrence of ESBL producers is linked mainly to anthropogenic activities and intensive agriculture (Fig. 1). The predominance of CTX-M group 1 enzymes (Fig. 2) correlated conspicuously with results of studies in Switzerland investigating the occurrence of blaESBL (i) in clinical isolates (26), (ii) in isolates from healthy human carriers (15), and (iii) in isolates from food-producing animals (6). CTX-M15 was predominantly found in humans, and CTX-M1 was the predominant CTX-M type in farming animals, such as chicken, cattle, and pigs. Furthermore, CTX-M-15 is currently the most common CTX-M type worldwide (1, 27), and it is often associated with the pandemic E. coli strain B2:ST131 (28).
The representatives of CTX-M group 9 producers isolated in this study mostly turned out to express CTX-M-14, and, to a lesser extent CTX-M-27. Aside from CTX-M-15, CTX-M-14 is the most common ESBL found in Enterobacteriaceae. Recently, CTX-M-27-producing E. coli strains were also isolated from great cormorants (Phalacrocorax carbo) in the Czech Republic and Slovakia (29) and in Switzerland (30). Three of the four CTX-M-27-producing isolates belonged to the worldwide pandemic multiresistant E. coli B2:ST131 that is strongly associated with potentially severe infection in humans and animals (28). Additionally, four E. coli B2:ST131 isolates harboring blaCTX-M-15 and one E. coli B2:ST131 isolate harboring blaCTX-M-14 were detected.
Furthermore, a few less-frequent CTX-M types such as CTX-M-55 and CTX-M-79 were found in this study (Fig. 2). CTX-M-55, an Ala77Val ceftazimidase variant of CTX-M-15, was first isolated in Thailand (31). CTX-M-79, an Asp287Asn variant of CTX-M-55 (Ambler numbering), was first detected in China (32). So far, this enzyme had been isolated only from humans and farmed fish in China and from cattle in Ohio, USA (33, 34). Interestingly, SHV-12, first reported in Switzerland in 1997 (35), had been predominant in human clinical isolates in Switzerland and disseminated worldwide during the following decades but was detected in the present study in only three environmental isolates.
It is worrisome that most ESBL-producing strains are frequently cross-resistant to other classes of antimicrobial agents. This is due to the fact that blaESBL genes are commonly located on conjugative plasmids that also harbor genes conferring resistance to other antibiotic classes such as quinolones and aminoglycosides (36). It is of particular concern that all 74 ESBL isolates described in this study expressed a multiresistance phenotype.
The presence of a carbapenem-resistant strain harboring blaVIM isolated from one river in Switzerland is of further concern, since carbapenems are crucial back-up antibiotics in human clinical use. What is more, the prevalence of carbapenemase producers in our study may have been underestimated, since we had used Brilliance CRE agar, which was most recently proved to lack sufficient sensitivity in detecting OXA-48-type carbapenemase producers (37). OXA-48 is of particular concern, because it appears to have become the most important type in central Europe. Carbapenemase-producing strains have hitherto been detected in rivers in the United States (38), China (20), France (39), and Portugal (40), indicating that a global environmental dissemination is currently ongoing.
The spread of ESBL- and carbapenemase-producing Enterobacteriaceae in surface waters is highly worrisome. Appropriate measures urgently need to be enforced in order to reduce the anthropogenic burden of antibiotic resistance in the environment, such as judicious use of antibiotics in human and veterinary medicine as well as in agriculture. In addition, improvement of water status is of major concern. New strategies for the treatment of wastewaters, e.g., the use of sand filters (41) or more-stringent chlorine disinfection, need to be taken into consideration to prevent resistant bacteria from being released into the aquatic environment.
ACKNOWLEDGMENTS
We thank Guido Bloemberg (Institute for Medical Microbiology, University of Zurich) for providing control strains expressing various carbapenemases, Ekkehard Altpeter (Swiss Federal Office of Public Health) for advice regarding geographical data, Ronald Schmidt (Geographical Institute, University of Zurich) for constructing and designing Fig. 1, and Helga Abgottspon and Christine Gallati for providing technical assistance. Moreover, we thank the laboratories of the cantons BL, ZG, and AG for their help in the collection of some samples.
This study was funded in part by the Swiss Federal Office for Public Health, Department of Communicable Diseases.
Footnotes
Published ahead of print 1 March 2013
REFERENCES
- 1. Coque TM, Baquero F, Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 13(47): pii=19044 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19044 [PubMed] [Google Scholar]
- 2. Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 54: 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paterson DL, Bonomo RA. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18: 657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geser N, Stephan R, Korczak BM, Beutin L, Hächler H. 2012. Molecular identification of extended-spectrum β-lactamase genes from Enterobacteriaceae isolated from healthy human carriers in Switzerland. Antimicrob. Agents Chemother. 56: 1609–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortés P, Blanc V, Mora A, Dahbi G, Blanco JE, Blanco M, Lopez C, Andreu A, Navarro F, Alonso MP, Bou G, Blanco J, Llagoster M. 2010. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl. Environ. Microbiol. 76: 2799–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geser N, Stephan R, Hächer H. 2012. Occurrence and characteristics of extended-spectrum β-lactamases (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet. Res. 8: 21 doi:10.1186/1746-6148-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ewers C, Grobbel M, Bethe A, Wieler LH, Guenther S. 2011. Extended-spectrum beta-lactamases-producing gram-negative bacteria in companion animals: action is clearly warranted! Berl. Munch. Tierarztl. Wochenschr. 124: 94–101 [PubMed] [Google Scholar]
- 8. Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17: 1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer J, Rodríguez I, Schmoger S, Friese A, Roesler U, Helmuth R, Guerra B. 2012. Escherichia coli producing VIM-1 carbapenemase isolated on a pig farm. J. Antimicrob. Chemother. 67: 1793–1795 [DOI] [PubMed] [Google Scholar]
- 10. Poirel L, Barbosa-Vasconcelos A, Simoes RR, Da Costa PM, Liu W, Nordmann P. 2012. Environmental KPC-producing Escherichia coli isolates in Portugal. Antimicrob. Agents Chemother. 56: 1662–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mollet C, Drancourt M, Raoult D. 1997. rpoB sequence analysis as a novel basis for bacterial identification. Mol. Microbiol. 26: 1005–1011 [DOI] [PubMed] [Google Scholar]
- 12. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. CLSI document M100-S18 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13. Pitout JD, Thomson KS, Hanson ND, Erhardt AF, Moland ES, Sanders CC. 1998. Beta-lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 42: 1350–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woodford N, Fagan EJ, Ellington MJ. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 57: 154–155 [DOI] [PubMed] [Google Scholar]
- 15. Geser N, Stephan R, Kuhnert P, Zbinden R, Käppeli U, Cernela N, Hächler H. 2011. Fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in swine and cattle at slaughter in Switzerland. J. Food Prot. 74: 446–449 [DOI] [PubMed] [Google Scholar]
- 16. Ellington MJ, Kistler J, Livermore DM, Woodford N. 2007. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J. Antimicrob. Chemother. 59: 321–322 [DOI] [PubMed] [Google Scholar]
- 17. Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70: 119–123 [DOI] [PubMed] [Google Scholar]
- 18. Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66: 4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60: 1136–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H, Shu W, Chang X, Chen J, Guo Y, Tan Y. 2010. The profile of antibiotics resistance and integrons of extended-spectrum beta-lactamase producing thermotolerant coliforms isolated from the Yangtze River basin in Chongqing. Environ. Pollut. 158: 2459–2464 [DOI] [PubMed] [Google Scholar]
- 21. Dhanji H, Murphy NM, Akhigbe C, Doumith M, Hope R, Livermore DM, Woodford N. 2011. Isolation of fluoroquinolone-resistant O25b:H4-ST131 Escherichia coli with CTX-M-14 extended-spectrum β-lactamase from UK river water. J. Antimicrob. Chemother. 66: 512–516 [DOI] [PubMed] [Google Scholar]
- 22. Girlich D, Poirel L, Nordmann P. 2011. Diversity of clavulanic acid-inhibited extended-spectrum β-lactamases in Aeromonas spp. from the Seine River, Paris, France. Antimicrob. Agents Chemother. 55: 1256–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tacao M, Correia A, Hentriques I. 2012. Resistance to broad-spectrum antibiotics in aquatic systems: anthropogenic activities modulate the dissemination of blaCTX-M-like genes. Appl. Environ. Microbiol. 78: 4134–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lupo A, Coyne S, Berendonk TU. 2012. Origin and evolution of antibiotic resistance: the common mechanisms of emergence and spread in water bodies. Front. Microbiol. 18: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filippini M, Masiero G, Moschetti K. 2006. Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy 78: 77–92 [DOI] [PubMed] [Google Scholar]
- 26. Lartigue MF, Zinsius C, Wenger A, Bille J, Poirel L, Nordmann P. 2007. Extended-spectrum β-lactamases of the CTX-M type now in Switzerland. Antimicrob. Agents Chemother. 51: 2855–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cantòn R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front. Microbiol. 3: 110 doi:10.3389/fmicb.2012.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogers BA, Sidjabat HE, Paterson DL. 2011. Escherichia coli O25b:ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 66: 1–14 [DOI] [PubMed] [Google Scholar]
- 29. Tausova D, Dolejska M, Cizek A, Hanusova L, Hrusakova J, Svoboda O, Camlik G, Literak I. 2012. Escherichia coli with extended-spectrum β-lactamase and plasmid-mediated quinolone resistance genes in great cormorants and mallards in Central Europe. J. Antimicrob. Chemother. 67: 1103–1107 [DOI] [PubMed] [Google Scholar]
- 30. Zurfluh K, Nüesch-Inderbinen M, Stephan R, Hächler H. 2013. Higher generation cephalosporin resistant Escherichia coli in feral birds in Switzerland. Int. J. Antimicrob. Agents 91: 296–297 [DOI] [PubMed] [Google Scholar]
- 31. Kiratisin P, Apisarnthanarak A, Saifon P, Laesripa C, Kitphati R, Mundy LM. 2007. The emergence of a novel ceftazidime-resistant CTX-M extended-spectrum β-lactamase, CTX-M-55, in both community-onset and hospital-acquired infections in Thailand. Diagn. Microbiol. Infect. Dis. 58: 349–355 [DOI] [PubMed] [Google Scholar]
- 32. Tian SF, Chen BY, Chu YZ, Wang S. 2008. Prevalence of rectal carriage of extended-spectrum β-lactamase-producing Escherichia coli among elderly people in community settings in China. Can. J. Microbiol. 54: 781–785 [DOI] [PubMed] [Google Scholar]
- 33. Wittum TE, Mollenkopf DF, Daniel JB, Parkinson AE, Mathews JL, Fry PR, Abley MJ, Gebreyes WA. 2010. CTX-M-type extended-spectrum β-lactamases present in Escherichia coli from the feces of cattle in Ohio, United States. Foodborne Pathog. Dis. 7: 1575–1579 [DOI] [PubMed] [Google Scholar]
- 34. Jiang HX, Tang D, Liu YH, Zhang XH, Zeng ZL, Xu L, Hawkey PM. 2012. Prevalence and characteristics of β-lactamase and plasmid-mediated quinolone resistance genes in Escherichia coli isolated from farmed fish in China. J. Antimicrob. Chemother. 67: 2350–2353 [DOI] [PubMed] [Google Scholar]
- 35. Nüesch-Inderbinen MT, Kayser F, Hächler H. 1997. Survey and molecular genetics of SHV β-lactamases in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob. Agents Chemother. 41: 943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gniadkowski M. 2001. Evolution and epidemiology of extended-spectrum β-lactamases (ESBLs) and ESBL-producing microorganisms. Clin. Microbiol. Infect. 7: 597–608 [DOI] [PubMed] [Google Scholar]
- 37. Girlich D, Poirel L, Nordmann P. 2013. Comparison of the SUPERCARBA, CHROMagar KPC, and Brilliance CRE screening media for detection of Enterobacteriaceae with reduced susceptibility to carbapenems. Diagn. Microbiol. Infect. Dis. 75: 214–217 [DOI] [PubMed] [Google Scholar]
- 38. Aubron C, Porel L, Ash RJ, Nordmann P. 2005. Carbapenemase-producing Enterobacteriaceae, U.S. rivers. Emerg. Infect. Dis. 11: 260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Girlich D, Poirel L, Nordmann P. 2010. Novel Ambler class A carbapenem-hydrolyzing β-lactamase from a Pseudomonas fluorescens isolate from the Seine River, Paris, France. Antimicrob. Agents Chemother. 54: 328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob. Chemother. 67: 1597–1606 [DOI] [PubMed] [Google Scholar]
- 41. LaPara TM, Firl SJ, Onan LJ, Ghosh S, Yan T, Sadowsky MJ. 2006. Municipal wastewater treatment: a novel opportunity to slow the proliferation of antibiotic-resistant bacteria? CURA Reporter 36: 18–23 [Google Scholar]