Abstract
In a gelatin-dextran mixture, changing the (relative and/or absolute) concentration of the components leads to the formation of different microstructures. Confocal laser scanning microscopy illustrated that the nature of the microstructure determines the location and morphology of Escherichia coli colonies. Observations indicate that bacterial growth preferentially occurs in the dextran phase, regardless of the microstructure.
TEXT
It is generally accepted that food structure has an influence on bacterial growth (1). In the past decades, a myriad of research has been conducted on growth of bacteria in solid-food (model) systems (2–7). These studies used only one gelling agent, resulting in a homogeneous microstructure. In reality, food products are most often heterogeneous, as different phases (e.g., fat, water, proteins, etc.) are present. In addition, most studies are macroscopic, focusing on the overall population, whereas bacterial growth occurs at the level of the individual bacteria. In this study, the effect of a heterogeneous microstructure on the growth of Escherichia coli JM-109 DE3 was investigated by means of a nondestructive technique, i.e., confocal laser scanning microscopy (CLSM). This work is a first step in the process of unraveling the mechanism of bacterial growth in heterogeneous microstructures, i.e., microstructures consisting of different phases (8).
For the study, a loop of an E. coli JM-109 DE3 pRSETb-Venus stock culture, kindly provided by the Department of Chemistry, KU Leuven, was transferred to an Erlenmeyer flask containing 20 ml of lysogeny broth medium enriched with 20 μl of ampicillin and placed for 7 h at 37°C to obtain the preculture. Media were prepared by mixing 0.185 g brain heart infusion (BHI) (Oxoid, United Kingdom) and 0.146 g NaCl (AnalaR Normapur; VWR, Belgium) with different ratios of gelatin (from bovine skin, type B; Sigma, USA) and dextran (from Leuconostoc spp.; Sigma, Denmark) (Mr, ∼500,000) in glass tubes with a screw cap (see Table 1 for the composition of the different mixtures). After addition of 5 ml of distilled water, samples were placed in a water bath (GR 150 S12; Grant, United Kingdom) at 70°C for 12 min. In the next step, 10 μl of 0.01% rhodamine B (R953; Aldrich, Germany) and 5 μl of ampicillin were added and the mixture was filter sterilized by pushing it through a 0.2-μm-pore-size filter (Filtropur S 0.2; Sarstedt, Germany) with the aid of a syringe (10-ml Norm-Ject; Henke Sass Wolf, Germany). Samples were inoculated with 10 μl of preculture. Well chambers (Nunc Lab-Tek [USA] chambered borosilicate coverglass system) were filled with 300 μl of inoculated medium and allowed to solidify at room temperature. After approximately 40 h, images were taken with a commercial laser scanning microscope (FV 1000; Olympus). The fluorescent probe rhodamine B and Venus fluorescent protein were used to visualize, respectively, the gelatin phase (see, e.g., reference 9) and the bacterial cells. The associated excitation wavelengths were 561 and 488 nm, respectively, and emission maxima were at 625 and 528 nm. The emission ranges recorded were 570 to 670 nm and 505 to 555 nm. A 60× oil immersion objective was used with a numerical aperture of 1.35. Digital image files were acquired in .tif format at a resolution of 512 by 512 pixels. This results in an image resolution of 0.414 μm per pixel. Experiments are performed twice, whereas multiple images were taken from each mixture. The images in Fig. 1 are the images most representative of the observed phenomena.
Table 1.
Composition of different mixtures
| Mixture | Weight (g) of component: |
||
|---|---|---|---|
| Gelatin (G) | Dextran (D) | Salt | |
| 1 (1G/1D) | 0.125 | 0.125 | 0.146 |
| 2 (2G/1D) | 0.250 | 0.125 | 0.146 |
| 3 (3G/1D) | 0.375 | 0.125 | 0.146 |
| 4 (4G/1D) | 0.500 | 0.125 | 0.146 |
| 5 (2G/2D) | 0.250 | 0.250 | 0.146 |
| 6 (3G/2D) | 0.375 | 0.250 | 0.146 |
| 7 (4G/2D) | 0.500 | 0.250 | 0.146 |
| 8 (2G/2D NSa) | 0.250 | 0.250 | |
NS, no salt.
Fig 1.
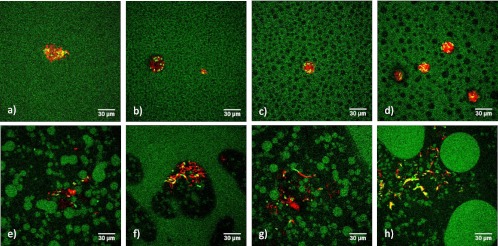
Growth of E. coli JM-109 DE3 (red) in gelatin (G [green])-dextran (D [black]) mixtures. (a) 1G/1D; (b) 2G/1D; (c) 3G/1D; (d) 4G/1D; (e) 2G/2D; (f) 3G/2D; (g) 4G/2D; (h) 2G/2D with no added salt.
Influence of the gelatin/dextran ratio on the microstructure.
Visualizations of the microstructures listed in Table 1 are shown in Fig. 1 (gelatin is shown in green). Starting from a 1:1 ratio of gelatin and dextran (mixture 1 [Fig. 1a]), increasing the concentration of gelatin led to the formation of a heterogeneous structure. Whereas the 1:1 ratio showed a uniform mixture, dextran spheres were formed in a gelatin matrix when the gelatin concentration was increased (Fig. 1c and d). Doubling the concentration of both gelling agents to a 2:2 ratio (mixture 5) led to phase inversion, i.e., the formation of gelatin spheres in a dextran matrix (Fig. 1e). Increasing the gelatin concentration further induced bigger spheres and even parts in the microstructure where the dextran spheres emerged in a gelatin matrix (Fig. 1f and g) similarly to the first mixtures. Whereas the 2:2 ratio with added salt (mixture 5) consisted of a dextran matrix with gelatin spheres of more or less the same size (Fig. 1e), leaving out the salt led to a combination of a few big droplets with a large amount of smaller droplets (Fig. 1h).
Preferential growth of E. coli in a gelatin-dextran mixture.
Fig. 1 also presents the E. coli colonies (red) in the gelatin-dextran mixture. Some diffusion of rhodamine B, used for the staining of the gelatin phase, into the bacterial cells is observed. As a result, bacterial cells can also appear as green or yellow rods in the microstructure.
Depending on the microstructure, colonies evolved differently. In the microstructures of mixtures 1 to 4, colonies formed spheres (Fig. 1a to d). As the gelatin concentration increased and a heterogeneous structure arose, colonial growth occurred in the dextran phase. When the dextran concentration (Fig. 1e to h) was doubled, phase inversion occurred and colonies no longer grew as regular demarcated spheres. Instead, the colonies appeared as diffuse strings permeating the dextran phase while avoiding the gelatin phase. In mixtures where both microstructures occurred (gelatin in a dextran matrix and dextran in a gelatin matrix; see, e.g., Fig. 1f and g), bacteria grew only in the dextran phase, regardless the microstructure.
The literature reports that several bacteria can metabolize dextran and use it as a substrate (10, 11), which could explain the preference of the bacteria for the dextran phase. However, for this specific strain, no references were found. Testing growth of E. coli in “poor” growth media with and without the addition of dextran did not show any difference in growth curves; i.e., no growth occurred after 24 h. If the bacteria had been able to metabolize dextran, the addition of dextran to the poor medium would have resulted in growth. As this did not occur, the experimental results indicate that dextran cannot be metabolized by E. coli JM-109 DE3.
In previous experiments, we were able to visualize E. coli microcolonies in gelatin only (not published). This means that gelatin does not form a microstructure that physically excludes E. coli cells as happens with acid-modified corn starch in gelatin gels (12). The presence of the microcolonies also proves that cell multiplication is not restricted by the presence of the gelatin.
It can be concluded that the location and morphology of E. coli JM-109 DE3 colonies are influenced by the microstructure in a gelatin-dextran mixture. Images taken with CLSM illustrate that E. coli bacteria have a preferential phase for growth, i.e., the dextran phase. The preference ascribed to dextran cannot be attributed to (i) an ability to metabolize dextran molecules, (ii) exclusion of E. coli cells by the gelatin, or (iii) restriction of cell multiplication in the gelatin phase. The preference of E. coli cells for the dextran phase is probably due to the more favorable physical properties in the dextran phase.
ACKNOWLEDGMENTS
This work was supported by project PFV/10/002 (Center of Excellence OPTEC—Optimization in Engineering) of the KU Leuven Research Council, Knowledge Platform KP/09/005 (www.scores4chem.be) of the KU Leuven Industrial Research Fund, project G.0930.13 of the Fund for Scientific Research—Flanders, and the Belgian Program on Interuniversity Poles of Attraction, initiated by the Belgian Federal Science Policy Office. J.F.V.I. holds the chair Safety Engineering sponsored by the Belgian chemistry and life sciences federation essenscia. J.H. gratefully acknowledges financial support in the form of long-term structural funding “Methusalem” from the Flemish Government and from the Hercules Foundation (HER/08/021). K.B. is supported by a research grant of the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT). C.C.D. is supported by the Fonds voor Wetenschappelijk Onderzoek (FWO).
Footnotes
Published ahead of print 22 February 2013
REFERENCES
- 1. Wilson P, Brocklehurst T, Arino S, Thuault D, Jakobsen M, Lange M, Farkas J, Wimpenny J, Van Impe JF. 2002. Modelling microbial growth in structured foods: towards a unified approach. Int. J. Food Microbiol. 73:275–289 [DOI] [PubMed] [Google Scholar]
- 2. Antwi M, Geeraerd AH, Vereecken KM, Jenné R, Bernaerts K, Van Impe JF. 2006. Influence of a gel microstructure as modified by gelatin concentration on Listeria innocua growth. Innov. Food Sci. Emerg. 7:124–131 [Google Scholar]
- 3. Theys TE, Geeraerd AH, Verhulst A, Poot K, Van Bree I, Devlieghere F, Moldenaers P, Wilson D, Brocklehurst T, Van Impe JF. 2008. Effect of pH, water activity and gel micro-structure, including oxygen profiles and rheological characterization, on the growth kinetics of Salmonella Typhimurium. Int. J. Food Microbiol. 128:67–77 [DOI] [PubMed] [Google Scholar]
- 4. Mertens L, Van Derlinden E, Dang TDT, Cappuyns AM, Vermeulen A, Debevere J, Moldenaers P, Devlieghere F, Geeraerd AH, Van Impe JF. 2011. On the critical evaluation of growth/no growth assessment of Zygosaccharomyces bailii with optical density measurements: liquid versus structured media. Food Microbiol. 28:736–745 [DOI] [PubMed] [Google Scholar]
- 5. Farber JM, McKellar RC, Ross WH. 1995. Modelling the effects of various parameters on the growth of Listeria monocytogenes on liver pâté. Food Microbiol. 12:447–453 [Google Scholar]
- 6. Noriega E, Laca A, Díaz M. 2010. Development of a structure-based model for the competitive growth of Listeria innocua in minced chicken breasts. Int. J. Food Microbiol. 142:44–52 [DOI] [PubMed] [Google Scholar]
- 7. Jeanson S, Chadoeuf J, Madec MN, Aly S, Floury J, Brocklehurst TF, Lortal S. 2011. Spatial distribution of bacterial colonies in a model cheese. Appl. Environ. Microbiol. 77:1493–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hornbogen E. 1985. Strength of alloys with heterogeneous microstructures. Czech. J. Phys. 35:193–205 [Google Scholar]
- 9. Tromp RH, van de Velde F, van Riel J, Paques M. 2001. Confocal scanning light microscopy (CSLM) on mixtures of gelatine and polysaccharides. Food Res. Int. 34:931–938 [Google Scholar]
- 10. Gibson GR, Willems A, Reading S, Collins MD. 1996. Fermentation of non-digestible oligosaccharides by human colonic bacteria. Proc. Nutr. Soc. 55:899–912 [DOI] [PubMed] [Google Scholar]
- 11. Khalikova E, Susi P, Korpela T. 2005. Microbial dextran-hydrolyzing enzymes: fundamentals and applications. Microbiol. Mol. Biol. Rev. 69:306–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marfil PHM, Anhê ACBM, Telis VRN. 2012. Texture and microstructure of gelatin/corn starch-based gummy confections. Food Biophys. 7:236–243 [Google Scholar]


