Abstract
Twenty-eight-day-old weaned pigs were fed diets with a low (LZn), medium (MZn), or high (MZn) Zn concentration (50 to 80, 150, or 2,500 mg Zn/kg of diet, respectively) provided as zinc oxide (ZnO)(24 pigs per group). They were infected orally with Salmonella enterica serovar Typhimurium DT104 on day 32. Salmonellae were cultivated from feces (up to 42 days postinfection [dpi]) and organs (2 and 42 dpi). Activation of the adaptive systemic and mucosal immune systems was investigated by recording anti-Salmonella IgG levels and levels of B and T lymphocyte subpopulations in blood and gut-associated lymphatic tissue. Growth performance was recorded as well. Salmonellae were shed at higher levels and for longer periods in the HZn group (P < 0.05), with no differences in the tissues. At 2 dpi, the relative percentages of CD4+ T helper cells (P < 0.01) and of CD2+ T and NK cells (P < 0.01) in blood were reduced from the relative cell counts obtained at 0 dpi, irrespective of the Zn group. The lowest percentage of cytotoxic T cells was found 14 dpi in the HZn group relative to the MZn (P < 0.05) and LZn (P < 0.01) groups. Supplementation of the feed with 2,500 mg Zn/kg of diet immediately after weaning could positively affect the immune responses of piglets infected with Salmonella Typhimurium, but for a short period only. After 2 weeks, all positive effects disappeared, and rather negative effects, such as higher shedding of salmonellae, lower T cell frequencies, and worse performance, occurred. Thus, supplementation with ZnO at high levels in the pig industry should be limited to 2 to 3 weeks.
INTRODUCTION
Zinc (Zn) is a ubiquitous trace mineral of proven importance for the function of >300 enzymes (1), including those active in immune cells. Therefore, zinc is active in a variety of cellular functions and has a strong ability to influence the immune system (2). Zn deficiency in humans has been shown to result in immunodeficiency and increased morbidity caused by infectious agents, whereas supplementation of food with Zn in areas where Zn deficiency is common can improve the health of the children by reducing diarrhea (3, 4). Growing pigs require 50 mg Zn/kg of diet according to National Research Council recommendations (5), or 80 to 100 mg Zn/kg dry matter (DM) of feed according to the German Association for Nutrition Physiology (6). Zn occurs naturally in many feed components, but calcium, copper, protein, and phytic acid all reduce its bioavailability (7). Among different techniques, supplementation of the diet with organic and inorganic Zn has become standard (8). Because of environmental concerns, the maximal level of Zn allowed in the diets of pigs was set to 150 mg/kg of diet in the European Union irrespective of the origin of the Zn (9). Dietary zinc oxide (ZnO) added at high levels (i.e., 2,000 to 3,000 mg Zn/kg of diet) to the diets of weaned pigs has been shown to reduce the incidence of diarrhea and to improve growth performance (7, 10, 11). One of the proposed modes of action leading to the improved performance of weaned piglets and the reduced incidence of diarrhea is the effect of ZnO on the gastrointestinal microbiome (12, 13, 14). Salmonellae are among the intestinal pathogens that can cause disease in growing pigs, and many pigs are natural carriers of Salmonella spp. Salmonellae enter the body by infection and transit the enterocytes (15, 16). After they leave the enterocytes, salmonellae are caught by macrophages and other cells of the gut-associated lymphatic tissue (GALT) and are transferred first to regional mesenteric lymph nodes and later to other lymphatic organs (16). They can survive there, resulting in a carrier state and providing a possible source of reinfection for pigs and for humans (17, 18). Salmonella enterica serovar Typhimurium is the predominant causative agent responsible for approximately 12% of salmonellosis outbreaks in the European Union (19).
In the present study, the influence of different dietary ZnO concentrations on the immune responses of pigs after infection with Salmonella enterica serovar Typhimurium DT104 was investigated. In order to assess the activation of the adaptive systemic and mucosal immune systems, we determined the anti-S. Typhimurium immunoglobulin G (IgG) levels, as well as the levels of B and T lymphocyte subpopulations in samples of circulating blood and different tissues of the GALT. Additionally, the effect of ZnO on the performance of weaned pigs was studied.
MATERIALS AND METHODS
Animals.
German Landrace piglets (n = 72) of both sexes were weaned at the age of 28 days and were allocated to pens in a biosecure, environmentally controlled experimental facility. The piglets were randomly assigned to one of three groups and were allocated to 6 pens per group (4 pigs in each pen). Each dietary group was fed a common basal maize-wheat-barley-soybean diet with different zinc levels, calculated to be 50 to 80 (low zinc concentration [LZn]), 150 (medium zinc concentration [MZn]), or 2,500 (high zinc concentration [HZn]) mg Zn/kg of feed. The Zn supplementation source was analytical-grade ZnO (Sigma-Aldrich, Taufkirchen, Germany). Beginning at weaning (at the age of 28 days), the respective feed was fed semi ad libitum twice a day for 1 h in the form of a mixture with water, in order to avoid refusals. Water was provided ad libitum via drinking nipples. Four days after weaning (i.e., day 32 of age), all piglets were infected with S. Typhimurium DT104 via an oral tube leading into the throat. The feeding troughs and the pens' floors and walls were cleaned twice daily by brushing and flushing with lukewarm water. Two days postinfection (2 dpi), two animals from each pen were euthanized by an overdose of pentobarbiturates (Narcoren; Merial GmbH, Germany) under general azaperone (Stresnil; Janssen Animal Health, Germany)-ketamine (10% ketamine; Bremer Pharma GmbH, Germany) anesthesia. The remaining piglets were euthanized 6 weeks after infection, i.e., 42 dpi. The experiment was approved by the local animal welfare authority (Landesamt für Gesundheit und Soziales, Berlin, Germany) under identification (ID) number G0348/09.
Sampling.
After infection with S. Typhimurium, the following clinical and zootechnical parameters were recorded for all pigs: (i) general condition and fecal score (from 1 to 5, where 1 stands for liquid and 5 stands for firm feces), (ii) rectal temperature, (iii) shedding of salmonellae in feces, which was determined daily for 5 days postinfection and then twice a week, (iv) prevalence of anti-Salmonella IgG in blood samples, which were obtained before infection and then 2, 7, 10, 14, 17, 21, 24, 28, 35, and 42 dpi, (v) weekly body weight. During necropsy, palatine tonsils and jejunal and colonic mesenteric lymph nodes were collected for the cultivation of S. Typhimurium. For the characterization of immune cells, mesenteric ileocecal lymph nodes, as well as ileal Peyer's patches, were collected from 8 piglets per group at 2 dpi and from 12 piglets per group at 42 dpi. In addition, blood samples collected before infection and 2, 7, 10, 14, and 42 dpi were analyzed for their relative percentages of immune cell populations.
Bacterial strains.
S. Typhimurium DT104 was chosen for the infection because it was obtained from a swine with sepsis (17). The strain was characterized by multiple resistances against antibiotics, including resistance to nalidixic acid (NAL). The resistance to NAL was used later for selective cultivation of the strain from the samples. The S. Typhimurium was cultured in buffered peptone water (BPW) containing 50 μl NAL/ml (BPW-NAL) at 37°C for 20 to 22 h with shaking, to reach an optical density (OD) of 0.69 to 0.72, corresponding to 2 × 109 to 3 × 109 CFU/ml, which was subsequently confirmed by plating. The culture was set up a day before infection to enable collection of the broth immediately before it was provided to the piglets. Each piglet was infected with 5 ml of such culture broth (1.0 × 1010 to 1.5 × 1010 CFU in total).
Quantitation of Salmonella bacteria.
The quantitative detection of S. Typhimurium in feces was performed using a spiral plater (Whitley, Meintrup DWS, Germany) with a detection limit of 2 × 102 CFU/g. For each sample, 100-μl portions from five dilutions were streaked onto three xylose-lysine-deoxycholate (XLD) agar plates supplemented with 50 μg/ml of NAL (XLD-NAL) and were incubated at 37°C for 20 to 24 h. For the quantitation of S. Typhimurium in internal organs, tissue samples were immersed in 95% ethanol, flamed, minced aseptically, and homogenized with BPW-NAL (1:10) in filter bags using a stomacher for 2 min at high speed. S. Typhimurium was quantified in all samples by plating 400 μl of filtrates using a spiral plater. In addition, for all samples, a mini-most probable number (mini-MPN) method was applied in triplicate to reduce the detection limit of salmonellae and to obtain more accurate CFU numbers (20). Fivefold dilutions in BPW-NAL were performed in two 24-well culture plates. After incubation at 37°C for 16 to 20 h, a droplet from each dilution was also transferred to a modified semisolid Rappaport-Vassiliadis (MSRV) medium (Becton Dickinson GmbH, Heidelberg, Germany) in 24-well culture plates. The plates were checked after 24 h of incubation at 41°C for the presence of swarming typical of salmonellae. Afterwards, MPNs were calculated. All wells recorded as positive or equivocal were further checked for the presence of salmonellae by picking the MSRV agar with the swarm, transferring it to XLD-NAL agar plates, and incubating as described above, with final confirmation by real-time PCR (21).
In order to investigate whether bacteremia occurred, 2 to 4 ml of collected blood was poured into 9 ml of BPW-NAL and was incubated for 20 to 24 h at 37°C. Three drops of the cultures were then spotted onto MSRV agar plates and were incubated for 24 to 48 h at 41°C. The CFU and MPNs obtained were transformed into log/g to obtain nearly normal distribution of trait values. Mean values and standard deviations are given below.
FCM.
Cells were isolated and purified from tissue and blood as described elsewhere (18). Triplet staining with either (i) CD4 directly conjugated to fluorescein isothiocyanate (FITC; SouthernBiotech), CD8 directly conjugated to phycoerythrin (PE; SouthernBiotech), and CD25 (Biozol) or (ii) TcR1-N4, CD2, and IgM (all from VMRD) was performed using 1 × 106 cells for each reaction in a volume of 30 μl of phosphate-buffered saline (PBS) for 30 min on ice in the dark. After a washing step, the cell suspensions were exposed to fluorescence-labeled secondary antibodies for 15 min. The detailed procedure is described elsewhere (22). Flow cytometry (FCM) was performed for 50,000 lymphocytes per sample using a BD FACSCalibur flow cytometer. The lymphocytes were obtained within one specific lymphocyte gate corresponding to their forward and side scatter signals. For immune cell characterization, only living lymphocytes, negative for propidium iodide (PI) staining (0.5 μg/ml), were taken. T helper cells (CD4+ CD25+/− CD8−/dim), cytotoxic T cells (CD8α/β+ CD4−), and B cells (membrane IgM+ CD2−/dim) were identified.
Serology.
Blood samples of all animals were collected from the jugular vein/cranial vena cava. Blood was incubated at 37°C for 2 h and was then centrifuged at 1,400 × g for 15 min, and serum was collected and stored at −20°C until analysis.
The presence of anti-Salmonella antibodies was tested for by use of the Salmotype PigScreen enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Labor Diagnostik Leipzig, Leipzig, Germany). This test recognizes anti-Salmonella immunoglobulin G. The IgG levels were calculated using a reference standard method and are presented as percentages of the optical density [OD%, calculated according to the following formula: sample OD% value = 72.1 × (OD sample−mean OD negative control)/(mean OD positive control−mean OD negative control)].
Statistical analysis.
Statistical analysis was performed using R, version 2.11.1, and SPSS, version 12.0.2 (SPSS, Inc., Chicago, IL). For the phenotypes of immune cells, 12 samples per group (combined effects of time [dpi] and zinc concentrations in the diet) were analyzed. All values higher than twice the interquartile range (IQR), below the first quartile, and above the third quartile were identified as outliers. Outliers had no significant effects on test statistics, since <1% of the data were outliers. To test the effects of supplementation with different concentrations of zinc on the phenotypes, we applied analysis of variance (ANOVA), with zinc supplementation in the diet and days postinfection as fixed effects. The following linear model was used to assess the variation (V) in every trait for each tissue: Vtrait = Vdiet group + Vdays postinfection + residual error. If ANOVA revealed significance, Student's t test was performed at each time point to specify the results. To test the effects of the three levels of zinc in the diet on the zootechnical parameters, multivariate analysis of variance (MANOVA) was performed by applying SPSS, version 12.0.2 (SPSS, Inc., Chicago, IL), with a post hoc Tukey test. The CFU counts of salmonellae were log transformed to obtain normal distribution of the data. The amounts of S. Typhimurium in feces and organs were compared using the nonparameteric Kruskal-Wallis test, because salmonellae were not continuously detected in all samples. The effect of treatment was considered significant at a P value of <0.05. The Mann-Whitney U test was further performed to elucidate which values differed with a P value of <0.01. Pearson's correlation coefficients were calculated for the correlation between IgG and log counts of S. Typhimurium in feces. Box-and-whisker plots were chosen for graphical presentation of the results. The boxes indicate the medians (horizontal lines) and the lower and upper quartiles (bottoms and tops of the boxes). The vertical bars (“whiskers”) in the box plots indicate the minimal and maximal values recorded. Outliers are indicated by circles and asterisks in the plots.
RESULTS
One animal intended for the MZn group died at weaning; thus, only 23 animals were in this group, and 11 animals were euthanized at 2 dpi. One animal from the HZn group died within the 4th week of the study due to reasons unrelated to the Salmonella infection.
Clinical parameters.
The infection with S. Typhimurium caused no or very mild clinical signs. None of the animals developed diarrhea (scores 1 and 2). Increased rectal body temperatures (≥40°C) were measured in 7, 4, and 8 piglets in the LZn, MZn, and HZn groups, respectively, at 1 dpi. The rectal temperatures were physiological for all animals at 2 dpi.
Zootechnical parameters.
The mean body weight (BW) at weaning was 7.0 ± 1.58 kg in the LZn group, 7.4 ± 1.35 kg in the MZn group, and 7.4 ± 1.36 kg in the HZn group (Table 1). A significant effect of the feeding groups during the study was detectable only at 14 dpi (the BW was 9.3 ± 2.24 kg for the LZn group, 11.3 ± 1.57 kg for the MZn group, and 11.7 ± 1.85 kg for the HZn group [P < 0.05]). Later, the LZn group caught up in body weight: no difference in BW between the groups could be observed for the rest of the experiment. Additionally, the average gain (AG) was no longer significantly different from 21 dpi on.
Table 1.
Mean values for performance parameters of pigletsa
| Parameterb | Valuec |
Pooled SEMd | Pe | ||
|---|---|---|---|---|---|
| LZn | MZn | HZn | |||
| BW (kg) | |||||
| −4 dpi | 7.0 | 7.4 | 7.4 | 0.18 | 0.503 |
| 0 dpi | 7.1 | 8.2 | 8.1 | 0.17 | 0.109 |
| 2 dpi | 7.4 | 8.4 | 8.0 | 0.18 | 0.139 |
| 7 dpi | 8.2 | 9.1 | 9.7 | 0.26 | 0.065 |
| 14 dpi | 9.3a | 11.3b | 11.7b | 0.35 | 0.010 |
| 21 dpi | 12.1 | 13.6 | 13.3 | 0.36 | 0.237 |
| 28 dpi | 15.3 | 16.2 | 15.9 | 0.47 | 0.748 |
| 35 dpi | 19.6 | 20.3 | 20.5 | 0.59 | 0.808 |
| 42 dpi | 24.0 | 25.2 | 25.7 | 0.74 | 0.649 |
| AG (kg) | |||||
| 2–7 dpi | 1.58a | 1.27a | 3.42b | 0.255 | 0.000 |
| 7–14 dpi | 2.23a | 4.43b | 3.92b | 0.321 | 0.005 |
| 14–21 dpi | 5.68a | 4.55ab | 3.32b | 0.323 | 0.004 |
| 21–28 dpi | 6.33 | 5.17 | 4.72 | 0.315 | 0.089 |
| 28–35 dpi | 8.58 | 8.33 | 8.43 | 0.271 | 0.938 |
| 35–42 dpi | 8.90 | 9.83 | 9.53 | 0.416 | 0.673 |
Piglets were weaned at the age of 28 days, fed a diet low in Zn (LZn) or supplemented with 150 mg Zn/kg (MZn) or 2,500 mg Zn/kg (HZn) as ZnO, and infected with Salmonella Typhimurium on day 32.
BW, body weight, based on individual data; AG, average gain within a pen (2 pigs/pen) over the period given.
Values followed by different superscript letters differ significantly (P < 0.05) by the Tukey test.
The pooled SEM corresponds to the overall value for LZn, MZn, and HZn.
P values of multivariate analysis of variance (MANOVA) performed to test the effects of the three levels of zinc in the diet with significance level of P < 0.05 (bold).
Detection of Salmonella Typhimurium in feces and organs.
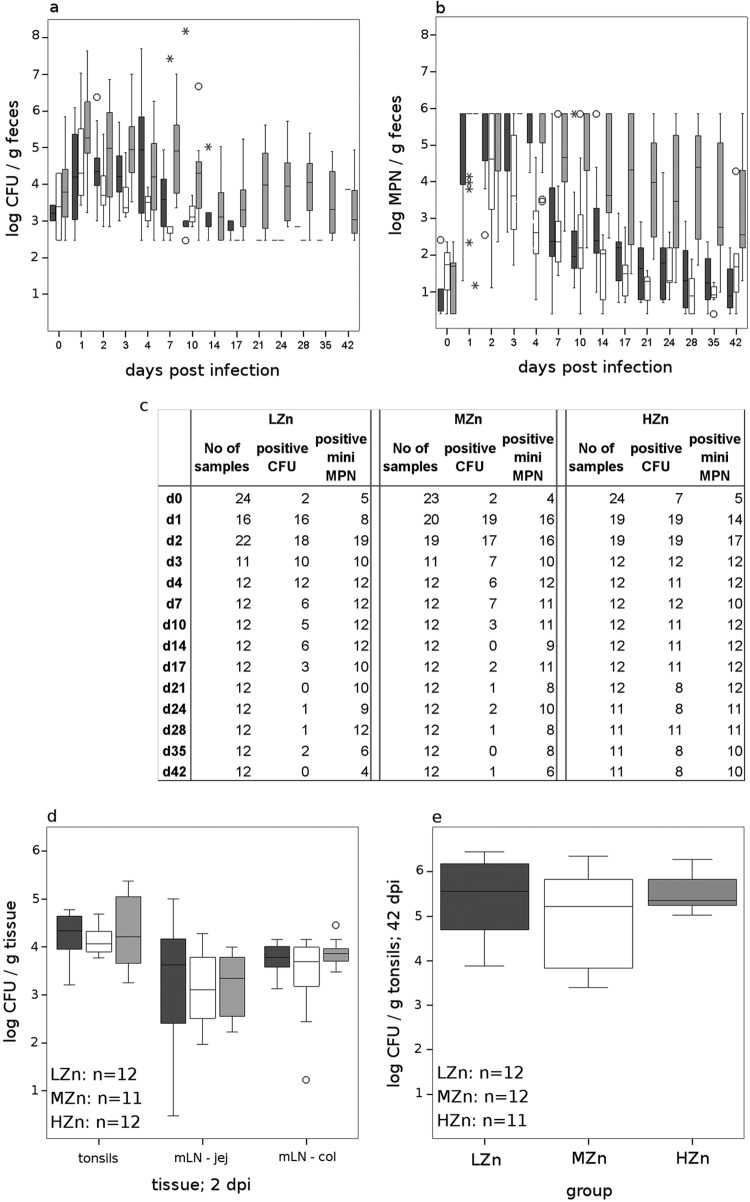
ZnO had an effect on the fecal salmonella counts measured on XLD-NAL plates (P, <0.05 by the Kruskal-Wallis test). All animals shed salmonellae during the first week postinfection, and higher log CFU/g of feces was recorded for the HZn group on 1, 2, 3, and 7 dpi (Fig. 1a). From 10 dpi on, few or no animals in the LZn and MZn groups shed salmonellae above the detection limit of 200 CFU/g, but all animals in the HZn group did. Therefore, no statistical calculation was possible.
Fig 1.
Box plots of log-transformed CFU (a) and mini-MPNs (b) of Salmonella Typhimurium in fecal samples and in organs (d and e) after oral infection of pigs fed a diet with 60 to 80 mg Zn/kg (LZn), 150 mg Zn/kg (MZn), or 2,500 mg Zn/kg (HZn) as ZnO. The upper limit of the mini-MPN method was 6 log. Boxes indicate the medians (horizontal lines) and the lower and upper quartiles (bottoms and tops of boxes). The vertical bars in the box plots indicate the minimal and maximal values recorded. Circles and asterisks indicate outliers. Dark gray boxes, LZn; white boxes, MZn; light gray boxes, HZn. jej, jejunum; col, colon. (c) Numbers of fecal samples analyzed and numbers of samples positive by XLD-NAL plating and by the mini-MPN method. d, day postinfection. (d and e) The number of organ samples analyzed for each group is given at the bottom left of each box plot. Salmonellae could not be reliably counted in the mesenteric lymph nodes (mLN) at 42 dpi; thus, only values for tonsils are shown at this time point.
More animals tested positive for salmonellae (<100 MPN/g) from 10 dpi by use of the mini-MPN method than by use of XLD-NAL plating. However, during the first week after infection, almost all animals were beyond the maximal level of the test (Fig. 1b); thus, statistical calculations were performed starting from 10 dpi. MPNs differed between groups (P, <0.05 by the Kruskal-Wallis test). The MPNs in the HZn group were higher than those in both the other groups (P, <0.01 by the Mann-Whitney U test).
Differences in the numbers of salmonellae in organs were calculated only for results obtained by XLD-NAL plating, because all samples reached the upper limit if the mini-MPN method was used. The numbers of salmonellae in the mesenteric lymph nodes of the jejunum and colon 42 dpi reached more than 10,000 CFU/g and could not be counted exactly. Thus, only tonsils were tested further at 42 dpi. No differences in the salmonella counts were observed between groups, either at 2 or at 42 dpi (Fig. 1c and d).
Humoral immunity—anti-Salmonella IgG.
The piglets were already positive for antibodies (Ab) against Salmonella antigens at weaning. Hence, the mothers very likely had been infected with Salmonella previously and therefore had passed some maternal Salmonella-specific antibodies to their offspring, but since these antibodies were not able to opsonize the strain of S. Typhimurium used in a microagglutination test in vitro (data not shown), these antibodies likely had no effect on the protective immunity of the piglets. However, there was high individual variation. The levels of the Ab (anti-Salmonella IgG) decreased within 10 days after infection (Fig. 2). Until 28 dpi, no anti-Salmonella IgG or only a low level could be recorded, but at later time points, the level started to increase. There was a tendency for the HZn group to have higher levels of anti-Salmonella IgG than the other groups at 42 dpi (P = 0.098). No correlation was found between the levels of anti-Salmonella IgG and the counts of salmonellae shed in feces.
Fig 2.
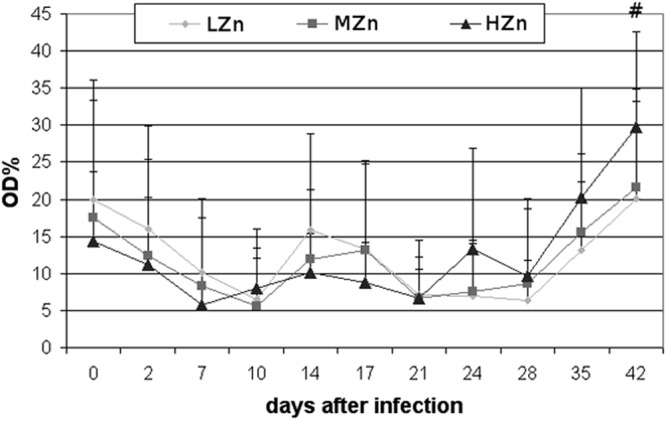
Anti-Salmonella IgG in the serum of pigs weaned at the age of 28 days and fed a diet with 60 to 80 (LZn), 150 (MZn), or 2,500 (HZn) mg Zn/kg as ZnO before and after oral infection with S. Typhimurium at the age of day 32. Mean values plus standard deviations for the percentage of optical density (OD%) are shown. The pound sign (#) indicates the tendency (P = 0.098) for the HZn group to have higher levels of anti-Salmonella IgG than the MZn and LZn groups at 42 dpi. The following numbers of samples were analyzed: for the LZn group, 24 samples on days 0 and 2 and 12 samples between days 7 and 42; for the MZn group, 23 samples on days 0 and 2 and 12 samples between days 7 and 42; for the HZn group, 24 samples on days 0 and 2, 12 samples between days 7 and 21, and 11 samples on days 24 to 42.
Phenotyping of immune cell populations.
The relative percentages of CD4+ T helper cells (P < 0.01) and CD2+ cells, which represent T and NK cells (P < 0.01), in blood were reduced at 2 dpi from the relative cell counts obtained before infection, irrespective of the zinc feeding group (Fig. 3). ANOVA revealed significant ZnO effects on the CD8+ cytotoxic T cell population (P = 0.01) in blood and also a tendency for an effect of the ZnO level in the feed on the ileal lymph nodes (P = 0.06) over the whole experiment. In contrast, no ZnO effect was detectable in the ileal Peyer's patches.
Fig 3.
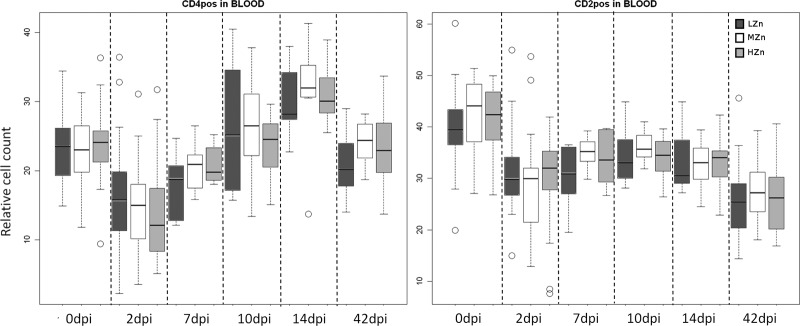
CD4+ T helper (left) and CD2+ T and NK (right) cell counts, relative to those of the living lymphocyte population, in the blood of pigs receiving different Zn concentrations in their diets before and after challenge with S. Typhimurium. Dark gray bars, low Zn concentrations (LZn) in the diet (about 50 to 80 mg/kg of feed); white bars, medium Zn levels (MZn) in the diet (150 mg Zn/kg); light gray bars, high Zn levels (HZn) in the diet (2,500 mg Zn/kg). pos, positive. The numbers of samples analyzed were as follows: before infection (0 dpi) and at 2 dpi, 24 in the LZn group, 23 in the MZn group, and 24 in the HZn group; at 7, 10, and 14 dpi, 12 in each group; at 42 dpi, 12 in the LZn and MZn groups and 11 in the HZn group). All piglets were weaned at the age of 28 days and were orally infected with S. Typhimurium on day 32. The vertical bars in the box plots indicate the minimal and maximal values recorded. Circles represent outliers.
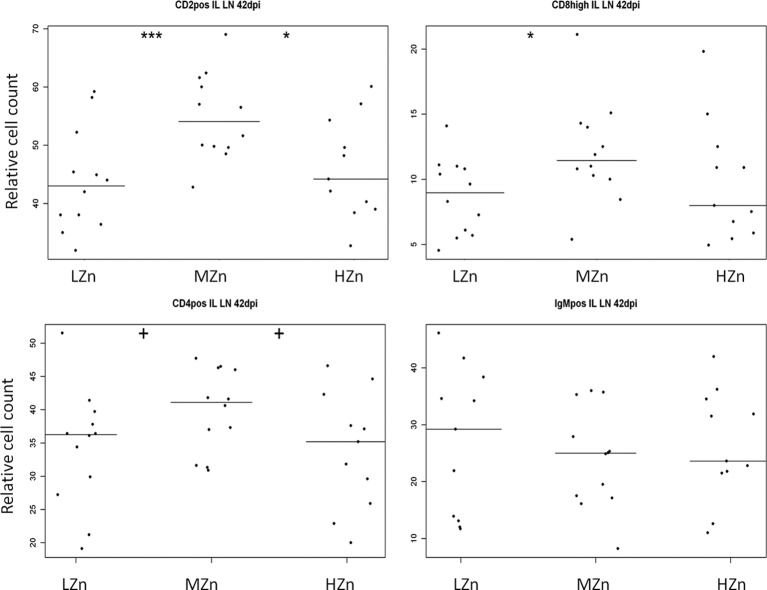
Fourteen days postinfection, the HZn group had the lowest percentage of cytotoxic T cells (P, <0.05 for comparison to the MZn group and <0.01 for comparison to the LZn group) (Fig. 4). Tendencies in the same direction were found at 7 and 10 dpi. At the end of the study, 42 dpi, levels of all T cell populations examined in the ileal lymph nodes (i.e., CD2+, CD4+, and CD8high cells) were either significantly lower or tended to be lower in the LZn and HZn feeding groups than in the MZn group, but no differences were observed within the B cell population examined (IgM-positive cells) (Fig. 5).
Fig 4.
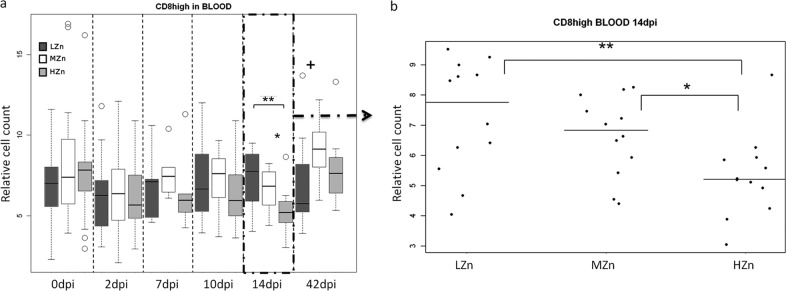
CD8+ cytotoxic T cell counts, relative to those of the living lymphocyte population, in the blood of piglets receiving different Zn concentrations in their diets before and after challenge with S. Typhimurium (a) and 14 dpi (b). (a) Dark gray bars, low Zn concentrations (LZn) in the diet (about 50 to 80 mg/kg of feed); white bars, medium Zn levels (MZn) in the diet (150 mg Zn/kg); light gray bars, high Zn levels (HZn) in the diet (2,500 mg Zn/kg). The numbers of samples analyzed were as follows: before infection (0 dpi) and at 2 dpi, 24 in the LZn group, 23 in the MZn group, and 24 in the HZn group; at 7, 10, and 14 dpi, 12 in each group; and at 42 dpi, 12 in the LZn and MZn groups and 11 in the HZn group. (b) Relative CD8+ cytotoxic T cell counts in LZn, MZn, and HZn piglets at 14 dpi (n = 12). All piglets were weaned at the age of 28 days and were orally infected with S. Typhimurium on day 32. The plus sign indicates a tendency, and asterisks indicate significance (+, 0.05 < P < 0.1; *, 0.01 < P < 0.05; **, P < 0.01). The vertical bars in the box plots indicate the minimal and maximal values recorded. Circles represent outliers.
Fig 5.
Counts, relative to those of the living lymphocyte population, of CD2+ T and NK cells, CD8high cytotoxic T cells, CD4+ T helper cells, and IgM-positive (IgMpos) B cells in ileal lymph nodes (IL LN) 42 days after S. Typhimurium infection of 12 piglets fed a low-Zn (LZn) diet (60 to 80 mg Zn/kg as ZnO), 12 piglets fed a medium-Zn (MZn) diet (150 mg Zn/kg as ZnO), and 11 piglets fed a high-Zn (HZn) diet (2,500 mg Zn/kg as ZnO). Each dot represents the finding for one animal. Each horizontal line indicates the median for the animals in one feeding group. Plus signs indicate a tendency (0.05 < P < 0.1); asterisks indicate significant differences (*, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, P < 0.001).
DISCUSSION
In the present study, we examined the effect of zinc oxide supplementation of the diet with respect to the immune responses of weaned piglets after a challenge with S. Typhimurium. The zinc dosages used were approximately the maximal level of Zn allowed in the diets of pigs in the European Union (9), a level below this, and a very high concentration of 2,500 mg/kg of feed.
Unexpectedly, some piglets tested positive for salmonellae immediately on the day of infection. Nalidixic acid-resistant salmonellae are not common in pigs, but their numbers were low and similar in all groups, which may indicate earlier infections in suckling piglets. Serotyping of isolates also revealed S. Typhimurium DT104, which was not surprising, because this serotype and phage type dominate in pigs (19, 23). Statistical calculations revealed no differences between groups in the numbers of salmonellae shed on the day of infection. Later on, after infection, all animals shed high numbers of salmonellae. Additionally, we found no differences in the immune parameters measured at 2 dpi between piglets that tested positive and negative for S. Typhimurium before the administration of S. Typhimurium DT104. This was in line with our expectations, because the memory function of the immune system is not yet well developed at the age of 4 weeks (24). Therefore, we consider that this primary infection had no influence on the outcome of the study or on the effect of zinc oxide.
A high level of ZnO, 2,500 mg Zn/kg of diet, caused increased body weight gain of pigs within the first week after infection. Supplementation of the diet with ZnO to either 150 or 2,500 mg Zn/kg of feed was also beneficial for weight gain during the 2nd and 3rd weeks after infection, but no additional effect of 2,500 mg Zn/kg of diet was recorded at this time. Later, the piglets compensated for the growth differences, and beneficial effects of ZnO supplementation were no longer observed. The results obtained in this study were in agreement with those of other studies showing improvement in growth performance after weaned piglets were fed high doses of ZnO (7, 25, 26, 27). In one of our previous studies with a similar feeding regime, improvement of performance caused by 2,500 mg Zn/kg as ZnO could be observed only within the 3rd week after weaning (unpublished data). Obviously, environmental and sanitary conditions, which differ between experiments, affect the effects of dietary supplements (28), and this may also be true for the effects of ZnO. Evidence for the antimicrobial activity of zinc in vitro (29) and for modification of the intestinal microbiome by high levels of dietary ZnO (13, 14, 30) has been reported. For example, a diet containing 2,500 mg Zn/kg caused a 0.5- to 2-log reduction in the number of intestinal Campylobacter species in weaned piglets (31). In our study, in contrast, no reduction, but an increase, in the shedding of Salmonella Typhimurium was observed in the group fed 2,500 mg Zn/kg of diet. This observation is in agreement with the 2011 report of Vahjen et al. (13), who observed increased enterobacterial counts and diversity in the ilea of pigs fed high levels of ZnO. Thus, the increased Zn concentration in the gastrointestinal milieu could suppress a part of the microbial population, allowing better growth or survival of the salmonellae, and thus could lead to prolonged shedding.
Surprisingly, many of the piglets also shed high numbers of other bacteria that grew on XLD-NAL plates, forming yellow colonies with or without a black center, with yellow discoloration of the XLD agar, typical for Escherichia coli. The presence of E. coli was further confirmed by biochemical methods, i.e., API 20E (bioMérieux, France) (data not shown). These E. coli bacteria were also isolated from jejunal mesenteric lymph nodes and, to a lesser extent, from tonsils. Because the study focused on Salmonella, the isolated E. coli bacteria were not investigated further. Resistance to nalidixic acid has been found in 61 to 68% of commensal E. coli strains in humans and in 58% of isolates from pigs (32, 33). Thus, it can be assumed that in the pigs used in the present study, there were multiple commensal E. coli strains carrying this resistance. There has been repeated evidence that enteropathogens, such as S. Typhimurium or E. coli, use the inflammation state in the intestine to infect the host (15, 16, 34). Furthermore, the inflammatory state in the gut can boost horizontal gene transfer between the infectious agent and the residual gut microbiome (35), which could also play a role in the observed bloom of the E. coli resistance to NAL. Resistance to NAL is caused by a mutation in the gyr gene (36), which, among other genes coding for resistance to antibiotics in S. Typhimurium DT104, is clustered in class 1 integrons on Salmonella genomic island 1 (SGI1) (37). SGI1 was detected in S. Typhimurium DT104 as circular extrachromosomal DNA (38), which can be transferred in the presence of a helper plasmid providing the mating apparatus (39).
In contrast to previous studies performed under the same experimental conditions, where salmonellae were observed sporadically in the mesenteric lymph nodes 28 dpi (17, 18), massive Salmonella counts in the colonic mesenteric lymph nodes and in the tonsils were observed in this study even at 42 dpi, irrespective of the Zn level in the diet. At the same time, no clinical signs were observed. This might suggest that the immune system was able to sequester the bacteria but was not strong enough to kill them. Additionally, the high ZnO level in the diet had no protective effect against invasion of the host by enteric pathogens, as evidenced by the fact that the same levels of salmonellae were observed in the organs.
Salmonellae possess several defense mechanisms allowing them to survive within host cells (15, 16, 40). Host antibodies opsonize circulating bacteria, preparing them for lysis. The maternal anti-Salmonella IgG was not able to opsonize the strain of S. Typhimurium used in this study for the microagglutination test in vitro (data not shown) and thus likely had no effect on the piglets' immune defenses but possibly delayed the synthesis of the host IgG. In contrast to the findings of previous studies (17, 18), the piglets' own antibodies, which were able to opsonize the infection strain in vitro (data not shown), were synthesized from 21 to 28 days after infection. The observed tendency in the group fed high dietary ZnO levels to have increased anti-Salmonella IgG levels 42 dpi needs further investigation, because the titer of antibodies reached no plateau at this time.
The relative counts of the CD2+ T and NK cells as well as the CD4+ T helper cells circulating in the blood decreased after the Salmonella challenge; thus, their migration to the infection site in the intestine could be expected. However, this remains speculative, since no data on the cell counts in the lymph nodes before infection could be obtained.
A recent study has shown an ability of Salmonella Typhimurium to induce downregulation of major histocompatibility complex class II (MHC II) molecules on porcine alveolar macrophages due to regulation by Salmonella pathogenicity island 1 (SPI-1) and SPI-2 (41). For this reason, S. Typhimurium could escape the lysis mechanisms applied by host immune cells (42) by hiding itself in host cells, because the host cells, especially dendritic cells, are no longer able to present Salmonella antigens through MHC II molecules to the T helper cells. This mechanism could explain our findings that the relative percentages of CD2+ T and NK cells and of CD4+ T helper cells are diminished. Additionally, Van Parys et al. (41) found in 2012 that MHC I molecules are not affected. In line with those findings, we observed no changes in the cytotoxic T cell population. There were no differences between the zinc feeding groups at 2 dpi: all three different feeding groups showed the same decreases in the relative percentages of CD2+ T and NK cells and of CD4+ T helper cells. Therefore, one could assume that it was not Zn supplementation, but S. Typhimurium, that was responsible for the observed decreases in the level of T cells. However, the piglets were fed the Zn-supplemented diets for only a short time, while it takes about 14 days to observe differences in tissue Zn concentrations (7). Indeed, at 14 dpi, a lower frequency of CD8high cytotoxic T cells in blood was observed for the HZn group. Thus, increased homing of the cytotoxic T cells to the infection site could have occurred in this group. The transient and potentially beneficial effect of increased Zn intake diminished after a longer time. Six weeks of feeding the high level of ZnO resulted in decreases in the numbers of different T cells in the mesenteric lymph nodes, indicating a suppressive effect of Zn, comparable to the situation at the edge of deficiency in the LZn group.
In summary, supplementation of the feed with ZnO at a level of 2,500 mg Zn/kg of diet immediately after weaning could positively affect piglets infected with Salmonella Typhimurium, but the beneficial effect may last for a short period only. After 2 weeks of feeding the high dose of 2,500 mg Zn/kg of diet, all positive effects disappear, and rather negative effects occur, such as higher shedding of salmonellae, lower T cell frequencies, and a lower average body weight gain. Thus, when ZnO is used at high levels in the pig industry, it should be fed no longer than 2 to 3 weeks.
ACKNOWLEDGMENTS
We acknowledge the animal welfare officer Mechthild Ladwig for her fruitful input during the preparation of the animal trial. We thank Robert Pieper, Freie Universität Berlin, for providing the piglets and the diets. We further thank the team of Stefanie Banneke, of the Research Institute for Risk Assessment, Berlin, Germany, for the excellent animal care provided during the experiment, and Enno Luge and Maiken Kroll for technical support during sampling. We also thank all the technical staff of the lab of the Unit Molecular Diagnostics and Genetics for their valuable engagement.
This study was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG]) within Collaborative Research Group (Sonderforschungsbereich [SFB]) 852/1, “Nutrition and intestinal microbiota–host interactions in the pig.”
The authors are solely responsible for the data presented here and do not represent the opinions either of the DFG or of any other public or commercial entity.
Footnotes
Published ahead of print 22 February 2013
REFERENCES
- 1. Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. 2012. Zinc and human health: an update. Arch. Toxicol. 86:521–534 [DOI] [PubMed] [Google Scholar]
- 2. Fraker PJ, King LE, Laakko T, Vollmer TL. 2000. The dynamic link between the integrity of the immune system and zinc status. J. Nutr. 130:1399S–1406S [DOI] [PubMed] [Google Scholar]
- 3. Gupta DN, Mondal SK, Ghosh S, Rajendran K, Sur D, Manna B. 2003. Impact of zinc supplementation on diarrhoeal morbidity in rural children of West Bengal, India. Acta Paediatr. 92:531–536 [PubMed] [Google Scholar]
- 4. Rahman MM, Vermund SH, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. 2001. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ 323:314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Research Council 1998. Nutrient requirements of swine, 10th rev ed, p 56 National Academy Press, Washington, DC [Google Scholar]
- 6. Gesellschaft für Ernährungsphysiologie der Haustiere Ausschuss für Bedarfsnormen 2006. Energie- und Nährstoffbedarf landwirtschaftlicher Nutztiere, vol 4 Empfehlungen zur Energie- und Nährstoffversorgung der Schweine, 10th ed DLG-Verlag, Frankfurt am Main, Germany [Google Scholar]
- 7. Poulsen HD. 1995. Zinc oxide for weanling piglets. Acta Agric. Scand. Sect. A 45:159–167 [Google Scholar]
- 8. Richards JD, Zhao J, Harrell RJ, Atwell CA, Dibner JJ. 2010. Trace mineral nutrition in poultry and swine. Asian-Aust. J. Anim. Sci. 23:1527–1534 [Google Scholar]
- 9. European Community 26 July 2003. Commission Regulation (EC) no. 1334/2003 of 25 July 2003 amending the conditions for authorisation of a number of additives in feedingstuffs belonging to the group of trace elements. Off. J. Eur. Union L 187:11–15 http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003R1334:EN:HTML [Google Scholar]
- 10. Molist F, Hermes RG, de Segura AG, Martin-Orue SM, Gasa J, Manzanilla EG, Perez JF. 2011. Effect and interaction between wheat bran and zinc oxide on productive performance and intestinal health in post-weaning piglets. Br. J. Nutr. 105:1592–1600 [DOI] [PubMed] [Google Scholar]
- 11. Shelton NW, Tokach MD, Nelssen JL, Goodband RD, Dritz SS, Derouchey JM, Hill GM. 2011. Effects of copper sulfate, tri-basic copper chloride, and zinc oxide on weanling pig performance. J. Anim. Sci. 89:2440–2451 [DOI] [PubMed] [Google Scholar]
- 12. Højberg O, Canibe J, Poulsen HD, Hedemann MS, Jensen BB. 2005. Influence of dietary zinc oxide and copper sulfate on the intestinal ecosystem in newly weaned piglets. Appl. Environ. Microbiol. 71:2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vahjen W, Pieper R, Zentek J. 2011. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. J. Anim. Sci. 89:2430–2439 [DOI] [PubMed] [Google Scholar]
- 14. Pieper R, Vahjen W, Neumann K, Van Kessel AG, Zentek J. 2012. Dose-dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 96:825–833 [DOI] [PubMed] [Google Scholar]
- 15. Kaiser P, Hardt WD. 2011. Salmonella Typhimurium diarrhea: switching the mucosal epithelium from homeostasis to defense. Curr. Opin. Immunol. 23:456–463 [DOI] [PubMed] [Google Scholar]
- 16. Hallstrom K, McCormick BA. 2011. Salmonella interaction with and passage through the intestinal mucosa: through the lens of the organism. Front. Microbiol. 2:88 doi:10.3389/fmicb.2011.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szabó I, Wieler LH, Tedin K, Scharek-Tedin L, Taras D, Hensel A, Appe B, Noeckler K. 2009. Influence of a probiotic strain of Enterococcus faecium on Salmonella enterica serovar Typhimurium DT104 infection in a porcine animal infection model. Appl. Environ. Microbiol. 75:2621–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kreuzer S, Janczyk P, Assmus J, Schmidt MF, Brockmann GA, Nockler K. 2012. No beneficial effects evident for Enterococcus faecium NCIMB 10415 in weaned pigs infected with Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 78:4816–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Food Safety Authority 2010. The Community Summary Report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in the European Union in 2008. EFSA J. 8(1):1496 doi:10.2903/j.efsa.2010.1496 [Google Scholar]
- 20. Fravalo P, Hascoet Y, le Fellic M, Queguiner S, Petton J, Salvat G. 2003. Convenient method for rapid and quantitative assessment of Salmonella enterica contamination: the mini-MSRV MPN technique. J. Rapid Methods Automat. Microbiol. 11:81–88 [Google Scholar]
- 21. Malorny B, Paccassoni E, Fach P, Bunge C, Martin A, Helmuth R. 2004. Diagnostic real-time PCR for detection of Salmonella in food. Appl. Environ. Microbiol. 70:7046–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kreuzer S, Machnowska P, Assmus J, Sieber M, Pieper R, Schmidt MF, Brockmann GA, Scharek-Tedin L, Johne R. 2012. Feeding of the probiotic bacterium Enterococcus faecium NCIMB 10415 differentially affects shedding of enteric viruses in pigs. Vet. Res. 43:58 doi:10.1186/1297-9716-43-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Boxstael S, Dierick K, Van Huffel X, Uyttendaele M, Berkvens D, Herman L, Bertrand S, Wildemauwe C, Catry B, Butaye P, Imberechts H. 2012. Comparison of antimicrobial resistance patterns and phage types of Salmonella Typhimurium isolated from pigs, pork and humans in Belgium between 2001 and 2006. Food Res. Int. 45:913–918 [Google Scholar]
- 24. Sinkora M, Butler JE. 2009. The ontogeny of the porcine immune system. Dev. Comp. Immunol. 33:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carlson MS, Hill GM, Link JE. 1999. Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: effect on metallothionein and mineral concentrations. J. Anim. Sci. 77:1199–1207 [DOI] [PubMed] [Google Scholar]
- 26. Hollis GR, Carter SD, Cline TR, Crenshaw TD, Cromwell GL, Hill GM, Kim SW, Lewis AJ, Mahan DC, Miller PS, Stein HH, Veum TL. 2005. Effects of replacing pharmacological levels of dietary zinc oxide with lower dietary levels of various organic zinc sources for weanling piglets. J. Anim. Sci. 83:2123–2129 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Tang JW, Ma WQ, Feng J, Feng J. 26 June 2009. Dietary zinc glycine chelate on growth performance, tissue mineral concentrations, and serum enzyme activity in weanling piglets. Biol. Trace Elem. Res. [Epub ahead of print.] doi:10.1007/s12011-009-8437-3 [DOI] [PubMed] [Google Scholar]
- 28. Janczyk P, Pieper R, Smidt H, Souffrant WB. 2010. Effect of alginate and inulin on intestinal microbial ecology of weanling pigs reared under different husbandry conditions. FEMS Microbiol. Ecol. 72:132–142 [DOI] [PubMed] [Google Scholar]
- 29. Surjawidjaja JE, Hidayat A, Lesmana M. 2004. Growth inhibition of enteric pathogens by zinc sulfate: an in vitro study. Med. Princ. Pract. 13:286–289 [DOI] [PubMed] [Google Scholar]
- 30. Jensen-Waern M, Melin L, Lindberg R, Johannisson A, Petersson L, Wallgren P. 1998. Dietary zinc oxide in weaned pigs—effects on performance, tissue concentrations, morphology, neutrophil functions and faecal microflora. Res. Vet. Sci. 64:225–231 [DOI] [PubMed] [Google Scholar]
- 31. Bratz K, Gölz G, Alter T, Bücker R, Janczyk P, Nöckler K. 2012. Effects of dietary zinc oxide and Enterococcus faecium NCIMB 10415 supplementation on Campylobacter coli levels in weaned piglets. Int. J. Med. Microbiol. 302:56 (Abstract.) [Google Scholar]
- 32. Mosquito S, Ruiz J, Pons MJ, Durand D, Barletta F, Ochoa TJ. 2012. Molecular mechanisms of antibiotic resistance in diarrhoeagenic Escherichia coli isolated from children, Int. J. Antimicrob. Agents 40:544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsu S-C, Chiu T-H, Pang J-C, Hsuan-Yuan C-H, Chang G-N, Tsen H-Y. 2006. Characterisation of antimicrobial resistance patterns and class 1 integrons among Escherichia coli and Salmonella enterica serovar Choleraesuis strains isolated from humans and swine in Taiwan. Int. J. Antimicrob. Agents 27:383–391 [DOI] [PubMed] [Google Scholar]
- 34. Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Bäumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, Pickard DJ, Barthel M, Westendorf AM, Krogfelt KA, Walker AW, Ackermann M, Dobrindt U, Thomson NR, Hardt WD. 2012. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. U. S. A. 109:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruiz J, Castro D, Goni P, Santamaria JA, Borrego JJ, Vila J. 1997. Analysis of the mechanism of quinolone resistance in nalidixic acid-resistant clinical isolates of Salmonella serotype Typhimurium. J. Med. Microbiol. 46:623–628 [DOI] [PubMed] [Google Scholar]
- 37. Boyd DA, Peters GA, Ng L, Mulvey MR. 2000. Partial characterization of a genomic island associated with the multidrug resistance region of Salmonella enterica Typhimurium DT104. FEMS Microbiol. Lett. 189:285–291 [DOI] [PubMed] [Google Scholar]
- 38. Vo ATT, van Duijkeren A, Gaastra W, Fluit AC. 2010. Antimicrobial resistance, class 1 integrons, and genomic island 1 in Salmonella isolates from Vietnam. PLoS One 5:e9440 doi:10.1371/journal.pone.0009440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doublet B, Boyd D, Mulvey MR, Cloeckaert A. 2005. The Salmonella genomic island 1 is an integrative mobilizable element. Mol. Microbiol. 55:1911–1924 [DOI] [PubMed] [Google Scholar]
- 40. Misselwitz B, Barrett N, Kreibich S, Vonaesch P, Andritschke D, Rout S, Weidner K, Sormaz M, Songhet P, Horvath P, Chabria M, Vogel V, Spori DM, Jenny P, Hardt WD. 2012. Near surface swimming of Salmonella Typhimurium explains target-site selection and cooperative invasion. PLoS Pathog. 8:e1002810 doi:10.1371/journal.ppat.1002810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Parys A, Boyen F, Verbrugghe E, Leyman B, Bram F, Haesebrouck F, Pasmans F. 2012. Salmonella Typhimurium induces SPI-1 and SPI-2 regulated and strain dependent downregulation of MHC II expression on porcine alveolar macrophages. Vet. Res. 43:52 doi:10.1186/1297-9716-43-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Müller AJ, Kaiser P, Dittmar KE, Weber TC, Haueter S, Endt K, Songhet P, Zellweger C, Kremer M, Fehling HJ, Hardt WD. 2012. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe 11:19–32 [DOI] [PubMed] [Google Scholar]