Abstract
Rhodococcus opacus PD630, which is known for its ability to accumulate large amounts of triacylglycerols (TAG), was metabolically engineered, and a cellobiose utilization pathway was introduced. Activities of β-glucosidases were determined, and recombinant strains accumulated fatty acids up to 39.5 ± 5.7% (wt/wt) of cell dry mass from cellobiose.
TEXT
Today's first-generation biofuels are mainly derived from edible plant parts, such as sugar cane and corn, and their production is associated with numerous economical, ecological, and social problems (1). The microbial conversion of abundant lignocellulosic biomass into so-called second-generation biofuels in an economically competitive process is regarded as a key challenge for sustainable fuel production (2, 3). Cellulose, the homopolymer of 1,4-β-linked d-glucose, is the major component of lignocellulose (40 to 50%). The key steps in cellulose degradation and subsequent fermentation include the saccharification of the polymeric substrate into simple sugars, usually mediated by the action of at least three enzymes (endoglucanase [E.C. 3.2.1.4], exoglucanase [E.C. 3.2.1.91], and β-glucosidase [E.C. 3.2.1.21]) that act in a synergistic manner (4, 5). For fermentation of cellulosic materials, these enzymes are usually produced in a separate process and thereby represent the second highest raw material expense factor in such fermentation process after the feedstock itself (6). The process involving simultaneous saccharification and fermentation (SSF), also known as consolidated bioprocessing (CBP), is regarded as potential alternative to dedicated enzyme production, as it combines both saccharification of lignocellulosic materials and fermentation of the released sugars in one microorganism (4, 7). However, several challenges must still be overcome to achieve economically viable production processes (8), and to date no production of cellulosic biofuels on a commercial scale has been established (9).
Rhodococcus opacus strain PD630 is the model oleaginous prokaryote for accumulation and biosynthesis of lipids, which serve as carbon and energy storage and can account for up to 87% of the cell dry mass in this strain (10–12). It has been considered as a production strain for high-value triacylglycerols (TAGs) from renewable resources for the production of biodiesel, monoalkyl esters of short-chain alcohols, and long-chain fatty acids, due to its high substrate tolerance, its ability to be cultivated to high densities, and its rapid growth, which make it favorable over other production organisms (12–14). Unfortunately, this strain does not use cellobiose (1,4-β-d-glucopyranosyl-d-glucopyranose), the main product of cellulases, as the sole carbon and energy source (15, 16).
Strategies to establish cellobiose utilization in R. opacus PD630.
All bacteria, plasmids, and primers used in this study are listed in Tables 1 and 2. Previous studies concluded that the cellobiose deficiency of R. opacus PD630 is due to a missing active glycoside hydrolase enzyme (15). In order to establish cellobiose utilization in R. opacus PD630, three different strategies were applied. The first strategy, employing two extracellular β-glucosidases (BGL) from Rhodococcus erythropolis DSM43066 and Gordonia polyisoprenivorans VH2, which can both use cellobiose as the sole carbon and energy source, aimed at the extracellular cleavage of cellobiose and subsequent uptake of the generated glucose. The second strategy attempted to complement the lack of a suitable cytoplasmic β-glucosidase. Despite being noncellulolytic, Escherichia coli is known to possess cryptic genes for cellobiose utilization (21) and also a characterized periplasmic β-glucosidase enzyme (BglX) (22), which was chosen for this study. The third strategy conferred both a transporter and a β-glucosidase, using the bglABC operon of Thermobifida fusca, to R. opacus. This operon, first described and partially characterized by Spiridonov and Wilson (23), comprises genes encoding two ABC sugar transport proteins (BglA and BglB) and a cytoplasmic β-glucosidase (BglC).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli Mach1-T1 | F− ϕ80(lacZ)ΔM15 ΔlacX74 hsdR(rK− mK+) ΔrecA1398 endA1 tonA | Invitrogen (Karlsbad, Germany) |
| R. opacus PD630 | TAG-producing strain | 10 |
| R. erythropolis DSM43066 | Cellobiose utilization | 17 |
| T. fusca DSM43792 | Cellobiose utilization | 18 |
| G. polyisoprenivorans VH2 | Cellobiose utilization | 19 |
| Plasmids | ||
| pEC-K18mob2 | 20 | |
| pEC-K18mob2::bglRE | bglRE as a BamHI/XbaI fragment | This study |
| pEC-K18mob2::bglGI | bglGI as an XbaI fragment | This study |
| pEC-K18mob2::bglXEC-SP | bglXEC-SP as an EcoRI/KpnI fragment | This study |
| pEC-K18mob2::bglABCTF | bglABCTF as an EcoRI/XbaI fragment | This study |
| pEC-K18mob2::bglABTF | bglABTF as an EcoRI/SacI fragment | This study |
| pEC-K18mob2::bglBCTF | bglBCTF as an EcoRI/XbaI fragment | This study |
Table 2.
Oligonucleotides used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| FbglRER_BamHI | AAAGGATCCGGGAGCTCCTTGATGGCACTGACGTGCCTGCT |
| RbglRER_XbaI | AAATCTAGATCATCGAGTAGCCGTACAGCTGCG |
| FbglVH2_XbaI | AAATCTAGAGGAAGAGGACCCCATGAGCCGACCTCACCACC |
| RbglVH2_XbaI | AAATCTAGACTAGAGCTGTGCCCGCGGCC |
| FbglX_EcoRI | AAAGAATTCGGGAGCTCCTTGATGGATTTATTCGGCAACCATCCATTAA |
| RbglX_KpnI | AAGGTACCTTACAGCAACTCAAACTCGCCTTTCTTAACG |
| FbglABC_EcoRI | AAAGAATTCGGCCGTCCTCTCTTCCATCTGACATCTGACCTCTC |
| RbglABC_XbaI | AAATCTAGAGCCGCCGGGACGGCGAGATTTTGACCTATC |
| RbglAB_SacI | AAAGAGCTCTCACTTAATGGCACCTTCCATGATCC |
| FbglBC_EcoRI | AAGAATTCGGGAGCTCCTTGATGGCTGCGACTTCGACCCC |
Restriction sites are underlined.
For cloning of the respective genes, the coding regions of bglRE from R. erythropolis, bglGI from G. polyisoprenivorans, and bglABCTF, bglABTF, and bglBCTF from T. fusca were amplified by PCR using the oligonucleotides FbglRER_BamHI and RbglRER_XbaI for bglRE, FbglVH2_XbaI and RbglVH2_XbaI for bglGI, FbglABC_EcoRI and RbglABC_XbaI for bglABCTF, FbglABC-EcoRI and RbglAB_SacI for bglABTF, and FbglBC_EcoRI and RbglABC_XbaI for bglBCTF (Table 2). Additionally, the bglXEC gene from E. coli was amplified by PCR using the oligonucleotides FbglX-EcoRI and RbglX_KpnI, thereby omitting the signal peptide for periplasmic location. The truncated gene was designated bglXEC-SP. For expression experiments, all fragments were ligated to the E. coli/Corynebacterium glutamicum shuttle vector pEC-K18mob2 (20) using the respective restriction enzymes (Table 2), yielding plasmids pEC-K18mob2::bglRE, pEC-K18mob2::bglGI, pEC-K18mob2::bglXEC-SP, pEC-K18mob2::bglABCTF (Fig. 1), pEC-K18mob2::bglABTF, and pEC-K18mob2::bglBCTF. All plasmids were analyzed by sequencing and later transferred to R. opacus PD630 by electroporation according to the previously described protocol (24).
Fig 1.
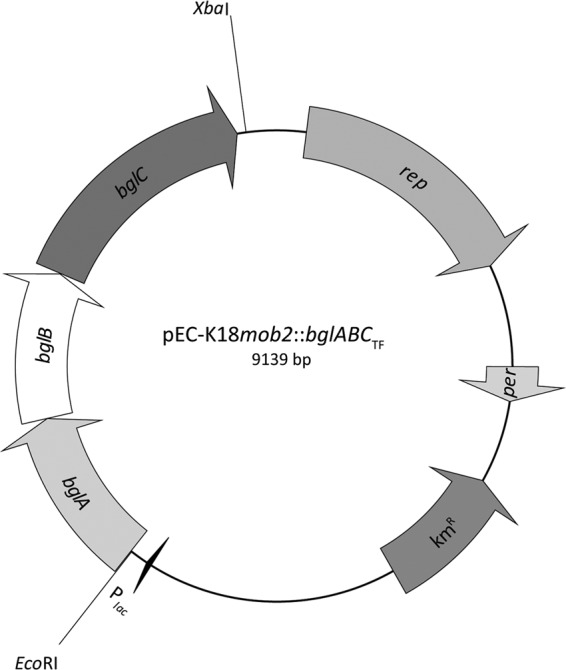
Physical map of the constructed plasmid pEC-K18mob2::bglABCTF. Relevant cleavage sites and structural genes are indicated (rep, origin of replication; per, positive effector of replication; kmR, kanamycin resistance cassette; Plac, lac promoter; bglABC operon encoding two sugar transporters (bglAB, accession no. YP_288996 and YP_288997) and a β-glucosidase (bglC, accession no. YP_288998) from T. fusca.
Recombinant strains were cultivated both on solid and in liquid MSM (14) with 1% (wt/vol) glucose and/or cellobiose as the carbon source. Only recombinant strains harboring pEC-K18mob2::bglABCTF exhibited significant growth with cellobiose, whereas all other strains grew like the wild type. When R. opacus pEC-K18mob2::bglABCTF was cultivated in liquid MSM containing different concentrations (1, 1.7, or 4%, wt/vol) of cellobiose as the sole carbon source, similar growth for all cultures was observed (μ = 0.021 to 0.025 h−1) (Fig. 2). However, cultures with 1.7 and 4% (wt/vol) cellobiose exhibited a shorter lag phase and higher final optical densities than cultures with 1% (wt/vol) cellobiose.
Fig 2.
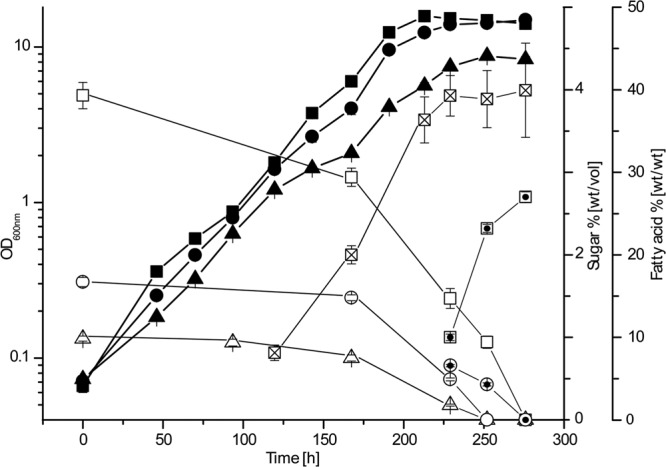
Growth of the recombinant strain R. opacus PD630 pEC-K18mob2::bglABCTF in the presence of different cellobiose concentrations. Cells were cultivated in liquid MSM containing 1, 1.7, or 4% (wt/vol) cellobiose as the sole carbon source. ▲, 1% (wt/vol) cellobiose; ●, 1.7% (wt/vol) cellobiose; ■, 4% (wt/vol) cellobiose. Cellobiose concentrations in the medium: △, 1% (wt/vol) culture; ○, 1.7% (wt/vol) culture; □, 4% (wt/vol) culture. Glucose concentrations in the medium: ⊙, 1.7% (wt/vol) culture; ⊡, 4% (wt/vol) culture. Fatty acid content: ☒, 4% (wt/vol) culture. Error bars indicate standard deviations of triplicate measurements.
High-performance liquid chromatography (HPLC) analysis of medium cellobiose contents was done as follows. Culture media were centrifuged at 14,000 × g to remove cells. Supernatants were filtered using Spartan 0.2-μm filters (Whatman, Dassel, Germany) and applied to a Eurokat Pb column (30GX350EKN; Knauer, Berlin, Germany) using water-acetonitrile (95:5) as the eluent at 75°C and a flow rate of 1 ml min−1. The HPLC system used comprises a Kontron system 522 pump and an HPLC 560 autosampler (Kontron, München, Germany) as well as a Sedex 80 LT-ELS detector (Sedere, Alfortville, France). Determination of cellobiose contents of the culture supernatants revealed that cellobiose was utilized only by growing cells of strain R. opacus PD630 pEC-K18mob2::bglABCTF. After 250 h cultivation, cellobiose was completely consumed in the 1% culture (Fig. 2), whereas in the 1.7 and 4% cultures, glucose accumulated in the medium after a cultivation period of 229 h, most likely as a result of cell death and subsequent leakage of BglC into the medium. Glucose levels reached 0.66 ± 0.01% and 2.73 ± 0.06% (wt/vol) in the 1.7 and 4% cultures, respectively. The latter was higher than what was expected; however, the low remaining volume led to accelerated evaporation of the medium. In general, growth of recombinant R. opacus PD630 pEC-K18mob2::bglABCTF was considerably slower with cellobiose than that of wild-type R. opacus PD630 with sucrose or glucose (μ = 0.088 h−1 and μ = 0.072 h−1, respectively [25]). Taking into account the fact that low growth rates could also be observed in complex media, this provides evidence that expression of bglABCTF represents a strong metabolic burden to the cells, thereby decreasing consumption and growth rates. Interestingly, the additional stabilization of the plasmid by kanamycin resulted in a significantly shorter lag-phase of recombinant strains (data not shown).
BGL enzyme assays.
Activity of β-glucosidases was determined as described by Adin et al. (26). Enzyme assays of the soluble protein fractions obtained from cells of the recombinant strain of R. opacus PD630 harboring pEC-K18mob2::bglABCTF or pEC-K18mob2::bglXEC-SP demonstrated the presence of active BGL (0.881 ± 0.011 U mg−1 and 0.003 ± 0.0001 U mg−1, respectively, at 30°C), whereas no activity was detected in the soluble protein fraction of the control strain PD630 harboring plasmid pEC-K18mob2. This finding confirmed the assumption, made by Holder et al., that a functional glycosyl hydrolase enzyme is lacking in R. opacus PD630 (15), although the experimental results further demonstrated that a suitable sugar permease is also required for growth. Interestingly, the activity of BglXEC-SP was surprisingly low, demonstrating that the removal of the signal peptide significantly affected the activity of the enzyme. However, it must also be taken into account that the heterologous expression of a gene with no actinomycete origin and thus a different G+C content (54% for bglX) might negatively influence its expression level in R. opacus PD630. Consistent with the growth characteristics of recombinant strains, no BGL activity was detected in culture supernatants or in the soluble protein fractions of strains which expressed the genes for the extracellular β-glucosidases BglGI and BglRE.
Analysis of storage lipids.
Determination of the TAG content was performed as described in detail elsewhere (14, 27). Qualitative analysis by thin-layer chromatography revealed a spot corresponding to the triolein standard for all three cellobiose cultivations. In addition, the fatty acid content of cells cultivated with 4% (wt/vol) cellobiose was determined at different time points (Fig. 2), which increased from 8.13 ± 0.94% (wt/wt) at 167 h to 39.27 ± 2.45% (wt/wt) after 229 h of cultivation and remained almost constant until the cultivation ended. These values are clearly lower than the lipid levels that can be achieved with sucrose or gluconate as carbon sources, but they reflect on the one hand the basic metabolic rate for nitrogen accompanied by the long cultivation time and on the other hand the elevated nitrogen content of the medium (0.1% [wt/vol]) that was employed to stimulate cell growth.
Conclusions.
In the present study, we successfully conferred the ability to utilize cellobiose to R. opacus PD630. However, future investigations are needed to improve both growth and lipid storage of recombinant strains. Experiments already indicate that the reduction of the bglABC expression level by, e.g., the addition of genes upstream of the bglABC operon or expression by low-copy-number vectors resulted in both faster growth and increased levels of lipid accumulation by recombinant strains (unpublished data). In addition, especially with regard to SSF, i.e., combination with cellulase genes, the integration of the cellobiose utilization genes into the genome of R. opacus PD630 is desirable.
ACKNOWLEDGMENT
We are grateful to NesteOil for funding this study.
Footnotes
Published ahead of print 22 February 2013
REFERENCES
- 1. Tilman D, Socolow R, Foley JA, Hill J, Larson E, Lynd L, Pacala S, Reilly J, Searchinger T, Somerville C, Williams R. 2009. Beneficial biofuels—the food, energy, and environment trilemma. Science 325:270–271 [DOI] [PubMed] [Google Scholar]
- 2. Lee SK, Chou H, Ham TS, Lee TS, Keasling JD. 2008. Metabolic engineering of microorganisms for biofuels production: from bugs to synthetic biology to fuels. Curr. Opin. Biotechnol. 19:556–563 [DOI] [PubMed] [Google Scholar]
- 3. Stephanopoulos G. 2007. Challenges in engineering microbes for biofuels production. Science 315:801–804 [DOI] [PubMed] [Google Scholar]
- 4. Lynd LR, van Zyl WH, McBride JE, Laser M. 2005. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16:577–583 [DOI] [PubMed] [Google Scholar]
- 5. Mosier NS, Hall P, Ladisch CM, Ladisch MR. 1999. Reaction kinetics, molecular action, and mechanisms of cellulolytic proteins. Adv. Biochem. Eng. Biotechnol. 65:23–40 [DOI] [PubMed] [Google Scholar]
- 6. Bokinsky G, Peralta-Yahya PP, George A, Holmes BM, Steen EJ, Dietrich J, Soon Lee T, Tullman-Ercek D, Voigt CA, Simmons BA, Keasling JD. 2011. Synthesis of three advanced biofuels from ionic liquid-pretreated switchgrass using engineered Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 108:19949–19954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang YH, Lynd LR. 2004. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol. Bioeng. 88:797–824 [DOI] [PubMed] [Google Scholar]
- 8. Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan J, Wyman CE. 2008. How biotech can transform biofuels. Nat. Biotechnol. 26:169–172 [DOI] [PubMed] [Google Scholar]
- 9. Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS. 2012. Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol. 30:274–282 [DOI] [PubMed] [Google Scholar]
- 10. Alvarez HM, Mayer F, Fabritius D, Steinbüchel A. 1996. Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch. Microbiol. 165:377–386 [DOI] [PubMed] [Google Scholar]
- 11. Alvarez HM, Steinbüchel A. 2002. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60:367–376 [DOI] [PubMed] [Google Scholar]
- 12. Kurosawa K, Boccazzi P, de Almeida NM, Sinskey AJ. 2010. High-cell-density batch fermentation of Rhodococcus opacus PD630 using a high glucose concentration for triacylglycerol production. J. Biotechnol. 147:212–218 [DOI] [PubMed] [Google Scholar]
- 13. Finnerty WR. 1992. The biology and genetics of the genus Rhodococcus. Annu. Rev. Microbiol. 46:193–218 [DOI] [PubMed] [Google Scholar]
- 14. Wältermann M, Luftmann H, Baumeister D, Kalscheuer R, Steinbüchel A. 2000. Rhodococcus opacus strain PD630 as a new source of high-value single-cell oil? Isolation and characterization of triacylglycerols and other storage lipids. Microbiology 146:1143–1149 [DOI] [PubMed] [Google Scholar]
- 15. Holder JW, Ulrich JC, DeBono AC, Godfrey PA, Desjardins CA, Zucker J, Zeng Q, Leach AL, Ghiviriga I, Dancel C, Abeel T, Gevers D, Kodira CD, Desany B, Affourtit JP, Birren BW, Sinskey AJ. 2011. Comparative and functional genomics of Rhodococcus opacus PD630 for biofuels development. PLoS Genet. 7:e1002219 doi:10.1371/journal.pgen.1002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santos SC, Alviano DS, Alviano CS, Goulart FR, de Padula M, Leitao AC, Martins OB, Ribeiro CM, Sassaki MY, Matta CP, Bevilaqua J, Sebastian GV, Seldin L. 2007. Comparative studies of phenotypic and genetic characteristics between two desulfurizing isolates of Rhodococcus erythropolis and the well-characterized R. erythropolis strain IGTS8. J. Ind. Microbiol. Biotechnol. 34:423–431 [DOI] [PubMed] [Google Scholar]
- 17. Goodfellow M, Alderson G, Lacey J. 1979. Numerical taxonomy of Actinomadura and related actinomycetes. J. Gen. Microbiol. 112:95–111 [DOI] [PubMed] [Google Scholar]
- 18. McCarthy AJ, Cross T. 1984. A taxonomic study of Thermomonospora and other monosporic actinomycetes. J. Gen. Microbiol. 130:5–25 [Google Scholar]
- 19. Linos A, Steinbüchel A, Spröer C, Kroppenstedt RM. 1999. Gordonia polyisoprenivorans sp. nov., a rubber-degrading actinomycete isolated from an automobile tire. Int. J. Syst. Bacteriol. 49:1785–1791 [DOI] [PubMed] [Google Scholar]
- 20. Tauch A, Kirchner O, Loffler B, Gotker S, Pühler A, Kalinowski J. 2002. Efficient electrotransformation of Corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr. Microbiol. 45:362–367 [DOI] [PubMed] [Google Scholar]
- 21. Kachroo AH, Kancherla AK, Singh NS, Varshney U, Mahadevan S. 2007. Mutations that alter the regulation of the chb operon of Escherichia coli allow utilization of cellobiose. Mol. Microbiol. 66:1382–1395 [DOI] [PubMed] [Google Scholar]
- 22. Yang M, Luoh SM, Goddard A, Reilly D, Henzel W, Bass S. 1996. The bglX gene located at 47.8 min on the Escherichia coli chromosome encodes a periplasmic beta-glucosidase. Microbiology 142:1659–1665 [DOI] [PubMed] [Google Scholar]
- 23. Spiridonov NA, Wilson DB. 2001. Cloning and biochemical characterization of BglC, a beta-glucosidase from the cellulolytic actinomycete Thermobifida fusca. Curr. Microbiol. 42:295–301 [DOI] [PubMed] [Google Scholar]
- 24. Kalscheuer R, Arenskötter M, Steinbüchel A. 1999. Establishment of a gene transfer system for Rhodococcus opacus PD630 based on electroporation and its application for recombinant biosynthesis of poly(3-hydroxyalkanoic acids). Appl. Microbiol. Biotechnol. 52:508–515 [DOI] [PubMed] [Google Scholar]
- 25. Voss I, Steinbüchel A. 2001. High cell density cultivation of Rhodococcus opacus for lipid production at a pilot-plant scale. Appl. Microbiol. Biotechnol. 55:547–555 [DOI] [PubMed] [Google Scholar]
- 26. Adin DM, Visick KL, Stabb EV. 2008. Identification of a cellobiose utilization gene cluster with cryptic beta-galactosidase activity in Vibrio fischeri. Appl. Environ. Microbiol. 74:4059–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brandl H, Gross RA, Lenz RW, Fuller RC. 1988. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 54:1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]


