Abstract
Chlorine was assessed as a reconditioning agent and wash water disinfectant in the fresh-cut produce industry. Artificial fresh-cut lettuce wash water, made from butterhead lettuce, was used for the experiments. In the reconditioning experiments, chlorine was added to artificial wash water inoculated with Escherichia coli O157 (6 log CFU/ml). Regression models were constructed based on the inactivation data and validated in actual wash water from leafy vegetable processing companies. The model that incorporated chlorine dose and chemical oxygen demand (COD) of the wash water accurately predicted inactivation. Listeria monocytogenes was more resistant to chlorine reconditioning in artificial wash water than Salmonella spp. and Escherichia coli O157. During the washing process with inoculated lettuce (4 log CFU/g), in the absence of chlorine, there was a rapid microbial buildup in the water that accumulated to 5.4 ± 0.4 log CFU/100 ml after 1 h. When maintaining a residual concentration of 1 mg/liter free chlorine, wash water contamination was maintained below 2.7, 2.5, and 2.5 log CFU/100 ml for tap water and artificial process water with COD values of 500 and 1,000 mg O2/liter, respectively. A model was developed to predict water contamination during the dynamic washing process. Only minor amounts of total trihalomethanes were formed in the water during reconditioning. Total trihalomethanes accumulated to larger amounts in the water during the wash water disinfection experiments and reached 124.5 ± 13.4 μg/liter after 1 h of execution of the washing process in water with a COD of 1,000 mg O2/liter. However, no total trihalomethanes were found on the fresh-cut lettuce after rinsing.
INTRODUCTION
Consumer demand for convenient, ready-to-eat, fresh-cut produce has continuously increased (1). However, fresh produce is relatively vulnerable to pathogen contamination (2), and leafy greens are among the most frequently implicated fresh produce in produce-associated outbreaks (2–4), which have been related mostly to Escherichia coli O157:H7 or Salmonella spp. (5, 6).
Minimal processing of lettuce consists of a series of steps including harvesting, cold storage, trimming, shredding, washing/rinsing, draining, packaging, cold storage, and distribution (7). The washing is done to remove dirt, foreign materials, tissue fluids from cut surfaces, and microorganisms. As fresh-cut lettuce does not undergo intensive inactivation or preservation treatments during processing, washing is the only processing step that reduces the microbial load on the lettuce (6, 8). Washing with potable water removes microorganisms to some degree, a process which can be enhanced by using sanitizers for disinfection of produce, i.e., decontamination (9).
Chlorine is the most used water disinfectant in general and also specifically in the fresh-cut produce industry for wash, spray, or flume waters due to the low cost, the reliable availability, the good effectiveness against suspended vegetative bacteria and some enteric viruses, and the minimal impact on the nutritional and sensorial quality of the produce (3, 6, 10). However, the efficiency of chlorine as a sanitizer for lettuce decontamination is generally limited to 1- to 2-log reductions, even at high chlorine concentrations (2, 7, 11). The common applied free chlorine concentrations in washing processes are in the range of 50 to 200 mg/liter. Contact times of 1 to 2 min and pH values of between 6 and 7.5 ensure the presence of chlorine in the hypochlorous acid form and yet minimize corrosion of equipment (7, 10). The use of high chlorine concentrations may cause the generation of chlorine gas in the production facilities and may lead to the production of excessive amounts of harmful disinfection by-products (DBPs) in the water (3, 12–15). Partially due to the possible generation of DBPs, the use of chlorine in fresh-cut produce washing is prohibited altogether in some European Union countries such as Germany, Switzerland, the Netherlands, Denmark, and Belgium (7, 8, 16).
However, washing of produce is a potential pathway for spreading contamination among crops (17), and the risk of cross-contamination is not removed by using large quantities of water (18). Chlorine is much more effective for inactivation of bacterial pathogens in wash water than for removal of these pathogens from fresh produce (11, 19). Therefore, chlorine was assessed in this study for two water disinfection strategies, with the aim of maintaining the microbial wash water quality without purposefully targeting fresh-cut lettuce. First, chlorination was used as a reconditioning treatment. This can be interpreted as a disinfection treatment of the water, separated from the washing bath, with subsequent reuse of the water. Second, chlorine was used as a wash water disinfectant in a dynamic washing process, as up until now, cross-contamination studies have been designed mostly as one-time-event experiments, i.e., adding free chlorine once initially and simulating a washing process at a single moment. Therefore, there is a lack of information concerning the impact, after prolonged exposure, of maintaining a fixed residual free chlorine concentration during a washing process on the microbial and chemical safety of the wash water and if accumulation of DBPs during the washing process leads to transfer to the lettuce. In both setups, the influence of physicochemical parameters was studied by disinfecting in artificial wash waters from butterhead lettuce (Lactuca sativa). During the trials, DBPs were measured to assess the influence of the disinfection processes on chemical safety. In addition, prediction models for E. coli O157 inactivation were made for both the reconditioning and wash water sanitation setups.
MATERIALS AND METHODS
Physicochemical measurements.
Chemical oxygen demand (COD) was measured according to the small-scale sealed-tube method (LCI 400; Hach Lange, Belgium). Turbidity was determined with a turbidimeter (HI98703; Hanna Instruments, Belgium); UV at 254 nm (UV254) was determined by using a UV-visible (UV-Vis) spectrophotometer (UV1601; Shimadzu, Belgium) and quartz cuvettes with a 1-cm path length (Hellma, Belgium), before and after filtration through a 0.45-µm-pore-size polytetrafluoroethylene filter [UV254(F)] (Macherey-Nagel, Belgium); NH4+ was measured with LCK304 from Hach Lange (Germany); Fe2+ and Mn2+ were measured with inductive coupled plasma (Vista-MPX; Varian); and free and total chlorine were measured by using the N,N-diethyl-p-phenylenediamine (DPD) colorimetric method (20).
Standardized process water.
Butterhead lettuce (Lactuca sativa) was purchased from a local market in Belgium and transported under refrigerated conditions to the laboratory for further handling. After discarding the outer leaves, 67 g of lettuce was put into a stomacher bag with a full-surface filter (0.5-mm pore size), 200 ml of tap water was added, and the mixture was homogenized for 2 min. The COD of this suspension was determined, and subsequently, the suspension was diluted with tap water to obtain standardized process water (SPW) containing the desired COD (500, 800, or 1,500 mg O2/liter). Before executing the disinfection experiments, the physicochemical parameters of this SPW were measured.
Wash water from fresh-cut produce companies.
Wash water from two fresh-cut produce companies was collected after 2 h of operation. Water was collected into sterile recipient containers and transported under refrigerated conditions to the laboratory, where it was stored at 4°C for a maximum of 24 h. At company 1, tap water was used as the water source during washing of salad mix and iceberg lettuce. Company 2 utilized bore hole water for processing butterhead lettuce, iceberg lettuce, endive, and radicchio.
Bacterial inoculation.
Two attenuated nalidixic acid-resistant E. coli O157 strains (LFMFP 662 and LFMFP 679) were used. The strains were grown at 37°C for 24 h in brain heart infusion broth (Oxoid, United Kingdom) containing 50 μg/ml nalidixic acid (Sigma-Aldrich, Belgium). Two gentamicin-resistant Salmonella strains were used: a Salmonella enterica serovar Thompson strain (LFMFP 687), which is a clinical isolate that was linked to an outbreak from cilantro (21), and a Salmonella enterica serovar Typhimurium strain (LFMFP 690), which is a natural streptomycin-resistant mutant of Salmonella Typhimurium SL1344 (78). The strains were grown in tryptone soya broth containing 15 μg/ml gentamicin (Sigma-Aldrich, Belgium) for 24 h at 37°C. Three Listeria monocytogenes strains, isolated from green and red peppers (LFMFP 207, LFMFP 233, and LFMFP 680), were used. The strains were grown in brain heart infusion broth for 24 h at 37°C. For each species, a cocktail was made by combining volumes of individual strains. Cocktails were centrifuged at 4°C at 1,800 × g for 10 min. The pellets were washed twice in phosphate buffer (pH 7), with intermittent centrifugation, and subsequently resuspended in phosphate buffer.
Inactivation experiments. (i) Buffered experiment.
Chlorine solutions containing 0.2, 0.3, 0.4, and 0.5 mg/liter free chlorine were made by diluting a chlorine stock solution (28.4 g/liter NaOCl; La Croix, Belgium) in phosphate buffer at pH 6.5 and maintained at a temperature of 5°C. E. coli O157 was added to 100 ml of these solutions to obtain 6 log CFU/ml. The solutions were continuously stirred, and microbial samples were taken after 15-, 30-, and 60-s contact times. Samples were immediately quenched by using Na2S2O3 (0.1 M). E. coli O157 was enumerated by the pour-plating method on Chromocult Coliform-agar (Merck, Belgium) containing 50 μg/ml nalidixic acid. To extend the limit of quantification (LOQ), E. coli O157 was also enumerated by using membrane filtration (22), in which the use of Tergitol 7 was replaced by Coliform Chromocult-agar (Merck, Germany) containing 50 μg/ml nalidixic acid, to increase the specificity of the analysis.
(ii) Reconditioning experiment.
The pH of the SPW was adjusted to 6.5 by using HCl (1 M). Volumes of the pathogens' cocktails were added to the SPW to obtain concentrations of 6 log CFU/ml. The SPW was continuously stirred during the experiment. Disinfection was executed at 5°C in volumes of 100 ml. The necessary amount of chlorine was added in 1 pulse. SPW with COD values of approximately 500, 800, and 1,500 mg O2/liter was exposed to chlorine concentrations of 20, 35, 50, and 75 mg/liter. Samples from the fresh-cut produce companies were chlorinated with 20, 35, and 50 mg/liter chlorine. All experiments were executed in triplicate. Samples for free chlorine measurements were taken simultaneously with the microbial samples and immediately analyzed. Microbial samples were quenched by using Na2S2O3 (0.1 M). E. coli O157 enumeration was done as described above for the buffered experiment, except that membrane filtration was not executed. Specifically, for the inactivation data used for modeling, the LOQ was lowered to 0.5 log CFU/ml by pour plating 3 ml of the nondiluted sample over three plates. Enumeration of Salmonella spp. and Listeria monocytogenes was executed with the spread-plating method (streaking 100 μl of serially diluted sample) on xylose-lysine-desoxycholate (XLD) medium (Oxoid, United Kingdom), containing 15 μg/ml gentamicin, and Brilliance Listeria agar (Oxoid, United Kingdom), respectively.
(iii) Wash water experiment.
Cut lettuce leaves were inoculated overnight with E. coli O157 to obtain ca. 4.0 log CFU/g and subsequently washed. The washing process consisted of washing portions of 50 g of lettuce for 1 min in a washing bath (volume of 4 liters) at temperatures below 7°C by manual stirring. The experiment was done in duplicate. The washing process was continued for 1 h by consecutively passing 50-g portions through the same washing bath. Each portion of lettuce was rinsed with tap water (product-to-water ratio of 1 kg to 1 liter) after washing. The pH of the washing water was reduced to 6.5 with HCl (1 M), and free chlorine was continuously added with a pump to maintain 1 mg/liter of free chlorine in the water. The level of free chlorine was measured each minute, and the flow of the pump was adapted accordingly. The experiment was performed in (i) tap water and (ii) SPW, the latter with COD values of approximately 500 or 1,000 mg O2/liter. The water loss resulting from the lettuce batches exiting the washing bath was measured, and after each batch, the washing bath was refilled with water with the respective COD value to maintain 4 liters in the washing bath for the duration of the trial. Water samples for microbial enumeration were taken. E. coli O157 was enumerated by using membrane filtration (22), in which the use of Tergitol 7 was replaced by Coliform Chromocult-agar (Merck, Germany) containing 50 μg/ml nalidixic acid. The LOQ for experiments in tap water or SPW with a COD of 500 mg O2/liter was 1.7 log CFU/100 ml, and for SPW with a COD of 1,000 mg O2/liter, it was 2.0 log CFU/100 ml. Enumeration of E. coli O157 on lettuce was performed by weighing 10 g of lettuce in a stomacher bag, which was homogenized for 1 min in 90 ml peptone water (Oxoid, United Kingdom). Chromocult Coliform-agar was used for enumeration of E. coli O157 (incubation at 37°C for 24 h).
Disinfection by-products.
The formation of total trihalomethanes (TTHMs), i.e., chloroform, bromoform, dichlorobromomethane, and dibromochloromethane, was measured. Samples were taken after the reconditioning experiment, i.e., after a 30-min contact time. In the washing bath experiment, a water sample was taken from the washing bath at the end of the 1-h trials and from the final batch of lettuce during each trial. The trihalomethanes both in the water and on the lettuce were analyzed as described previously by Lopez-Galvez et al. (23).
Statistics.
SPSS statistics 20 and Microsoft Excel were used for statistical analysis. The Kolmogorov-Smirnov test and Levene's test were used to assess normality and equality of variance (P ≥ 0.05), respectively. Differences between treatments, i.e., influence of physicochemical parameters, free chlorine, or bacterial species, were determined by analysis of variance (ANOVA). If normality or equality of variance could not be assumed, the Kruskal-Wallis or Brown-Forsythe test was used, respectively, as an alternative to ANOVA. If significant differences were found, the Tukey honestly significant difference (HSD) or Games-Howell post hoc test was used at a significance level of a P value of ≤0.05 for further analysis if the group variances were equal or unequal, respectively.
Modeling. (i) Reconditioning experiment.
For assessing the overall quantitative quality of the models, both the squared correlation coefficient (r2) for predicted values versus measured values and the ratio of prediction to deviation (RPD) were used. The RPD is the ratio of the standard deviation of the measured log reduction values to the root mean square error (RMSE) of the predicted values. The RPD expresses the increase of prediction accuracy compared to the use of the mean log reduction value to predict all chlorination trials. A ratio greater than 2 is necessary for a decent calibration, whereas a ratio below 1.5 indicates an insufficient prediction potential of the model (24). In order to provide additional qualitative information on the origin of the error, Theil's decomposition of the mean square error (MSE) was used. Decomposition of the MSE can be performed as follows:
| (1) |
where y̅m is the mean of measured data points, y̅ is the mean of modeled data points, and r is Pearson's coefficient of correlation.
| (2) |
| (3) |
Dividing equation 1 by the MSE allows a proportional representation of the three decomposed factors of the MSE:
| (4) |
| (5) |
Um measures the proportion of the MSE related to bias in the prediction model. Ur represents the proportion of the MSE that is caused by deviation of the regression line between measured and predicted data points from the 45° perfect-fit line. Ud represents random prediction errors that cannot be reduced. Ideally, Um and Ur would be zero, while Ud would equal the MSE.
(ii) Washing bath experiment.
The models were constructed in @RISK. Distributions were fitted to the measured parameters that were used for constructing the model, and distributions were chosen based on the lowest chi-square statistic value. Monte Carlo simulation was used to select random samples from the input distributions as input for the model. As such, a set of output samples (E. coli O157 wash water contamination) were obtained, and distributions were fitted to these output samples. For assessing the overall quality of the time series models in this experiment, Theil's inequality coefficient (TIC) was used (equation 6). TIC values range from 0 to 1, and values below 0.3 indicate a decent agreement of the model with the experimental data (25).
| (6) |
As with the reconditioning experiments, Theil's decomposition of the MSE was used to further assess the prediction quality of these models.
RESULTS
Inactivation of E. coli O157 in oxidant-demand-free buffer.
E. coli O157 reduction in oxidant-demand-free buffer with chlorine can be seen in Table 1. E. coli O157 was highly vulnerable to chlorine disinfection. The data in this study were fitted to three basic disinfection models: log-linear kinetics, Chick-Watson, and Hom (Table 2). The Chick-Watson model gave a value for n close to 1, meaning that only a slightly higher weight was given to the free chlorine concentration than to the contact time. Not surprisingly, applying log-linear kinetics, i.e., log linear as the concentration remained virtually constant in the chlorine-demand-free buffer, resulted in a similar prediction quality. For the Hom model, the m value was less than 1, suggesting the presence of some tailing effect. The fact that E. coli O157 was still detectable (without enrichment) after 2 min in the cases of 0.2, 0.3, and 0.4 mg/liter free chlorine allows the possibility of such a tailing effect. However, as these measurements were below the LOQ and the number of time points was limited, this could not be confirmed. The less complex Chick-Watson and log-linear models deviated somewhat from the perfect-fit line (Ur = 0.205 and 0.182, respectively).
Table 1.
Chlorine inactivation of E. coli O157 in oxidant-demand-free buffer
| Free chlorine concn (mg/liter) | Mean chlorine inactivation of E. coli O157 (log N/N0) ± SD with contact time (min) of: |
|||
|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | |
| 0.2 | −0.9 ± 0.1 | −1.6 ± 0.1 | −3.4 ± 0.3 | <−6.3a |
| 0.3 | −1.8 ± 0.6 | −2.7 ± 0.3 | −4.9 ± 0.2 | <−6.3a |
| 0.4 | −1.9 ± 0.2 | −2.7 ± 0.2 | −5.2 ± 0.4 | <−6.3a |
| 0.5 | −3.0 ± 0.2 | −5.4 ± 0.2 | <−6.3a | <−6.3 |
Detectable by direct plating yet below the limit of quantification.
Table 2.
Prediction quality of kinetic disinfection models in chlorine-demand-free buffer
| Model | k | n | m | r2 | Um | Ur | Ud | RPD |
|---|---|---|---|---|---|---|---|---|
| ln N/N0 = −kCt (log linear) | 37.3 | 0.82 | 0.059 | 0.182 | 0.759 | 2.11 | ||
| ln N/N0 = −kCnt (Chick-Watson) | 41.6 | 1.11 | 0.83 | 0.070 | 0.205 | 0.725 | 2.13 | |
| ln N/N0 = −kCntm (Hom) | 32.6 | 0.97 | 0.70 | 0.87 | 0.001 | 0.003 | 0.996 | 2.78 |
Use of chlorine as a reconditioning agent.
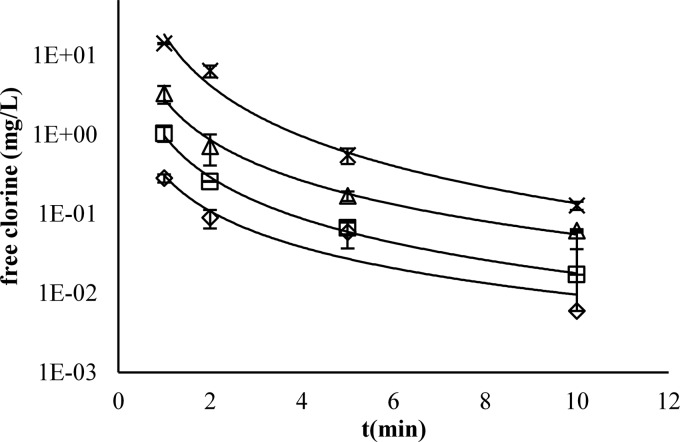
The measurements of selected physicochemical parameters of the SPW can be seen in Table 3. The consumption of free chlorine in the SPW occurred rapidly. At COD values of 800 and 1,500 mg O2/liter, 75 mg/liter of free chlorine was virtually completely consumed within 1 min (data not shown). Water with a COD value of 456 ± 1 mg O2/liter represents a best-case scenario, i.e., water with the best possible physicochemical quality in this study. It can be seen that even at this lower bound of organic load, free chlorine was consumed rapidly (Fig. 1). Within 2 min, 50 mg/liter free chlorine was reduced to below 0.1 mg/liter, and an initial addition of 100 mg/liter free chlorine resulted in a residual concentration below 1 mg/liter free chlorine after 5 min. Considerable amounts of total chlorine formed due to free chlorine decomposition (data not shown), which consisted predominantly of organic chloramines, due to the low NH4-N content.
Table 3.
Physicochemical characteristics of the used process watersa
| Parameter | Mean value ± SD |
||||
|---|---|---|---|---|---|
| SPW COD 500 mg O2/liter | SPW COD 800 mg O2/liter | SPW COD 1,500 mg O2/liter | Company 1 | Company 2 | |
| pH | 7.6 ± 0.1 | 7.5 ± 0.1 | 7.6 ± 0.02 | 7.34 ± 0.01 | 7.2 ± 0.1 |
| Turbidity (NTU) | 66.4 ± 5.4 | 118.4 ± 20.7 | 228.0 ± 50.5 | 13.8 ± 0.9 | 72.6 ± 6.6 |
| COD (mg O2/liter) | 510 ± 20 | 772 ± 20 | 1,430 ± 58 | 465 ± 2 | 1,405 ± 57 |
| UV254 | 1.58 ± 0.05 | 2.40 ± 0.06 | 3.74 ± 0.39 | 0.36 ± 0.03 | 1.08 ± 0.05 |
| UV254(F) | 0.82 ± 0.05 | 1.40 ± 0.11 | 2.12 ± 0.21 | 0.22 ± 0.02 | 0.70 ± 0.02 |
| Fe (mg/liter) | ND | 0.72 ± 0.07 | ND | 0.19 ± 0.005 | 0.22 ± 0.01 |
| Mn (mg/liter) | ND | 0.070 ± 0.0002 | ND | 0.013 ± 0.002 | 0.024 ± 0.003 |
| NH4-N (mg/liter) | ND | 0.45 ± 0.03 | ND | 1.13 ± 0.03 | 0.89 ± 0.05 |
ND, not determined; NTU, nephelometric turbidity units.
Fig 1.
Free chlorine consumption in SPW with a COD of 456 ± 1 mg O2/liter. ♢, 50 mg/liter chlorine; □, 60 mg/liter chlorine; △, 70 mg/liter chlorine; ×, 100 mg/liter chlorine.
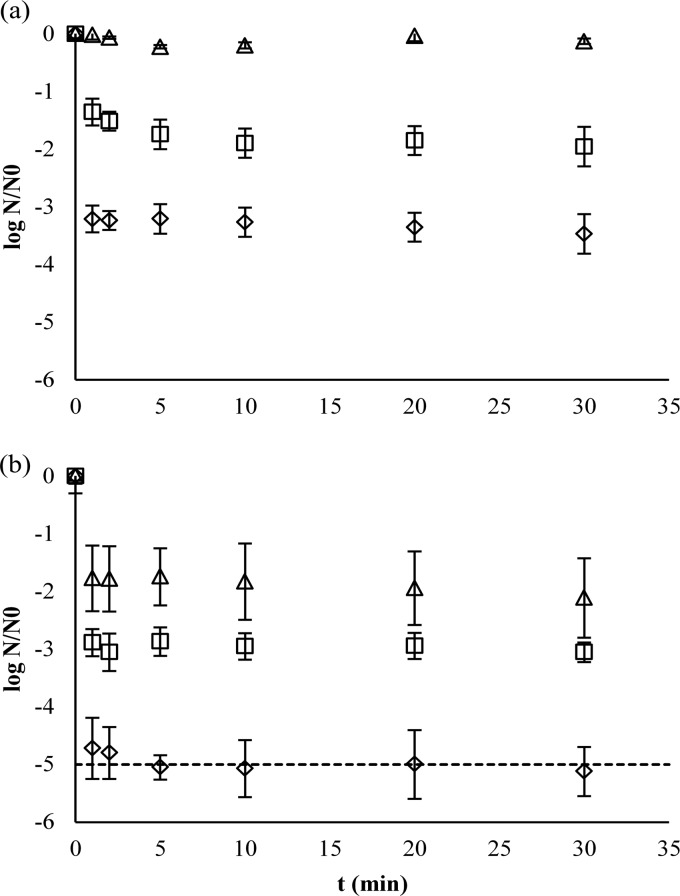
The inactivation of E. coli O157 occurred during the first minute of contact time (Fig. 2). This was due to the virtually complete free chlorine consumption within this first minute (Fig. 1), except for the case of the addition of 75 mg/liter in SPW with a COD of 500 mg/liter, the latter resulting in reductions below the LOQ (Fig. 3). No further significant inactivation occurred during the 30-min contact time (Fig. 4). Therefore, when disinfecting E. coli O157 in SPW with the studied amounts of COD and added free chlorine concentrations, the time factor could be discarded if a contact time above 1 min was maintained.
Fig 2.
Inactivation of E. coli O157 in SPW with a COD of 500 mg O2/liter. ♢, 20 mg/liter chlorine; □, 35 mg/liter chlorine; △, 50 mg/liter chlorine;  , LOQ.
, LOQ.
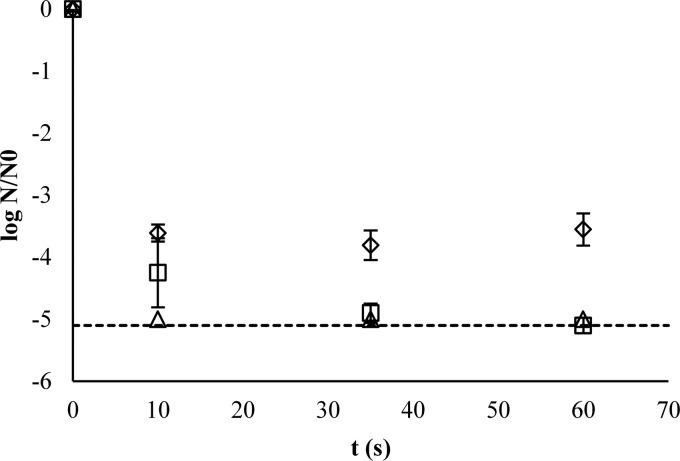
Fig 3.
Inactivation efficiencies of E. coli O157 in SPW using a 2-min contact time.  , LOQ; ∗, below the limit of detection.
, LOQ; ∗, below the limit of detection.
Fig 4.
Inactivation of E. coli O157 with 20 mg/liter free chlorine (a) and 35 mg/liter free chlorine (b) in SPW containing COD values of 500 mg O2/liter (♢), 800 mg O2/liter (△), and 1,500 mg O2/liter.  , LOQ.
, LOQ.
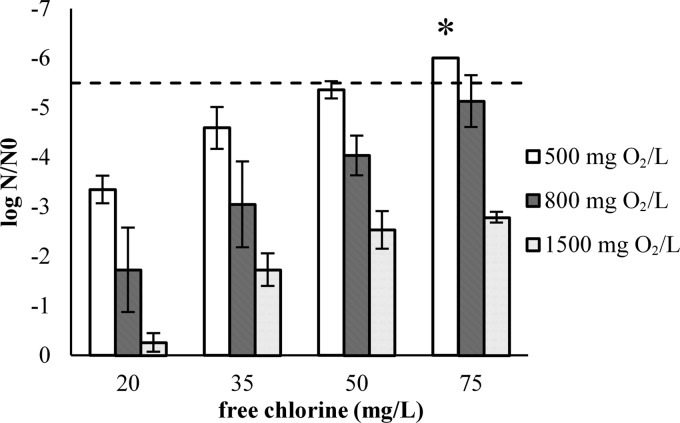
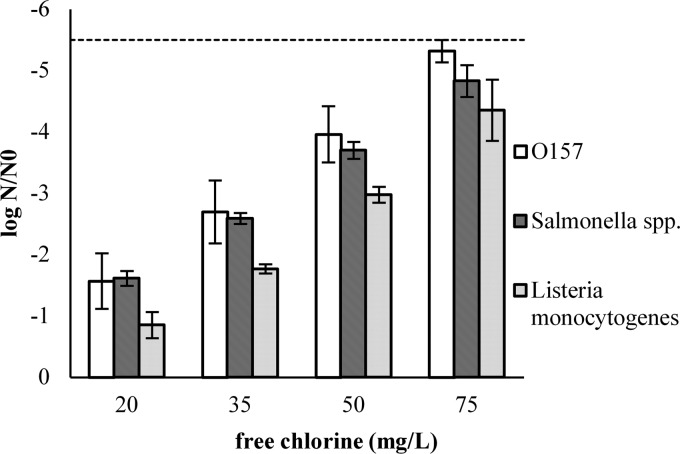
An overview of the inactivation experiments using a default contact time of 2 min is shown in Fig. 3. Inactivation was significantly influenced by both the added free chlorine and the COD of the SPW (P < 0.0005). When comparing the inactivation of E. coli O157 with those of other relevant bacterial pathogens, it was observed that the Listeria monocytogenes strains were significantly more resistant to chlorination in SPW than the Salmonella (P < 0.0005) and E. coli O157 (P < 0.0005) strains (Fig. 5). There was no significant difference in the inactivation of the latter two pathogens (P > 0.05).
Fig 5.
Inactivation of E. coli O157, Salmonella spp., and Listeria monocytogenes in standardized process water with a COD of 800 mg O2/liter (2-min contact time).  , LOQ.
, LOQ.
For the modeling, as the influence of contact time on inactivation was insignificant (P > 0.05) in the SPW, the contact time was fixed at 2 min. The data from the addition of 75 mg/liter free chlorine were discarded due to the presence of inactivation values below the LOQ, and the remaining data were used for construction of the model. These disinfection data were divided into a calibration set (n = 27) and a validation set (n = 13), while the data from the experiments with water from the fresh-cut produce companies (n = 18) were used as an additional external validation set. The linear, quadratic, and interaction terms were modeled with multilinear regression and, based on data from the SPW experiments (calibration set) (equation 7), selecting variables that showed statistical significance (P < 0.05):
| (7) |
where N is CFU/ml after chlorination, N0 is the initial CFU/ml, a to f are constants, X is the physicochemical parameter, and Cl2 is added free chlorine.
All linear models were constructed. Concerning the integration of second-order terms in the model, in all cases, only the square of the physicochemical parameter, e.g., COD2, contributed significantly to the regression models (Table 4). For the SPW, the physicochemical parameters that negatively influenced the chlorination efficiency all correlated significantly (P < 0.0005) with each other. This suggests that all parameters would predict the inactivation efficiency with somewhat similar efficiencies, and indeed, all models predicted the validation set of SPW samples with an RPD of ≥2. Due to this correlation, a model incorporating all physicochemical parameters was redundant and did not have additional predictive value. In fact, when performing regression on all physicochemical parameters, the model was reduced to the linear model based on UV254(F), excluding the other parameters. Concerning predicting actual process water, only the RPD values for the COD models were >2, meaning that all physicochemical parameters, except for COD, failed to predict the disinfection efficiency of chlorine in the actual process water from both fresh produce processing companies. A large systematic bias was observed for the models based on UV254, UV254(F), and turbidity (high Um) values. The linear model based on COD predicted the disinfection efficiency better than the quadratic model. The quadratic model had a considerable bias (Um) and deviated from the perfect-fit line (Ur), as well as having a lower RPD value. Therefore, the quadratic model suffered from overfitting, and the simpler linear model was more robust toward predicting the inactivation efficiency in the process water from the companies.
Table 4.
Prediction quality of the reconditioning models based on different physicochemical parameters
| Model and parameter | Intercept | Free chlorine | Xa | X2 | Validation with SPW |
Validation with wash water companies |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | Um | Ur | Ud | RPD | r2 | Um | Ur | Ud | RPD | |||||
| Linear | ||||||||||||||
| COD | −2.583 | −0.079 | 0.003 | 0.88 | 0.034 | 0.010 | 0.956 | 2.92 | 0.91 | 0.050 | 0.070 | 0.880 | 3.23 | |
| UV254(F) | −2.999 | −0.078 | 1.924 | 0.80 | 0.012 | 0.001 | 0.987 | 2.34 | 0.75 | 0.872 | 0.014 | 0.114 | 0.70 | |
| UV254 | −3.047 | −0.079 | 1.112 | 0.81 | 0.004 | 4.30 × 10−5 | 0.996 | 2.39 | 0.71 | 0.875 | 0.010 | 0.115 | 0.65 | |
| Turbidity | −2.380 | −0.067 | 0.014 | 0.77 | 0.006 | 0.014 | 0.980 | 2.15 | 0.77 | 0.733 | 0.067 | 0.199 | 0.96 | |
| 23 factorial design | ||||||||||||||
| COD | −6.761 | −0.087 | 0.013 | −5.18 × 10−6 | 0.86 | 0.057 | 0.034 | 0.909 | 2.68 | 0.91 | 0.305 | 0.356 | 0.339 | 2.04 |
| UV254(F) | −5.360 | −0.083 | 5.717 | −1.239 | 0.79 | 0.027 | 0.030 | 0.943 | 2.21 | 0.91 | 0.981 | 5.53 × 10−5 | 0.019 | 0.48 |
| UV254 | −7.604 | −0.081 | 4.807 | −0.658 | 0.81 | 0.022 | 0.042 | 0.935 | 2.34 | 0.90 | 0.986 | 0.003 | 0.011 | 0.29 |
| Turbidity | −4.502 | −0.079 | 0.053 | −0.0001 | 0.85 | 0.023 | 0.011 | 0.966 | 2.65 | 0.93 | 0.974 | 0.002 | 0.024 | 0.51 |
Denotes a physicochemical parameter.
Chlorine as fresh-cut lettuce wash water disinfectant.
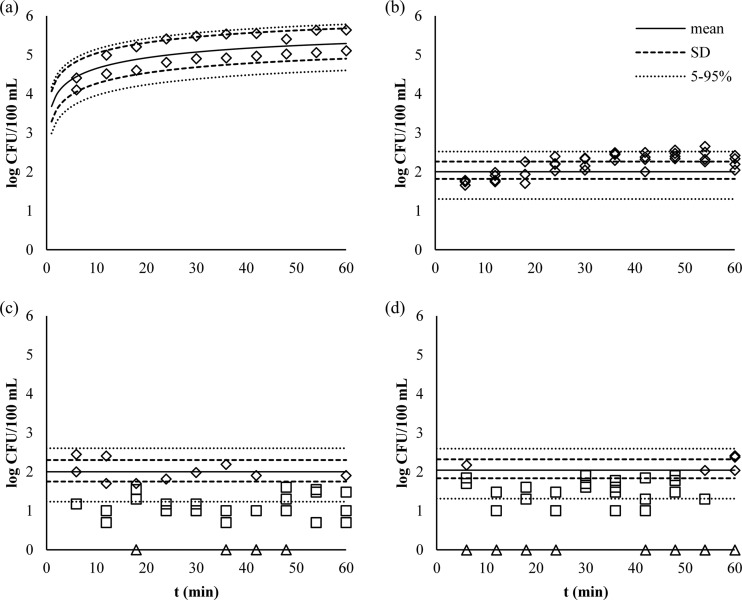
Washing of fresh-cut lettuce without the use of free chlorine caused a rapid microbial buildup in the wash water that reached 5.4 ± 0.4 log CFU/100 ml after 1 h (Fig. 6). Maintaining a residual concentration of 1 mg/liter free chlorine resulted in wash water contamination that was maintained below 2.7, 2.5, and 2.5 log CFU/100 ml for tap water and SPW with COD values of 500 and 1,000 mg O2/liter, respectively (Fig. 6). Microbial contamination was significantly correlated with time (r2 = 0.52; P < 0.0005) when chlorinating in tap water, whereas no correlation with time was present when chlorinating in SPW. When disinfecting wash water containing a COD of 500 or 1,000 mg O2/liter, a larger variation of E. coli O157 counts was observed than those in tap water, and there were overall lower E. coli O157 counts. Greater variation in free chlorine concentrations occurred in the presence of larger amounts of COD (Levene's statistic = 0.021 for tap water versus water with a COD of 500 mg O2/liter and 0.046 for water with a COD of 500 versus a COD of 1,000 mg O2/liter) (Table 5). The reduction of E. coli O157 on the lettuce due to washing with water was 0.5 ± 0.1 log CFU/g. Only in the case of washing with 1 mg/liter free chlorine in water with a COD of 1,000 mg O2/liter was the reduction on the lettuce significantly higher (P < 0.0005) during the 1-h duration of the experiment than for the wash with only water (Table 5). In all cases, rinsing had no additional influence on pathogen removal.
Fig 6.
Measured and modeled E. coli O157 contamination in the washing bath during the fresh-cut lettuce washing process without chlorine in tap water (a) and with 1.17 ± 0.26 mg/liter free chlorine in tap water (b), 1.16 ± 0.33 mg/liter free chlorine in water with a COD of 500 mg O2/liter (c), and 1.09 ± 0.39 mg/liter free chlorine in water with a COD of 1,000 mg O2/liter (d). Shown are E. coli O157 measurements more than the LOQ (♢), more than the limit of detection and less than the LOQ (□), and less than the limit of detection (△).
Table 5.
Measured chlorine consumption and DBP production in the washing bath experiment (n = 2)
| Parameter | Mean value ± SD |
||
|---|---|---|---|
| Tap water | Water with COD of 500 mg O2/liter | Water with COD of 1,000 mg O2/liter | |
| COD (mg O2/liter) | 36 ± 13 | 500 ± 25 | 1,017 ± 4 |
| Free chlorine, residual (mg/liter) | 1.17 ± 0.26 | 1.16 ± 0.33 | 1.09 ± 0.39 |
| Free chlorine dose (mg/liter/min) | 0.3 ± 0.02 | 2.6 ± 0.2 | 6.6 ± 1.2 |
| Chlorination breakpoint (mg/liter) | 1.9 ± 0.2 | 81.0 ± 14.4 | 244.5 ± 19.1 |
| Cumulative dose (mg/liter) | 17.1 ± 2.1 | 235.8 ± 23.6 | 609.0 ± 59.4 |
| Reduction of E. coli O157 on lettuce (log CFU/g) | 0.6 ± 0.2 | 0.6 ± 0.2 | 1.6 ± 0.2 |
| Total trihalomethanes (water) (μg/liter) | <6.3 | 27.8 ± 5.4 | 124.5 ± 13.4 |
| Chloroform | <6.3 | 27.8 ± 5.4 | 111.1 ± 17.3 |
| Bromodichloromethane | <6.3 | <6.3 | 13.4 ± 2.9 |
| Total trihalomethanes (lettuce) (μg/g) | <6.3 | <6.3 | <6.3 |
A model to assess E. coli O157 contamination in the wash water during fresh-cut lettuce washing was based on the following three assumptions: (i) free chlorine is free to inactivate bacteria, or otherwise stated, knowledge of the residual free chlorine in the washing bath can be used to estimate the microbial kill-off regardless of the physicochemical load; (ii) rinsing is considered to be insignificant for E. coli O157 removal and, as such, is not considered in the model; and (iii) the discrete experimental setup is interpreted as a continuous process where lettuce is continuously added instead of being added in intervals of 1 min. The following model was made for E. coli O157 contamination in the washing bath, to quantify the change in microbial load with respect to time:
| (8) |
where N is the E. coli O157 load in the washing bath (CFU/100 ml), L is contamination of lettuce entering the washing bath (CFU/g), R1 is the fraction of E. coli O157 transferred from the lettuce to the washing bath, R2 is lettuce entering the washing bath per unit of time (g/s), k and n are Chick-Watson constants for E. coli O157 inactivation in chlorine-demand-free buffer solution, Cl2 is residual free chlorine (mg/liter) in the washing bath, VL is the water volume loss per g of lettuce (ml/g), and V is the water volume of the washing bath.
Solving the differential equation with respect to the initial condition N(t = 0) = 0 CFU yields, in the absence and presence of free chlorine, equations 9 and 10, respectively:
| (9) |
| (10) |
The contamination in 100 ml is than calculated as
| (11) |
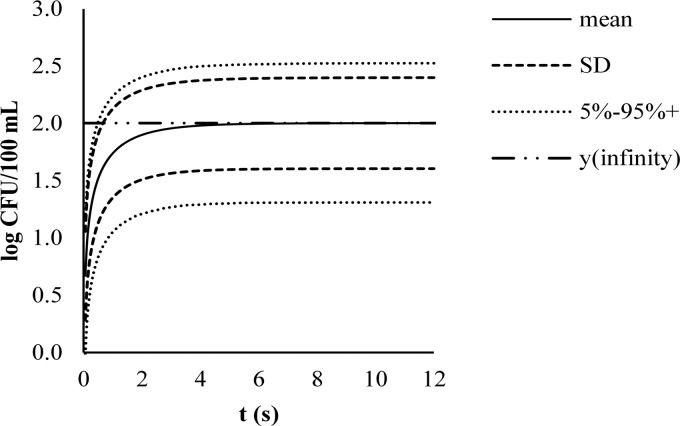
The model that predicted the contamination of the washing bath in the presence of free chlorine converged very rapidly to a static condition (Fig. 7). Therefore, the output of the model could be considered constant:
| (12) |
The model made a good prediction for contamination as a function of time in the absence of free chlorine (TIC = 0.028; Ud = 0.93), but in the presence of 1.17 ± 0.26 mg/liter free chlorine, there was some bias (Um = 0.29). Nonetheless, the TIC value was decent (0.077), indicating that the overall error was quite low (Table 6 and Fig. 6). The quality of the models of chlorination in water with a COD of 500 or 1,000 mg/liter was not assessed because of the large number of data points with values below the LOQ (Fig. 6). The difference in variability of the measured free chlorine residual concentrations between washing in wash water with different COD values had no significant impact on the model output, which predicted similar outputs for water disinfection in tap water and SPW with COD values of 500 and 1,000 mg/liter (2.0 ± 0.4 log CFU/100 ml).
Fig 7.
Model for E. coli O157 contamination during washing of fresh-cut lettuce in tap water with 1.17 ± 0.26 mg/liter free chlorine.
Table 6.
Prediction quality of the models of E. coli O157 contamination in tap water
| Mean free chlorine concn (mg/liter) ± SD | r2 | Um | Ur | Ud | TIC |
|---|---|---|---|---|---|
| 0.0 ± 0.0 | 0.58 | 0.02 | 0.05 | 0.93 | 0.028 |
| 1.17 ± 0.26 | 0 | 0.29 | 0 | 0.71 | 0.077 |
DBP formation during reconditioning and wash water disinfection.
DBPs were measured during the reconditioning trials. In all cases, only small amounts of TTHMs were measured. Only chloroform was present in quantifiable amounts: 7.8 ± 1.4 and 13.6 ± 2.9 μg/liter in water with a COD of 800 mg O2/liter when adding 100 or 150 mg/liter of chlorine, respectively, and 9.3 ± 3.4 and 13.5 ± 7.8 μg/liter in water with a COD of 1,500 mg O2/liter when adding 100 or 150 mg/liter of chlorine, respectively. In the wash water disinfection experiment, the free chlorine dose necessary to maintain a residual concentration of approximately 1 mg/liter increased substantially with increasing COD (Table 5). As with the reconditioning experiments, the amount of TTHMs formed was higher in SPW with higher COD values and with the addition of higher chlorine concentrations. Besides chloroform, small amounts of bromodichloromethane were also measured in SPW with a COD of 1,000 mg O2/liter.
DISCUSSION
That pathogenic and nonpathogenic E. coli strains are prone to chlorination in oxidant-demand-free environments and require low concentration × contact time values has been known for decades (26–28). Although the Hom model and the presence of some resistant cells suggested the possibility of some tailing, the experimental setup did not allow the confirmation of such an effect. Tailing phenomena can be explained by disinfectant decay or, from a microbial point of view, the presence of subpopulations with higher levels of resistance to chlorine (29). Modeling according to log-linear kinetics provided a decent prediction of E. coli O157 inactivation. A good fit to log-linear kinetics was observed previously by Lee et al. (30) for chlorination of E. coli (pH 8.5 at 4°C and 1 mg/liter free chlorine), i.e., when giving equal weights to free chlorine concentration and contact time.
COD load had a detrimental effect on disinfection efficiency. Higher organic loads lead to faster chlorine consumption, allowing less free chlorine to be in contact with the target microorganisms during the exposure period. This lowers the disinfection efficiency of chlorine, a fact well known for water and wastewater disinfection (31–33). The rapid decomposition of free chlorine was due to its reactivity with certain organic species. Chlorine decomposition can occur through oxidation, addition, and electrophilic substitution reactions with organic substances in the water. Usually, electrophilic substitution is the predominant mechanism. Chlorine reacts quite specifically with organic molecules, as can be seen from the great differences in reaction rates depending on the molecular structure of the target organic molecule. Reactions with double bonds, alcohols, and ketones are rather slow, whereas reactions with aliphatic amines, amino acids, and peptides (N atom of terminal amino function is the target) are fast. Reaction rates with compounds containing reduced sulfur moieties are especially high, and this includes the amino acids cysteine and methionine, proteins containing these amino acids, and the reducing compound glutathione (34, 35). Chlorine can also react with several inorganic species present in water, such as reduced iron, arsenic and manganese, halides, and sulfide, etc. (34). As the amounts of iron and manganese were small compared to the COD, these constituents most likely had no considerable influence on chlorine consumption. Although considerable amounts of organic chloramines were formed, there was only minor potential for inorganic chloramine formation. Organic chloramines possess little or no disinfection potential against E. coli (35, 36), whereas inorganic chloramines exhibit some disinfection efficiency but to a much lesser extent than free chlorine, although they are more stable (35). As the inactivation occurred in the first minute of the 30-min trials, it can be concluded that only free chlorine contributed to the inactivation of E. coli O157. Regression modeling showed that COD was, next to the added free chlorine dose, the predominant predictor of disinfection efficiency in fresh-cut lettuce wash waters. That the other physicochemical parameters were decent to good estimators in the SPW appeared to be due solely to the correlation between COD and the other parameters. This illustrates the importance of assessing models through external validation in actual wash waters. Particulate matter can protect microorganisms by incomplete penetration of chlorine in particles to which microorganisms are attached, which has been observed in wastewater studies (31, 37). However, in the case of these fresh-cut produce wash waters, the (dissolved) organic content, which reacts with chlorine, is very high and most likely the predominant detrimental influence on the chlorine efficiency.
The higher susceptibility to chlorine of E. coli O157 than of Listeria monocytogenes in this study was also observed in other studies (38, 39). Sublethal chlorination affects the physiological status of E. coli, and previous studies suggested that active transport and respiration systems for glucose and amino acids are targets for hypochlorous acid (40, 41). More recent studies further observed that actual destruction of membrane or cell wall integrity is not necessary for E. coli as well as Listeria monocytogenes inactivation and suggested that the primary action of chlorination is not on the cell surface of the prokaryotic cell but mostly on the interior (33, 42). As such, it can be considered that the difference in resistance between Gram-negative and Gram-positive species might be dependent predominately on differences in resistance to the mass transfer of chlorine across Gram-positive and -negative cell surface layers. On that note, a comparison between resistance of Gram-positive and Gram-negative species based solely on the thickness of the peptidoglycan layer is an oversimplification due to the spatial difference of these layers in the cell surface of both bacterial groups as well as the difference in composition of the Gram-positive and Gram-negative bacterial cell walls. This was noted previously by Dalrymple et al. (43) when reviewing the mechanisms of photocatalytic disinfection, but this reasoning also seems sound for chlorination. The higher level of resistance of Listeria monocytogenes than of Gram-negative pathogens illustrates the necessity for an accurate indicator organism (44, 45). If two or more pathogens form a significant risk in a given situation, the criteria should be designed to avoid the occurrence of the most resistant one, if practically and economically achievable. This vision has already been adopted in certain water quality criteria, e.g., the Long Term 2 Enhanced Surface Water Treatment Rule by the U.S. Environmental Protection Agency (EPA), which incorporated the necessity for drinking water production systems to fulfill a certain Cryptosporidium inactivation besides the conventional E. coli-based limits (46, 47).
When attempting to maintain 1 mg/liter of free chlorine during the washing bath trials, higher variations in free chlorine concentrations were observed in SPW with higher COD loads. Higher COD values necessitate the addition of more chlorine to maintain the residual concentration. This larger addition, in combination with a higher free chlorine consumption, causes higher free chlorine concentration gradients to occur throughout the washing bath. As such, greater variations in E. coli O157 levels can arise, as was observed in this study. This illustrates the practical difficulty when attempting to maintain very low (i.e., in the range of 1 mg/liter) free chlorine concentrations in wash water with high organic loads. When modeling inactivation in tap water containing 1 mg/liter of residual free chlorine, the bias observed between the model and the experimental data, i.e., the small observed increase in E. coli O157 contamination, suggests that one of the hypotheses made was not valid or that a significant influencing parameter was not considered in the model (e.g., insufficient prediction quality of disinfection kinetics), of which the output was virtually independent of time. Also, in the case of higher organic loads, the greater variations in E. coli O157 levels were not predicted by the model outputs. As the model made the assumption that knowledge of the residual free chlorine concentration is sufficient to predict inactivation, the higher chlorine gradients and the incompletely mixed character of the washing tank were not considered in the model. Maintaining 1 mg/liter free chlorine in the wash water avoided a high E. coli O157 buildup in the simulated continuous fresh-cut lettuce washing process, e.g., resulting in an average reduction of 3.2 log CFU/100 ml wash water after the 1-h simulated washing process in tap water. These findings confirm the good effectiveness of chlorination for wash water disinfection in fresh-cut lettuce washing observed in other recent studies (2, 6). However, reducing the E. coli O157 water contamination to low values does not necessarily imply that no cross-contamination can occur. Luo et al. (2) observed previously that although 5 mg/liter (1.7 mg/liter after chlorine dissipation due to organic matter) inactivated E. coli O157 to below the detection limit in the wash water, cross-contamination could not be completely avoided when simultaneously washing inoculated and uninoculated fresh-cut romaine lettuce. Tomas-Callejas et al. (6) made the same observations (though only with nonquantitative detection) when applying 25 mg/liter free chlorine (loss due to decomposition is not known) to avoid cross-contamination of E. coli O157 and Salmonella Typhimurium during processing of red chard. Suggested causes for cross-contamination in those studies were the presence of microenvironments with lower free chlorine concentrations and cross-contamination through direct contact of inoculated with uninoculated lettuce. The former seems realistic when maintaining low chlorine levels in washing baths. The latter, however, is less likely to occur in reality. Contrary to experimental situations, which apply mostly high inocula and a large abundance of inoculated lettuce, it can be assumed that, not considering extreme cases, only a minor part of the lettuce would be contaminated with pathogens and at lower contamination levels. As such, direct transfer seems insignificant. The good efficiency for wash water disinfection in this and previous studies is in strong contrast with the decontamination efficiency of free chlorine (48–50). Decontamination is hindered by the difficult removal of microorganisms infiltrated in hard-to-reach or damaged tissue or agglomerated in biofilms (48, 51, 52). This can be partially circumvented by washing the lettuce leaves in chlorine before actually cutting them because this avoids in part the infiltration of microorganisms during cutting due to the preceding microbial reduction. Furthermore, this reduction step is facilitated by the smaller amount of organics released into the wash water when washing whole produce. An additional log unit of E. coli O157:H7 was removed from romaine lettuce when chlorine washing was done before cutting and a subsequent second chlorine wash was done compared to cutting followed by two consecutive chlorine washing steps. As such, cross-contamination during cutting was significantly reduced (3).
The TTHM concentrations generated during the reconditioning trials were far below the allowed DBP concentrations in drinking water in the European Union (100 μg/liter TTHMs) and in the United States (80 μg/liter TTHMs) (53, 54). Higher concentrations were formed in the washing bath trials due to the continuous exposure of the wash water to free chlorine. Formation of TTHMs increases among others with increasing chlorine dose and organic load (55, 56). The results of this study and those of Lopez-Galvez et al. (23) confirmed this specifically for a lettuce-derived water matrix. In the case of water with a COD of 1,000 mg O2/liter, small amounts of bromodichloromethane were detected. Brominated organics are formed through a reaction of organic matter with hypobromous acid, which itself is formed because hypochlorous acid oxidizes bromide that is present in the water (57, 58). In the case of water with a COD of 1,000 mg O2/liter, DBPs in the water exceeded the European Union and U.S. TTHM drinking water limits. Nonetheless, in all cases, no measurable amounts of TTHMs were found on the lettuce after the rinsing step, even in the case where the TTHMs in the SPW exceeded the European Union legislative limit. The given setup would not pose any chemical danger to the consumer. This absence of DBPs on lettuce was also observed in other studies (23, 59). Nevertheless, the results of the washing bath trials, especially in the SPW containing a COD of 1,000 mg O2/liter, show that considerable amounts of THMs can be formed in the water due to prolonged chlorination. As such, formation of DBPs could still be a problem if legislation would pose limits on DBPs in wash water or the resulting wastewater, as besides the known drinking water limits, legal limits for treated wastewater are now also arising (e.g., by the Florida Department of Environmental Protection), to protect surface water quality and avoid accumulation in the environment (60).
To reduce the production of DBPs, especially when the water is reused multiple times, the implementation of a treatment that periodically removes organic matter might be a feasible strategy to prolong the use of a batch of water. A disinfection is normally an end treatment, because of the consumption of chemical disinfectants and production of DBPs, shielding and absorbance of UV irradiation by particles, and rapid clogging of membrane filters, etc. For large processing companies, it might be feasible to implement a multiple-chain water treatment system to treat the wastewater from all processing lines in order to be able to completely reuse all the wash water, and a multitude of physicochemical and biological treatments are available for removal of particles and dissolved matter (61–63). A more rapid alternative would be to apply granular or membrane filtration techniques, with pretreatment with a food-safe coagulant, which might significantly prolong water usage without actually requiring a complete water treatment chain. This could potentially be implemented by working with two batches of wash water, one used for washing while the other is being treated to reduce the physicochemical load, or by reconditioning overflow water that is otherwise discarded, to enable prolonged water usage. In addition, lowering the physicochemical load requires less chlorine to maintain the desired residual concentration in the washing tank, although it remains to be seen if a stand-alone pretreatment is available that is actually less costly than the cost reduction in the required chlorine dosage that it would provide.
When using chlorine for reconditioning, efficient inactivation of vegetative bacterial pathogens was achieved without having to maintain residual chlorine, and the needed dose depended on the amount of COD in the water. The very fast chlorine decomposition poses a design issue if a reconditioning system that does not rely on residual chlorine is chosen. In this case, it is of paramount importance that the water is exposed to these high chlorine concentrations before chlorine consumption occurs. Therefore, a reactor with a profound plug-flow design would probably be suitable to guarantee that virtually all water is in contact with these high chlorine concentrations before decomposition. As such, a lower concentration would be needed than when guaranteeing sufficient exposure to chlorine by aiming for presence of residual chlorine. The major disadvantage of reconditioning is the fact that there is no “in situ” prevention of cross-contamination. Once cross-contamination has occurred, even shortly after the event, rewashing of the newly infected lettuce in free chlorine solutions is unable to completely remove the newly attached E. coli O157 (2, 18, 64). Therefore, in situ wash water disinfection is highly recommended to put up a barrier against cross-contamination. Although maintaining 1 mg/liter of free chlorine does not keep the wash water free from E. coli O157, the results show that low chlorine concentrations are highly effective in eliminating E. coli O157 in the suspended state. It must also be noted that although eliminating cross-contamination may reduce the risk of outbreaks by avoiding the spread of contamination during produce washing, ultimately, it does not solve the problem of fresh produce food disease, and the best way of eliminating pathogens is to avoid contamination altogether during primary production, although this might be virtually impossible at the moment (10, 18, 65). The fact that chlorination of fresh-cut lettuce wash waters in the assessed model systems is feasible for inactivating vegetative bacterial pathogens does not make it necessarily effective against other pathogens under these conditions. Concerning viruses, norovirus is considered to be of the greatest concern in regard to fresh-cut produce (66, 67). Although there have been large fluctuations in inactivation of norovirus with free chlorine in various studies (dependent on the type of detection, if the viruses are aggregated or dispersed, and if free or total chlorine is measured), concentration × contact times from studies with free chlorine in low-organic-loaded waters show that norovirus and its more practical surrogate murine norovirus are both highly vulnerable to chlorination (31, 68, 69). Protozoan parasites, such as Cryptosporidium parvum and Cyclospora cayetanensis, have also been associated with outbreaks related to fresh produce consumption, and countries in Latin America have recognized them as pathogens of concern (4, 67, 70, 71). If produce wash water should be kept free of these pathogens, a chlorination system would fail due to the high level of resistance to chlorine (72, 73), or alternatively, excessively high free chlorine concentrations would be required. Ozone (74), UV (75), and, especially for avoiding cross-contamination in washing processes, the more stable chlorine dioxide (6, 32, 76, 77) are better alternatives to chlorine for inactivation of protozoan parasites.
Concluding remarks.
Research and applications of chlorine as a decontamination agent on fresh-cut lettuce are numerous. On the other hand, studies on chlorination to maintain wash water quality are limited. The results of this study show that chlorine is effective as a reconditioning agent to inactivate E. coli O157, Salmonella, and Listeria monocytogenes strains in the presence of fresh-cut lettuce wash water with a high COD load. Chlorine decomposition due to reaction with organic matter occurred rapidly, and as such, microbial inactivation occurred within 1 min. Maintaining 1 mg/liter residual free chlorine during fresh-cut lettuce washing illustrates that much lower free chlorine concentrations than those applied for decontamination effectively reduce E. coli O157 in the wash water as well as the practical difficulty in maintaining such low residual concentrations in the presence of organic matter. Both disinfection strategies were successful, but in order to avoid cross-contamination, online wash water chlorination is necessary. Models for predicting E. coli O157 inactivation during reconditioning and E. coli O157 wash water contamination during washing in the presence of residual free chlorine were made. These models provide insight into the parameters that influence the disinfection process, and the ultimate goal of these types of models is to be able to estimate the chlorine dosage necessary to achieve a desired microbial reduction when designing a water disinfection treatment for fresh-cut produce washing processes. Furthermore, this study shows that although TTHMs accumulated in the water due to chlorination during fresh-cut lettuce washing (up to 124.5 ± 13.4 μg/liter), this does not imply that TTHMs will be present on the product after rinsing, as no measurable amounts of TTHMs were detected on the fresh-cut lettuce during the experiments.
ACKNOWLEDGMENTS
The research leading to these results has been facilitated by the European Community's Seventh Framework Program (FP7) under grant agreement no. 244994 (project VEG-i-TRADE).
We also thank Bruno De Meulenaer (Ghent University) for the analysis of the TTHMs and Marlies De Ketele, Stephanie Hoornaert, and Jofre De Febrer Bonilla for their practical assistance.
Footnotes
Published ahead of print 8 February 2013
REFERENCES
- 1. Jacxsens L, Luning PA, van der Vorst J, Devlieghere F, Leemans R, Uyttendaele M. 2010. Simulation modelling and risk assessment as tools to identify the impact of climate change on microbiological food safety—the case study of fresh produce supply chain. Food Res. Int. 43:1925–1935 [Google Scholar]
- 2. Luo YG, Nou XW, Yang Y, Alegre I, Turner E, Feng H, Abadias M, Conway W. 2011. Determination of free chlorine concentrations needed to prevent Escherichia coli O157:H7 cross-contamination during fresh-cut produce wash. J. Food Prot. 74:352–358 [DOI] [PubMed] [Google Scholar]
- 3. Nou XW, Luo YG. 2010. Whole-leaf wash improves chlorine efficacy for microbial reduction and prevents pathogen cross-contamination during fresh-cut lettuce processing. J. Food Sci. 75:M283–M290 doi:10.1111/j.1750-3841.2010.01630.x [DOI] [PubMed] [Google Scholar]
- 4. Olaimat AN, Holley RA. 8 May 2012. Factors influencing the microbial safety of fresh produce: a review. Food Microbiol. doi:10.1016/j.fm.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 5. Golberg D, Kroupitski Y, Belausov E, Pinto R, Sela S. 2011. Salmonella Typhimurium internalization is variable in leafy vegetables and fresh herbs. Int. J. Food Microbiol. 145:250–257 [DOI] [PubMed] [Google Scholar]
- 6. Tomas-Callejas A, Lopez-Galvez F, Sbodio A, Artes F, Artes-Hernandez F, Suslow TV. 2012. Chlorine dioxide and chlorine effectiveness to prevent Escherichia coli O157:H7 and Salmonella cross-contamination on fresh-cut red chard. Food Control 23:325–332 [Google Scholar]
- 7. Tirpanalan O, Zunabovic M, Domig KJ, Kneifel W. 2011. Mini review: antimicrobial strategies in the production of fresh-cut lettuce products. Méndez-Vilas A. (ed), Science against microbial pathogens: communicating current research and technological advances, vol 1 Formatex Research Center, Badajoz, Spain: http://www.formatex.org/microbiology3/chapters1.html. Accessed 13 July 2012 [Google Scholar]
- 8. Artes F, Gomez P, Aguayo E, Escalona V, Artes-Hernandez F. 2009. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharvest Biol. Technol. 51:287–296 [Google Scholar]
- 9. Beuchat LR. 1998. Surface decontamination of fruits and vegetables eaten raw: a review. WHO/FSF/FOS/98.2. Food Safety Unit, World Health Organization, Geneva, Switzerland [Google Scholar]
- 10. Parish ME, Beuchat LR, Suslow TV, Harris LJ, Garret EH, Farber JM, Busta FF. 2003. Methods to reduce/eliminate pathogens from fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2(Suppl):161–178 [Google Scholar]
- 11. Sapers GM. 2001. Efficacy of washing and sanitizing methods for disinfection of fresh fruit and vegetable products. Food Technol. Biotechnol. 39:305–311 [Google Scholar]
- 12. Goslan EH, Krasner SW, Bower M, Rocks SA, Holmes P, Levy LS, Parsons SA. 2009. A comparison of disinfection by-products found in chlorinated and chloraminated drinking waters in Scotland. Water Res. 43:4698–4706 [DOI] [PubMed] [Google Scholar]
- 13. Hua G, Reckhow DA. 2007. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res. 41:1667–1678 [DOI] [PubMed] [Google Scholar]
- 14. Legay C, Rodriguez MJ, Serodes JB, Levallois P. 2010. Estimation of chlorination by-products presence in drinking water in epidemiological studies on adverse reproductive outcomes: a review. Sci. Total Environ. 408:456–472 [DOI] [PubMed] [Google Scholar]
- 15. Nieuwenhuijsen MJ, Toledano MB, Elliott P. 2000. Uptake of chlorination disinfection by-products; a review and a discussion of its implications for exposure assessment in epidemiological studies. J. Expo. Anal. Environ. Epidemiol. 10:586–599 [DOI] [PubMed] [Google Scholar]
- 16. Rico D, Martin-Diana AB, Barat JM, Barry-Ryan C. 2007. Extending and measuring the quality of fresh-cut fruit and vegetables: a review. Trends Food Sci. Technol. 18:373–386 [Google Scholar]
- 17. Holvoet K, Jacxsens L, Sampers I, Uyttendaele M. 2012. Insight into the prevalence and distribution of microbial contamination to evaluate water management in the fresh produce processing industry. J. Food Prot. 75:671–681 [DOI] [PubMed] [Google Scholar]
- 18. Lopez-Galvez F, Allende A, Selma MV, Gil MI. 2009. Prevention of Escherichia coli cross-contamination by different commercial sanitizers during washing of fresh-cut lettuce. Int. J. Food Microbiol. 133:167–171 [DOI] [PubMed] [Google Scholar]
- 19. Gil MI, Selma MV, Lopez-Galvez F, Allende A. 2009. Fresh-cut product sanitation and wash water disinfection: problems and solutions. Int. J. Food Microbiol. 134:37–45 [DOI] [PubMed] [Google Scholar]
- 20. Eaton AD, Clesceri LS, Rice EW, Greenberg AE. (ed). 2005. Standard methods for the examination of water and wastewater, 21st ed. American Public Health Association, Washington, DC [Google Scholar]
- 21. Brandl MT, Miller WG, Bates AH, Mandrell RE. 2005. Production of autoinducer 2 in Salmonella enterica serovar Thompson contributes to its fitness in chickens but not on cilantro leaf surfaces. Appl. Environ. Microbiol. 71:2653–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Organization for Standardization 2000. Water quality. Detection and enumeration of Escherichia coli and coliform bacteria. Part 1. Membrane filtration method. ISO 9308-1:2000 International Organization for Standardization, Geneva, Switzerland [Google Scholar]
- 23. Lopez-Galvez F, Allende A, Truchado P, Martinez-Sanchez A, Tudela JA, Selma MV, Gil MI. 2010. Suitability of aqueous chlorine dioxide versus sodium hypochlorite as an effective sanitizer for preserving quality of fresh-cut lettuce while avoiding by-product formation. Postharvest Biol. Technol. 55:53–60 [Google Scholar]
- 24. Karoui R, Pillonel L, Schaller E, Bosset JO, De Baerdemaeker J. 2007. Prediction of sensory attributes of European Emmental cheese using near-infrared spectroscopy: a feasibility study. Food Chem. 101:1121–1129 [Google Scholar]
- 25. Audenaert WTM, Callewaert M, Nopens I, Cromphout J, Vanhoucke R, Dumoulin A, Dejans P, Van Hulle SWH. 2010. Full-scale modelling of an ozone reactor for drinking water treatment. Chem. Eng. J. 157:551–557 [Google Scholar]
- 26. Hoff JC, Akin EW. 1986. Microbial resistance to disinfectants—mechanisms and significance. Environ. Health Perspect. 69:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rice EW, Clark RM, Johnson CH. 1999. Chlorine inactivation of Escherichia coli O157:H7. Emerg. Infect. Dis. 5:461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao T, Doyle MP, Zhao P, Blake P, Wu FM. 2001. Chlorine inactivation of Escherichia coli O157:H7 in water. J. Food Prot. 64:1607–1609 [DOI] [PubMed] [Google Scholar]
- 29. Li L. 2004. Effects of initial microbial density on disinfection efficiency in a continuous flow system and validation of disinfection batch kinetics in a continuous flow system. Doctoral dissertation. Drexel University, Philadelphia, PA [Google Scholar]
- 30. Lee ES, Yoon TH, Lee MY, Han SH, Ka JO. 2010. Inactivation of environmental mycobacteria by free chlorine and UV. Water Res. 44:1329–1334 [DOI] [PubMed] [Google Scholar]
- 31. Shin GA, Sobsey MD. 2008. Inactivation of norovirus by chlorine disinfection of water. Water Res. 42:4562–4568 [DOI] [PubMed] [Google Scholar]
- 32.US Environmental Protection Agency 1999. Alternative disinfectants and oxidants guidance manual. Office of Water, US Environmental Protection Agency, Washington, DC: http://www.epa.gov/ogwdw/mdbp/alternative_disinfectants_guidance.pdf. Accessed 21 March 2011 [Google Scholar]
- 33. Virto R, Manas P, Alvarez I, Condon S, Raso J. 2005. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl. Environ. Microbiol. 71:5022–5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deborde M, von Gunten U. 2008. Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: a critical review. Water Res. 42:13–51 [DOI] [PubMed] [Google Scholar]
- 35. Donnermair MM, Blatchley ER. 2003. Disinfection efficacy of organic chloramines. Water Res. 37:1557–1570 [DOI] [PubMed] [Google Scholar]
- 36. Jang H. 2009. Organic chloramines formation and its disinfection efficiency. Doctoral dissertation. Arizona State University, Phoenix, AZ [Google Scholar]
- 37. Dietrich JP, Loge FJ, Ginn TR, Basagaoglu H. 2007. Inactivation of particle-associated microorganisms in wastewater disinfection: modeling of ozone and chlorine reactive diffusive transport in polydispersed suspensions. Water Res. 41:2189–2201 [DOI] [PubMed] [Google Scholar]
- 38. Kim C, Hung YC, Brackett RE. 2000. Efficacy of electrolyzed oxidizing (EO) and chemically modified water on different types of foodborne pathogens. Int. J. Food Microbiol. 61:199–207 [DOI] [PubMed] [Google Scholar]
- 39. Park H, Hung YC, Chung D. 2004. Effects of chlorine and pH on efficacy of electrolyzed water for inactivating Escherichia coli O157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 91:13–18 [DOI] [PubMed] [Google Scholar]
- 40. Lisle JT, Pyle BH, McFeters GA. 1999. The use of multiple indices of physiological activity to access viability in chlorine disinfected Escherichia coli O157:H7. Lett. Appl. Microbiol. 29:42–47 [DOI] [PubMed] [Google Scholar]
- 41. McFeters GA, Camper AK. 1983. Enumeration of indicator bacteria exposed to chlorine. Adv. Appl. Microbiol. 29:177–193 [DOI] [PubMed] [Google Scholar]
- 42. Cho M, Kim J, Kim JY, Yoon J, Kim JH. 2010. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 44:3410–3418 [DOI] [PubMed] [Google Scholar]
- 43. Dalrymple OK, Stefanakos E, Trotz MA, Goswami DY. 2010. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B 98:27–38 [Google Scholar]
- 44. Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR, Rose JB. 2005. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 71:3163–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sinclair RG, Rose JB, Hashsham SA, Gerba CP, Haas CN. 2012. Criteria for selection of surrogates used to study the fate and control of pathogens in the environment. Appl. Environ. Microbiol. 78:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dotson AO, Rodriguez CE, Linden KG. 2012. UV disinfection implementation status in US water treatment plants. J. Am. Water Works Assoc. 104:77–78 [Google Scholar]
- 47.US Environmental Protection Agency 2012. Long term 2 enhanced surface water treatment rule (LT2). US Environmental Protection Agency, Washington, DC: http://water.epa.gov/lawsregs/rulesregs/sdwa/lt2/. Accessed 21 May 2012 [Google Scholar]
- 48. Keskinen LA, Burke A, Annous BA. 2009. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157:H7 from lettuce leaves. Int. J. Food Microbiol. 132:134–140 [DOI] [PubMed] [Google Scholar]
- 49. Kondo N, Murata M, Isshiki K. 2006. Efficiency of sodium hypochlorite, fumaric acid, and mild heat in killing native microflora and Escherichia coli O157:H7, Salmonella Typhimurium DT104, and Staphylococcus aureus attached to fresh-cut lettuce. J. Food Prot. 69:323–329 [DOI] [PubMed] [Google Scholar]
- 50. Stopforth JD, Mai T, Kottapalli B, Samadpour M. 2008. Effect of acidified sodium chlorite, chlorine, and acidic electrolyzed water on Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes inoculated onto leafy greens. J. Food Prot. 71:625–628 [DOI] [PubMed] [Google Scholar]
- 51. Koseki S, Yoshida K, Isobe S, Itoh K. 2001. Decontamination of lettuce using acidic electrolyzed water. J. Food Prot. 64:652–658 [DOI] [PubMed] [Google Scholar]
- 52. Niemira BA, Cooke PH. 2010. Escherichia coli O157:H7 biofilm formation on romaine lettuce and spinach leaf surfaces reduces efficacy of irradiation and sodium hypochlorite washes. J. Food Sci. 75:M270–M277 doi:10.1111/j.1750-3841.2010.01650.x [DOI] [PubMed] [Google Scholar]
- 53.Council of the European Union 1998. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. European Council, Brussels, Belgium: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31998L0083:EN:NOT [Google Scholar]
- 54.US Environmental Protection Agency 2009. National primary drinking water standards, EPA 816-F-09-0004, May 2009. US Environmental Protection Agency, Washington, DC: http://water.epa.gov/drink/contaminants/index.cfm%23Microorganisms. Accessed 12 February 2011 [Google Scholar]
- 55.World Health Organization 2000. Environmental health criteria 216, disinfectants and disinfectant by-products. World Health Organization, Geneva, Switzerland: http://www.who.int/ipcs/publications/ehc/ehc_216/en/. Accessed 20 May 2012 [Google Scholar]
- 56. Yang X, Shang C, Huang JC. 2005. DBP formation in breakpoint chlorination of wastewater. Water Res. 39:4755–4767 [DOI] [PubMed] [Google Scholar]
- 57. Gallard H, von Gunten U. 2002. Chlorination of natural organic matter: kinetics of chlorination and of THM formation. Water Res. 36:65–74 [DOI] [PubMed] [Google Scholar]
- 58. Westerhoff P, Chao P, Mash H. 2004. Reactivity of natural organic matter with aqueous chlorine and bromine. Water Res. 38:1502–1513 [DOI] [PubMed] [Google Scholar]
- 59.Committee on Toxicity 2006. COT statement on a commercial survey investigating the occurrence of disinfectants and disinfection by-products in prepared salads. Committee on Toxicity, London, United Kingdom: http://cot.food.gov.uk/pdfs/cotstatementwashaids200614.pdf. Accessed 21 June 2011 [Google Scholar]
- 60. Hua G, Yeats S. 2010. Control of trihalomethanes in wastewater treatment. Fla. Water Resour. J. 2010(April):6–12 [Google Scholar]
- 61. Crittenden JC, Trussell RR, Hand DW, Howe KJ, Tchobanoglous G. 2005. Water treatment principles and design, 2nd ed. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 62. Liu SX. 2007. Food and agricultural wastewater utilization and treatment. Blackwell Publishing, Ames, IA [Google Scholar]
- 63. Tchobanoglous G, Burton F, Stensel HD. 2003. Wastewater engineering: treatment and reuse, 4th ed. McGraw-Hill, New York, NY [Google Scholar]
- 64. Lopez-Galvez F, Gil MI, Truchado P, Selma MV, Allende A. 2010. Cross-contamination of fresh-cut lettuce after a short-term exposure during pre-washing cannot be controlled after subsequent washing with chlorine dioxide or sodium hypochlorite. Food Microbiol. 27:199–204 [DOI] [PubMed] [Google Scholar]
- 65. Lynch MF, Tauxe RV, Hedberg CW. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137:307–315 [DOI] [PubMed] [Google Scholar]
- 66. Baert L, Mattison K, Loisy-Hamon F, Harlow J, Martyres A, Lebeau B, Stals A, Van Coillie E, Herman L, Uyttendaele M. 2011. Norovirus prevalence in Belgian, Canadian and French fresh produce: a threat to human health? Int. J. Food Microbiol. 151:261–269 [DOI] [PubMed] [Google Scholar]
- 67.Food and Agriculture Organization of the United Nations, World Health Organization 2008. Microbial hazards in fruits and vegetables. World Health Organization, Geneva, Switzerland: http://www.fao.org/ag/agn/agns/files/FFV_2007_Final.pdf. Accessed 22 December 2011 [Google Scholar]
- 68. Cromeans TL, Kahler AM, Hill VR. 2010. Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Appl. Environ. Microbiol. 76:1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kitajima M, Tohya Y, Matsubara K, Haramoto E, Utagawa E, Katayama H. 2010. Chlorine inactivation of human norovirus, murine norovirus and poliovirus in drinking water. Lett. Appl. Microbiol. 51:119–121 [DOI] [PubMed] [Google Scholar]
- 70. Chaidez C, Soto M, Gortares P, Mena K. 2005. Occurrence of Cryptosporidium and Giardia in irrigation water and its impact on the fresh produce industry. Int. J. Environ. Health Res. 15:339–345 [DOI] [PubMed] [Google Scholar]
- 71. Ortega YR, Sanchez R. 2010. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 23:218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Duhain G, Minnaar A, Buys EM. 2012. Effect of chlorine, blanching, freezing, and microwave heating on Cryptosporidium parvum viability inoculated on green peppers. J. Food Prot. 75:936–941 [DOI] [PubMed] [Google Scholar]
- 73. Trussell RR. 1998. Modeling chlorine inactivation requirements of Cryptosporidium parvum oocysts—discussion. J. Environ. Eng. 124:1141–1142 [Google Scholar]
- 74. von Gunten U. 2003. Ozonation of drinking water: part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 37:1469–1487 [DOI] [PubMed] [Google Scholar]
- 75. Hijnen WAM, Beerendonk EF, Medema GJ. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 40:3–22 [DOI] [PubMed] [Google Scholar]
- 76. Korich DG, Mead JR, Madore MS, Sinclair NA, Sterling CR. 1990. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 56:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li HB, Finch GR, Smith DW, Belosevic M. 2001. Chlorine dioxide inactivation of Cryptosporidium parvum in oxidant demand-free phosphate buffer. J. Environ. Eng. 127:594–603 [Google Scholar]
- 78. Kroupitski Y, Pinto R, Brandl MT, Belausov E, Sela S. 2009. Interactions of Salmonella enterica with lettuce leaves. J. Appl. Microbiol. 106:1876–1885 [DOI] [PubMed] [Google Scholar]