Abstract
In order to elucidate the effects of lignin composition on the resistance of wood to degradation by decay fungi, wood specimens from two transgenic poplar lines expressing an Arabidopsis gene encoding ferulate 5-hydroxylase (F5H) driven by the cinnimate-4-hydroxylase promoter (C4H::F5H) that increased syringyl/guaiacyl (S/G) monolignol ratios relative to those in the untransformed control wood were incubated with six different wood decay fungi. Alterations in wood weight and chemical composition were monitored over the incubation period. The results showed that transgenic poplar lines extremely rich in syringyl lignin exhibited a drastically improved resistance to degradation by all decay fungi evaluated. Lignin monomer composition and its distribution among cell types and within different cell layers were the sole wood chemistry parameters determining wood durability. Since transgenic poplars with exceedingly high syringyl contents were recalcitrant to degradation, where wood durability is a critical factor, these genotypes may offer improved performance.
INTRODUCTION
In recent years the importance and utility of lignocellulosic substrates have become more significant to industry, since woody biomass is now considered not only a feedstock for the manufacture of traditional commodities (lumber, lumber products, and paper) and cellulose-based chemicals but also the starting feedstock for bioenergy (1). Wood and other lignocellulosic materials are formed from three main polymeric constituents: cellulose, lignin, and hemicelluloses (2). Lignin is a complex aromatic polymer which is assembled via free radical coupling of monolignol precursors derived from three p-hydroxycinnamyl alcohols with varying degrees of methoxylation, resulting in guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H) subunits (3). These monomers vary depending on wood species, tissue type, and environmental stress (4).
Wood decay fungi (Basidiomycetes, Ascomycetes, and mitosporic fungi) are among the few microorganisms capable of degrading the complex lignocellulosic matrix (5, 6). They are conventionally classified into one of three major groups: brown, white, and soft rot fungi. White rot fungi can be further divided into two subtypes: (i) those involved in the oxidative cleavage of lignin and structural polysaccharides at similar rates, often referred to as simultaneous degraders, and (ii) those (selective delignifiers) capable of removing lignin in advance of cellulose and hemicelluloses. The latter subgroup accounts for a much smaller proportion of the taxa. A phylogenetically related group of brown rot basidiomycetes can, unlike white rot fungi, metabolize structural polysaccharides without removing lignin, causing significant strength losses in the early stages of wood decay (7, 8). Although it is not degraded, there is an indication that the lignin can be highly modified in some cases, via demethylation and/or demethoxylation (9–13). During soft rot degradation, the enzyme-mediated decay of cellulose and hemicelluloses is accompanied by some lignin degradation (5, 14).
Many studies have demonstrated that white rot fungi possess lignocellulolytic enzymatic systems that, if used during pretreatment, can lead to energy reductions during mechanical pulping (15, 16), are beneficial for biodegradation of recalcitrant biopolymers (17), and/or increase the efficiency of bioconversion (18, 19). On the other hand, brown rot fungi have been shown to possess specialized endoglucanases that can be used in industrial processes (20, 21) and offer a unique opportunity for consolidated bioprocessing of lignocellulosic substrates. The synergy between brown rot pretreatment and saccharification has been suggested to be beneficial in achieving full sugar yields from lignocellulosic substrates in bioethanol production (22, 23). However, the inherent recalcitrance of lignin continues to pose challenges for industrial applications (24, 25).
In secondary plant cell walls of wood, the lignin matrix that surrounds the cellulosic components acts as a barrier to wood decay, preventing the access of extracellular enzymes to the more readily degradable cellulose and hemicellulose moieties. Earlier studies (26–28) clearly provided evidence that differences in lignin content alone cannot explain the variation in wood decay rates but that anatomical characteristics and cell wall ultrastructural differences are influential. Lignin remains a major factor and has been shown to affect cell wall degradation rates in a concentration- and composition-dependent manner (5, 29, 30). However, the most recent comprehensive research was done in 1994, studying the impact of the lignin composition on resistance to wood decay (31). Those authors analyzed the degradation of wood from different tree species by Trametes versicolor and meticulously described variation in decay patterns in tissue types. That work (31) used wood from different tree species to establish a model that effectively explained the impact of lignin monomer composition on wood decay rates. However, since variation in other wood characteristics and chemical, morphological, and/or ultrastructural features were not considered, the conclusions drawn remain ambiguous.
Advances in tree genetic engineering have facilitated the production of tree varieties with altered wood chemistry (32–35). Ferulate-5-hydroxylase (F5H) is a key rate-limiting enzyme required for the biosynthesis of syringyl (S) monolignols (36), and transgenic poplar lines overexpressing an Arabidopsis F5H gene under the control of the cinnamate-4-hydroxylase (C4H) promoter (C4H::F5H), exhibit substantially altered S lignin content (ranging from as high as 94 mol% syringyl units to 65 mol% in the wild-type [WT] trees) (33, 36).
These transgenic trees afford a unique opportunity to elucidate the impact of lignin monolignol composition on susceptibility to degradation by white and brown rot fungi, since all other wood phenotypes in these genotypes are similar to those in wild-type trees. The specific aims of this research were (i) to expand our understanding of the role played by lignin monomer composition in wood decay resistance and (ii) to examine the differences in fungal degradation of wood derived from transgenic poplar lines with increased S/G ratios when incubated with brown rot, selective white rot, and simultaneous white rot decay fungi.
MATERIALS AND METHODS
Plant material.
All hybrid poplar trees (Populus alba × tremula; P717) were propagated from apical explants grown in sterile tissue culture (3-week growth cycle in tissue culture), and rooted cuttings were potted into soil medium. Trees were then grown in a temperature-controlled greenhouse under a fixed 16-h photoperiod with supplemental lighting (radiant flux density of 300 W m−2). Daily watering with fertigated water was achieved by flood table irrigation. The trees were harvested 3 years after transfer to the greenhouse. Five clonally propagated trees of each transgenic line and wild-type trees were selected and individual wood samples collected from each stem at 10 cm above the base of the root collar. Bark and pith were removed from the samples and then oven dried (50°C) for 2 days. About 50 discs ranging from 12 to 30 mm in diameter were cut from stems into 10-mm lengths, and they were randomized and used for the incubation with wood decay fungi.
Wood samples.
Two transgenic and one wild-type line of hybrid poplar P717 (Populus alba × tremula) were included in the experiment. The transgenic lines employed overexpress the Arabidopsis ferulate-5-hydroxylase (F5H) gene under the control of the cinnamate-4-hydroxylase (C4H) promoter (C4H::F5H) and exhibit considerable increases in the lignin syringyl/guaiacyl (S:G) monomer ratio, without a significant effect on p-hydroxyphenyl monomer composition or the overall relative lignin content of the wood, as previously determined by thioacidolysis (33). Line “64” exhibits ca. 94 mol%, line “82” exhibits ca. 85 mol%, and the wild type (WT) exhibits ca. 65 mol% of syringyl lignin units, as determined by thioacidolysis.
Fungal isolates and culture conditions.
Three brown rot fungi, i.e., Postia placenta (strain MAD 698-R), Gloeophyllum trabeum (strain MAD 617), and Coniophora puteana (strain UBC 01), and three white rot fungi, i.e., Ceriporiopsis subvermispora (strain UBC 17-3), Phanerochaete chrysosporium (strain RP 78), and Trametes versicolor (strain S4-118 AG), were used in this study. Each fungal strain was cultured on malt extract agar (MEA) (Oxoid, United Kingdom) for 10 days prior to inoculation onto wood samples. Identification of pure cultures was confirmed using mycelium characteristics as observed on MEA plates.
Miniblock test.
A modified version of the European Standard EN 113 (37) was used for the miniblock test. For each tree line/fungus combination and incubation period, five transgenic (lines 64 and 82) wood specimens (“miniblocks”) were paired with five parallel control (wild-type) specimens. A total of 360 wood discs were used in the miniblock test.
Wood samples were dried at 105°C for 24 h, cooled in a desiccator containing phosphorus pentoxide, weighed, sterilized for 20 min at 121°C, dried at 65°C for 48 h, and cooled to room temperature.
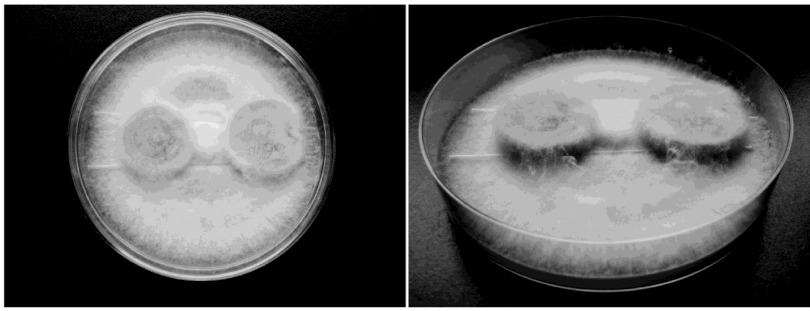
Two discs were placed on glass supports in a petri dish with actively growing mycelia (Fig. 1) and incubated at 22°C and 70% ± 5% relative humidity. Incubation periods were 4, 8, 12, and 16 weeks. Following incubation, discs were removed from the petri dishes, cleaned of associated mycelia, and reweighed. Before the discs were dried for measurement of weight loss, they were sampled at random points by removing chips of negligible weight. These were placed onto MEA to check whether the decay fungi were the only organisms present, and this was confirmed in all cases. The samples were then dried for weight loss measurement. Dry weight loss (WL) was calculated based on dry weights of discs before and after incubation according to the equation WL (%) = (W0 − W1)/W0 × 100%, where W0 is the original dry weight of the wood sample before incubation and W1 is the dry weight of the wood sample after incubation with decay fungi.
Fig 1.
Wood discs on glass supports incubated in petri dishes with actively growing mycelium.
Microscopy.
Specimens of wood discs were randomly selected to visualize the extent of degradation of both wild-type and transgenic line 64 poplar samples. Samples were prepared for scanning electron microscopy (SEM) by drying the specimens in a vacuum oven at 40°C and 1,000 Pa for 12 h, and they were then fixed in a holder using adhesive tape and sputtered coated with a 12-nm gold layer. The specimens were observed on a Hitachi S-2600N variable-pressure scanning electron microscope (VPSEM) at acceleration voltages of 10 and 15 kV.
Chemical analysis.
After weight loss values were calculated, one representative sample with the weight loss value closest to the mean value of each replicate group was selected for chemical analysis; 72 samples were therefore used to investigate variation in cell wall chemistry following incubation with each wood rot fungus. Samples were first ground in a Wiley mill to pass through a 40-mesh screen and then extracted for 12 h with hot acetone in a Soxhlet apparatus to remove extractives.
Lignin and carbohydrate contents were determined using a modified Klason procedure, where extracted ground stem tissue (0.2 g) was treated with 3 ml of 72% H2SO4 as described previously (33). The composition of neutral cell wall-associated carbohydrates (arabinose, rhamnose, galactose, glucose, mannose, and xylose) was determined using high-performance liquid chromatography (DX-600; Dionex, CA) equipped with an ion-exchange PA1 (Dionex) column, a pulsed amperometric detector (ED 40) with a gold electrode, and a Spectra AS 3500 autoinjector (Spectra-Physics, CA). The column was eluted with deionized water at a flow rate of 1 ml ml−1. Aliquots (20 μl) were injected after being passed through a 0.45-μm nylon syringe filter (Chromatographic Specialties Inc., Brockville, Ontario, Canada). Optimization of baseline stability and detector sensitivity was achieved by postcolumn addition of 0.2 M NaOH. Acid-soluble lignin was determined by UV absorbance at 205 nm according to TAPPI standard method UM-250 (38), while insoluble lignin was determined gravimetrically using medium-coarseness sintered glass crucibles.
Thioacidolysis.
Variation in lignin syringyl and guaiacyl monomer composition in samples selected for chemical analysis was assayed by thioacidolysis as described previously (39) using 10 mg of ground, extract-free oven-dried wood flour as the substrate. For each sample, 10 mg of ground, extract-free oven-dried wood flour was weighed into a 5-ml glass Wheaton vial with a Teflon-lined screw cap. One milliliter of freshly made reaction mixture (2.5% [vol/vol] boron trifluoride etherate [Sigma, St. Louis, MO] and 10% [vol/vol] ethanthiol [Sigma] in recently distilled dioxane) was added to each vial and blanketed with nitrogen gas prior to sealing. Vials were then placed together in a dry heating block (100°C) for 4 h with periodic (hourly) manual agitation. The reactions were halted by placing the reaction mixtures at −20°C for 5 min. An internal standard (5 mg ml−1 tetracosane [Sigma] in methylene chloride, 0.2 ml) was then added to each vial with enough 0.4 M sodium bicarbonate to bring the reaction pH to between 3 and 4 (ca. 0.3 ml, as determined by pH indicator paper). To extract the reaction products from the aqueous mixture, 2 ml of water and 1 ml of methylene chloride were added to each vial, which was then recapped, vortexed, and allowed to settle, phase separating the upper (aqueous) and lower (organic and containing lignin breakdown products) phases. An aliquot (1.5 ml) of the organic phase was removed with an autopipette, simultaneously cleared of residual water and filtered by passing through a Pasteur pipette packed with a small tissue paper plug and an inch (ca. 50 mg) of granular anhydrous sodium sulfate, and transferred directly into a 2-ml polypropylene microcentrifuge tube. Samples were then collectively evaporated to dryness in an Eppendorf Vacufuge (approximately 1.5 h at 45°C) and resuspended in 1 ml of methylene chloride. Samples were derivatized by combining 20 μl of resuspended sample with 20 μl of pyridine (Sigma) and 100 μl of N,O-bis(trimethylsilyl)acetamide (Sigma, St. Louis, MO). After incubation for at least 2 h at 25°C, 1 μl of this reaction product was analyzed by gas chromatography on a Hewlett-Packard 5890 series II instrument fitted with an autosampler, a splitless injector, a flame ionization detector (FID), and a 30-m RTX5ms 0.25-mm-internal-diameter capillary column. Injections were separated using helium as a carrier gas at 1 ml min−1. The inlet and detector temperatures were set to 250°C, while the oven profile consisted of an initial temperature of 130°C, holding for 3 min, temperature increase to 250°C, holding for 5 min, and cooling. Peak identification was consistent with that described previously (39).
Statistical analysis.
One-way analysis of variance (ANOVA) of the recorded dry weight losses was done for wood specimens, and the significance level was set at a P value of <0.05. A Tukey honestly significant difference (HSD) post hoc test was performed in SPSS to demonstrate differences in mean values.
RESULTS
Incubation with brown rot fungi. (i) Weight loss.
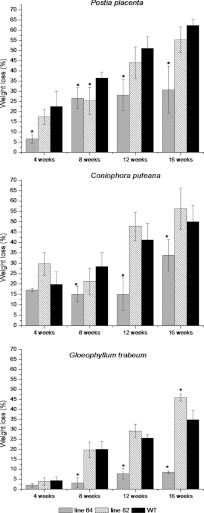
Most of the wood specimens were completely colonized by external mycelia after 16 weeks of incubation with brown rot fungi. Of the three fungal species, P. placenta showed the greatest extent of decomposition, resulting in a 62% weight loss in the wild type (Fig. 2). C. puteana induced the most extensive weight losses in transgenic line 82. The lowest weight losses after 16 weeks occurred in transgenic line 64 incubated with G. trabeum. Weight losses in line 64 were significantly lower than those in the corresponding wild-type controls when incubated with all three brown rot fungi. Poplar line 82 exhibited higher susceptibility to degradation by P. placenta than line 64, but this was not significantly different from results for samples taken from wild-type trees. Samples of transgenic poplar line 82 incubated with G. trabeum and C. puteana were, in contrast, more susceptible to degradation than the wild type.
Fig 2.
Dry weight losses of two transgenic poplar lines and the WT incubated with brown rot fungi G. trabeum, C. puteana, and P. placenta. Samples were incubated for 4, 8, 12, or 16 weeks (n = 5). Lines 64 and 82 have very high and high S/G ratios relative to that of the WT. Error bars represent standard deviations. Asterisks denote significant differences compared to the WT (P < 0.01).
(ii) Sugars.
Chemical analysis of the cell wall carbohydrates in decayed wood samples demonstrated an overall decreasing trend in total carbohydrates (Table 1). The most extensive losses were recorded for wild-type samples incubated with P. placenta (47% of glucose, 37% of xylose, and 73% of mannose). During the first 8 weeks of incubation, P. placenta degraded about 20 and 12%, respectively, of available xylose and glucose in wild-type trees but degraded none of the other carbohydrates. Incubation for an additional 8 weeks resulted in the loss of an additional 35% of glucose and 17% of xylose. At the same time, mannose was left almost intact during the first 12 weeks, and its rapid utilization (about 73%) took place only in the advanced stages of degradation. The greatest levels of glucose and xylose depletion were observed in line 82 incubated with C. puteana (losses of 48% and 31%, respectively), leaving rhamnose, galactose, and mannose not significantly affected. Mannose in line 64 showed the greatest extent of degradation among all the sugars (65% loss after a 16-week incubation with C. puteana), and only a 28% reduction in glucose was detected after 16 weeks with C. puteana. In contrast, G. trabeum employed a different strategy: it solubilized approximately 60% of the available mannose, 55% of galactose, and 30% of arabinose during the first 8 weeks of incubation but solubilized little or no glucose and xylose. No additional decreases in arabinose, rhamnose, galactose, or xylose were noted with prolonged incubation. However, additional glucose and mannose were removed to various degrees. The glucose content diminished by 29% in line 82 and only by approximately 4% in both the wild type and line 64 after 16 weeks of incubation with G. trabeum. Furthermore, decreasing sugar content correlated with increasing mass losses in test blocks in all fungus-wood substrate combinations.
Table 1.
Relative chemical compositions of hybrid poplar wood incubated with brown rot fungi
| Fungus | Hybrid line | Incubation period, wk | Carbohydrates, % |
Lignin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Gal | Glu | Xyl | Man | Insoluble, % | Soluble, % | Total, % | S/G ratio | S, % | |||
| C. puteana | 64 | 0 | 0.3 | 0.3 | 0.5 | 51.3 | 19.8 | 1.1 | 18.1 | 5.4 | 25.5 | 14.4 | 93.5 |
| 4 | 0.3 | 0.3 | 0.5 | 51.6 | 19.5 | 1.1 | 18.6 | 5.8 | 24.4 | 13.8 | 93.3 | ||
| 8 | 0.3 | 0.3 | 0.4 | 49.4 | 20.1 | 0.6 | 21.0 | 5.6 | 26.6 | 12.0 | 92.3 | ||
| 12 | 0.4 | 0.3 | 0.5 | 49.8 | 18.0 | 0.8 | 23.0 | 5.5 | 28.5 | 11.9 | 92.2 | ||
| 16 | 0.2 | 0.2 | 0.3 | 36.7 | 16.0 | 0.4 | 29.1 | 6.7 | 35.8 | 11.8 | 92.2 | ||
| 82 | 0 | 0.2 | 0.3 | 0.4 | 47.0 | 20.3 | 0.4 | 25.0 | 5.4 | 30.4 | 4.4 | 81.5 | |
| 4 | 0.2 | 0.3 | 0.4 | 47.4 | 19.1 | 0.3 | 25.6 | 5.2 | 30.7 | 4.4 | 81.6 | ||
| 8 | 0.2 | 0.3 | 0.3 | 52.0 | 15.6 | 0.7 | 19.8 | 4.3 | 24.1 | 4.4 | 81.6 | ||
| 12 | 0.1 | 0.2 | 0.3 | 31.1 | 14.9 | 0.2 | 28.3 | 4.7 | 33.0 | 4.4 | 81.5 | ||
| 16 | 0.1 | 0.3 | 0.3 | 26.8 | 13.3 | 0.3 | 40.1 | 4.7 | 44.8 | 4.0 | 80.0 | ||
| WT | 0 | 0.2 | 0.3 | 0.4 | 50.1 | 15.0 | 0.4 | 21.4 | 2.8 | 24.2 | 2.1 | 67.7 | |
| 4 | 0.2 | 0.3 | 0.4 | 49.2 | 15.3 | 0.5 | 22.7 | 3.0 | 25.7 | 2.1 | 67.3 | ||
| 8 | 0.2 | 0.3 | 0.5 | 47.9 | 14.2 | 0.9 | 24.0 | 3.3 | 27.3 | 2.1 | 68.2 | ||
| 12 | 0.2 | 0.2 | 0.5 | 40.7 | 13.4 | 0.6 | 26.0 | 3.6 | 29.6 | 2.2 | 68.8 | ||
| 16 | 0.2 | 0.2 | 0.3 | 35.6 | 13.4 | 0.4 | 32.4 | 3.8 | 36.2 | 2.3 | 69.7 | ||
| G. trabeum | 64 | 0 | 0.3 | 0.3 | 0.5 | 51.3 | 19.8 | 1.1 | 15.0 | 5.8 | 20.8 | 11.3 | 91.9 |
| 4 | 0.4 | 0.4 | 0.6 | 50.9 | 21.0 | 1.0 | 15.2 | 6.0 | 21.1 | 11.9 | 92.2 | ||
| 8 | 0.4 | 0.4 | 0.6 | 50.8 | 20.7 | 1.1 | 19.2 | 5.5 | 24.7 | 12.0 | 92.3 | ||
| 12 | 0.4 | 0.4 | 0.5 | 49.8 | 20.2 | 1.3 | 19.3 | 5.8 | 25.0 | 15.9 | 94.1 | ||
| 16 | 0.5 | 0.6 | 0.8 | 49.7 | 20.1 | 1.5 | 19.5 | 5.0 | 24.5 | 17.1 | 94.5 | ||
| 82 | 0 | 0.3 | 0.2 | 0.4 | 51.3 | 19.0 | 1.2 | 14.5 | 4.2 | 18.7 | 4.6 | 82.1 | |
| 4 | 0.2 | 0.2 | 0.4 | 51.4 | 18.8 | 1.2 | 14.9 | 4.2 | 19.1 | 4.6 | 82.2 | ||
| 8 | 0.1 | 0.2 | 0.2 | 50.8 | 17.6 | 0.6 | 16.4 | 4.4 | 20.8 | 4.8 | 82.7 | ||
| 12 | 0.1 | 0.2 | 0.2 | 43.2 | 15.2 | 0.7 | 19.0 | 4.4 | 23.4 | 4.8 | 82.8 | ||
| 16 | 0.1 | 0.2 | 0.3 | 36.8 | 14.3 | 0.4 | 30.4 | 4.7 | 35.1 | 4.9 | 83.0 | ||
| WT | 0 | 0.2 | 0.2 | 0.5 | 50.5 | 17.2 | 1.2 | 18.3 | 2.8 | 21.1 | 2.3 | 69.7 | |
| 4 | 0.2 | 0.2 | 0.6 | 50.6 | 17.2 | 1.4 | 15.1 | 2.9 | 18.0 | 2.3 | 69.2 | ||
| 8 | 0.1 | 0.2 | 0.2 | 50.3 | 17.1 | 0.6 | 20.8 | 2.9 | 23.7 | 2.3 | 69.4 | ||
| 12 | 0.1 | 0.1 | 0.2 | 49.8 | 14.3 | 0.5 | 22.0 | 2.9 | 24.9 | 2.2 | 69.1 | ||
| 16 | 0.1 | 0.2 | 0.2 | 48.6 | 14.2 | 0.4 | 24.1 | 3.1 | 27.3 | 2.3 | 69.7 | ||
| P. placenta | 64 | 0 | 0.5 | 0.3 | 0.5 | 51.2 | 19.9 | 0.5 | 15.0 | 4.9 | 19.9 | 15.2 | 93.8 |
| 4 | 0.5 | 0.2 | 0.6 | 50.1 | 20.2 | 0.5 | 14.7 | 5.0 | 19.7 | 15.4 | 93.9 | ||
| 8 | 0.2 | 0.2 | 0.2 | 46.3 | 18.7 | 0.6 | 21.1 | 5.2 | 26.3 | 16.8 | 94.4 | ||
| 12 | 0.4 | 0.4 | 0.6 | 43.9 | 17.7 | 0.7 | 24.0 | 5.5 | 29.5 | 17.1 | 94.5 | ||
| 16 | 0.2 | 0.3 | 0.4 | 41.2 | 17.6 | 0.9 | 27.0 | 5.9 | 32.9 | 18.0 | 94.7 | ||
| 82 | 0 | 0.3 | 0.4 | 0.3 | 52.8 | 18.1 | 0.6 | 14.5 | 4.2 | 18.7 | 5.1 | 83.6 | |
| 4 | 0.3 | 0.3 | 0.3 | 53.5 | 17.9 | 0.6 | 18.1 | 4.3 | 22.4 | 5.0 | 83.4 | ||
| 8 | 0.1 | 0.1 | 0.1 | 47.0 | 16.0 | 0.6 | 21.1 | 4.5 | 25.6 | 4.9 | 82.9 | ||
| 12 | 0.2 | 0.2 | 0.3 | 39.7 | 15.0 | 0.2 | 32.0 | 4.8 | 36.7 | 4.7 | 82.4 | ||
| 16 | 0.1 | 0.2 | 0.2 | 35.7 | 14.0 | 0.2 | 34.3 | 5.1 | 39.4 | 4.5 | 81.8 | ||
| WT | 0 | 0.2 | 0.2 | 0.3 | 53.4 | 18.3 | 0.9 | 18.5 | 2.8 | 21.3 | 2.3 | 69.7 | |
| 4 | 0.2 | 0.2 | 0.2 | 53.3 | 19.5 | 0.5 | 21.7 | 3.0 | 24.7 | 2.4 | 70.4 | ||
| 8 | 0.2 | 0.2 | 0.2 | 47.0 | 15.2 | 0.4 | 26.0 | 3.0 | 29.0 | 2.3 | 69.8 | ||
| 12 | 0.1 | 0.2 | 0.3 | 32.5 | 14.3 | 0.4 | 37.0 | 3.4 | 40.4 | 2.0 | 66.1 | ||
| 16 | 0.1 | 0.2 | 0.2 | 28.8 | 12.9 | 0.1 | 43.0 | 3.5 | 46.5 | 1.9 | 65.8 | ||
(iii) Lignin.
Incubation with brown rot fungi resulted in relative increases in both insoluble and soluble lignin fractions and hence an increase in the total lignin content relative to other cell wall polymers (Table 1). Both transgenic lines (“64” and “84”) were characterized by higher relative soluble lignin contents (5% and 4%, respectively) and reduced amounts of insoluble lignin, whereas the total lignin content (ca. 21%) was comparable to that of wild-type poplar trees. The maximum total lignin content was recorded for wild-type poplar after its 16-week incubation with P. placenta, which corresponded to the highest loss of carbohydrates and the highest mass loss. Following incubation with C. puteana, the relative soluble lignin fraction in line 64 increased by 14% and the insoluble lignin fraction by 56%, resulting in a 46% increase in total relative lignin content. The relative insoluble lignin content almost doubled in transgenic poplar line 64 after 16 weeks of incubation with G. trabeum, while the insoluble fraction content did not change. The two transgenic lines and wild-type trees exhibited a relative accumulation of both lignin fractions as a result of wood degradation by P. placenta.
Alterations in the S/G ratio, as estimated by thioacidolysis, differed depending on the wood substrate colonized by the fungal species. Although the content of syringyl monomers increased in wild-type wood incubated with C. puteana, transgenic poplar lines 64 and 82 exhibited slight reductions in the syringyl monomer content (Table 1). Degradation by G. trabeum did not cause any significant changes in S/G ratio in any poplar line. In contrast, the percentage of syringyl lignin monomers decreased slightly over the course of incubation with P. placenta, dropping by 7% in the wild type and 1.8% in line 82. In contrast, the syringyl content of transgenic line 64 incubated with G. trabeum increased by 2.3%. In this case, this slight increase was observed at a weight loss corresponding to 58.6%.
Incubation with simultaneous white rot fungi. (i) Weight loss.
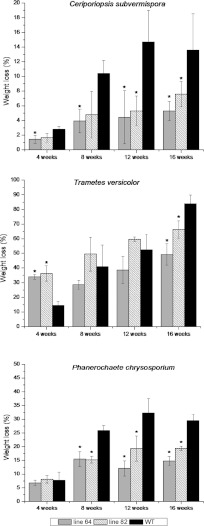
The simultaneous white rot fungi P. chrysosporium and T. versicolor induced the highest weight losses among all 360 samples (Fig. 3), with the greatest being a 84% reduction in the wild type exposed to T. versicolor for 16 weeks. During the incipient stages of degradation, transgenic lines 64 and 82 incubated with T. versicolor exhibited substantially higher weight losses than the corresponding wild type at the same incubation time; however, after 16 weeks, both transgenic lines appeared to be less susceptible to degradation, displaying significantly lower overall mass loss than the wild-type trees. Wood degradation by P. chrysosporium was somewhat less vigorous, with only 29.5% of mass loss in the wild type after 16 weeks. In both transgenic poplar lines, enhanced resistance to degradation by P. chrysosporium was also observed throughout the duration of the experiment.
Fig 3.
Dry weight losses of two transgenic poplar lines and the WT incubated with white rot fungi P. chrysosporium, T. versicolor, and C. subvermispora for 4, 8, 12, of 16 weeks (n = 5). Lines 64 and 82 have very high and high S/G ratios relative to that of the WT. Error bars represent standard deviations. Asterisks denote significant differences compared to the WT (P < 0.01).
(ii) Chemical composition.
Although P. chrysosporium caused a reduction in total lignin content in line 82 and the wild type, the more resistant line 64 showed a relative increase in both soluble and insoluble lignin fractions (Table 2). Apparently, the main targets of the enzymatic system of T. versicolor were cellulose and hemicelluloses, since the quantities of glucose and xylose dropped drastically in both transgenic lines and the wild type. As such, the relative lignin content increased to levels comparable to those in wood degraded by brown rot fungi.
Table 2.
Relative chemical compositions of hybrid poplar wood incubated with white rot fungi causing simultaneous rot
| Fungus | Hybrid line | Incubation period, wk | Carbohydrates, % |
Lignin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Gal | Glu | Xyl | Man | Insoluble, % | Soluble, % | Total, % | S/G ratio | S, % | |||
| P. chrysosporium | 64 | 0 | 0.5 | 0.3 | 1.0 | 54.0 | 14.5 | 1.6 | 14.2 | 5.3 | 19.5 | 14.2 | 93.4 |
| 4 | 0.4 | 0.4 | 1.1 | 54.1 | 14.3 | 1.7 | 14.0 | 5.2 | 19.1 | 13.8 | 93.2 | ||
| 8 | 0.5 | 0.5 | 1.2 | 53.3 | 17.3 | 1.6 | 15.3 | 5.6 | 20.9 | 15.5 | 93.9 | ||
| 12 | 0.5 | 0.4 | 1.1 | 55.3 | 16.4 | 1.3 | 16.5 | 5.4 | 21.9 | 15.4 | 93.9 | ||
| 16 | 0.5 | 0.4 | 0.9 | 52.1 | 16.6 | 1.4 | 16.3 | 6.5 | 22.7 | 15.2 | 93.8 | ||
| 82 | 0 | 0.4 | 0.3 | 1.1 | 54.0 | 18.2 | 1.3 | 18.0 | 4.4 | 22.4 | 4.9 | 83.1 | |
| 4 | 0.6 | 0.4 | 1.1 | 54.8 | 18.3 | 1.6 | 18.0 | 4.4 | 22.3 | 4.5 | 81.7 | ||
| 8 | 0.3 | 0.3 | 1.0 | 53.1 | 15.6 | 1.1 | 16.9 | 4.4 | 21.4 | 4.3 | 81.2 | ||
| 12 | 0.3 | 0.3 | 1.0 | 52.3 | 15.5 | 1.7 | 14.6 | 4.3 | 18.9 | 4.1 | 80.4 | ||
| 16 | 0.3 | 0.3 | 0.9 | 52.3 | 16.7 | 1.6 | 14.4 | 4.2 | 18.6 | 3.9 | 79.7 | ||
| WT | 0 | 0.3 | 0.2 | 0.5 | 53.4 | 14.6 | 1.9 | 19.0 | 2.8 | 21.8 | 2.0 | 66.7 | |
| 4 | 0.4 | 0.3 | 0.9 | 54.9 | 14.7 | 1.8 | 19.2 | 2.8 | 21.9 | 1.9 | 65.3 | ||
| 8 | 0.4 | 0.4 | 1.0 | 54.0 | 13.9 | 1.5 | 18.8 | 2.7 | 21.5 | 1.9 | 64.9 | ||
| 13 | 0.3 | 0.3 | 1.0 | 52.2 | 13.6 | 1.5 | 18.4 | 2.7 | 21.2 | 1.7 | 62.7 | ||
| 16 | 0.3 | 0.3 | 1.0 | 53.2 | 14.1 | 1.6 | 17.8 | 2.9 | 20.7 | 1.7 | 63.0 | ||
| T. versicolor | 64 | 0 | 0.3 | 0.3 | 1.0 | 52.1 | 18.0 | 1.6 | 14.3 | 5.2 | 19.5 | 11.2 | 91.8 |
| 4 | 0.4 | 0.3 | 0.9 | 52.9 | 18.2 | 1.3 | 15.2 | 5.4 | 20.5 | 10.6 | 91.3 | ||
| 8 | 0.5 | 0.4 | 0.7 | 52.7 | 16.9 | 0.9 | 16.4 | 5.0 | 21.4 | 10.0 | 90.9 | ||
| 12 | 0.4 | 0.5 | 0.7 | 50.5 | 15.7 | 1.1 | 16.8 | 5.6 | 22.4 | 9.7 | 90.6 | ||
| 16 | 0.5 | 0.5 | 0.7 | 46.1 | 14.2 | 1.3 | 20.8 | 5.9 | 26.7 | 9.7 | 90.6 | ||
| 82 | 0 | 0.5 | 0.3 | 1.1 | 52.1 | 17.8 | 0.7 | 17.5 | 4.0 | 21.5 | 4.5 | 81.8 | |
| 4 | 0.5 | 0.3 | 1.0 | 49.3 | 16.9 | 0.8 | 18.0 | 4.0 | 22.1 | 4.0 | 79.8 | ||
| 8 | 0.4 | 0.3 | 0.8 | 47.1 | 16.5 | 1.0 | 19.6 | 4.2 | 23.8 | 3.0 | 75.2 | ||
| 12 | 0.7 | 0.4 | 1.1 | 46.5 | 15.4 | 1.2 | 20.4 | 4.2 | 24.5 | 2.9 | 74.6 | ||
| 16 | 0.7 | 0.5 | 1.1 | 39.5 | 14.6 | 1.1 | 24.1 | 4.3 | 28.4 | 2.7 | 72.8 | ||
| WT | 0 | 0.3 | 0.3 | 1.1 | 50.0 | 15.1 | 1.7 | 17.1 | 2.4 | 19.5 | 2.1 | 67.7 | |
| 4 | 0.3 | 0.2 | 0.9 | 52.7 | 14.5 | 1.6 | 17.9 | 2.7 | 20.7 | 2.0 | 66.6 | ||
| 8 | 0.5 | 0.4 | 1.3 | 45.0 | 14.0 | 1.9 | 20.2 | 3.1 | 23.3 | 1.3 | 56.7 | ||
| 12 | 0.3 | 0.2 | 1.6 | 42.8 | 13.5 | 1.3 | 24.3 | 2.4 | 26.7 | 1.2 | 53.9 | ||
| 16 | 0.6 | 0.5 | 1.8 | 37.1 | 11.2 | 1.8 | 29.5 | 2.7 | 32.2 | 0.8 | 43.5 | ||
A noticeable reduction in syringyl monomer content occurred in wild-type trees incubated with both the simultaneous white rot fungi and in line 82 incubated with T. versicolor, whereas the syringyl content of the transgenic line 64 did not change substantially over the course of exposition.
Incubation with a selective delignifying white rot fungus. (i) Weight loss.
C. subvermispora, the only species tested causing selective delignification, induced lower weight losses than the other white rot fungi employed (Fig. 3). Following the longest incubation period (16 weeks), the greatest mass reduction was recorded for wild-type trees (15.2%), while both transgenic lines 64 and 82 were resistant to degradation, showing only 5.3% and 7.6% decreases in weight, respectively.
(ii) Chemical composition.
Most of the changes in chemistry induced by C. subvermispora degradation can be attributed to lignin decomposition. The insoluble lignin fraction decreased by 28.2% in the wild type after 16 weeks, whereas the soluble lignin fraction was augmented by 27% relative to other cell wall constituents (Table 3). The degradation of lignin in the two transgenic poplars followed a similar pattern: reductions in insoluble lignin and concurrent relative increases in the soluble fraction. The relative glucose and mannose contents increased in all three wood substrates. Xylose, on the other hand, displayed an inconsistent behavior; its relative content increased by 7% in the degraded substrate of line 64 and decreased by 24% and 15% in line 82 and the wild type, respectively.
Table 3.
Relative chemical composition of hybrid poplar wood incubated with C. subvermispora, a white rot fungus causing selective delignification
| Hybrid line | Incubation period, wk | Carbohydrates, % |
Lignin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Gal | Glu | Xyl | Man | Insoluble, % | Soluble, % | Total, % | S/G ratio | S, % | ||
| 64 | 0 | 0.4 | 0.3 | 1.0 | 51.1 | 18.8 | 1.2 | 16.5 | 5.1 | 21.6 | 14.1 | 93.4 |
| 4 | 0.5 | 0.4 | 0.8 | 51.4 | 19.3 | 1.1 | 16.6 | 5.1 | 21.6 | 14.0 | 93.3 | |
| 8 | 0.4 | 0.5 | 0.8 | 52.6 | 19.3 | 1.2 | 15.9 | 5.5 | 21.4 | 14.0 | 93.3 | |
| 12 | 0.4 | 0.6 | 0.7 | 53.8 | 20.2 | 1.4 | 15.4 | 5.5 | 20.9 | 14.0 | 93.3 | |
| 16 | 0.5 | 0.6 | 0.8 | 54.6 | 20.6 | 1.7 | 14.5 | 5.6 | 20.1 | 17.0 | 94.4 | |
| 82 | 0 | 0.5 | 0.3 | 0.8 | 51.0 | 21.4 | 1.0 | 17.5 | 4.0 | 21.5 | 5.0 | 83.3 |
| 4 | 0.6 | 0.4 | 0.8 | 50.2 | 22.1 | 1.0 | 17.3 | 4.1 | 21.5 | 5.1 | 83.6 | |
| 8 | 0.5 | 0.5 | 0.8 | 52.2 | 20.3 | 1.2 | 15.6 | 4.2 | 19.8 | 4.6 | 82.1 | |
| 12 | 0.4 | 0.3 | 0.8 | 53.2 | 19.5 | 1.4 | 14.9 | 4.3 | 19.2 | 4.7 | 82.5 | |
| 16 | 0.2 | 0.3 | 0.8 | 58.0 | 16.8 | 1.6 | 14.6 | 4.5 | 19.0 | 4.8 | 82.7 | |
| WT | 0 | 0.4 | 0.5 | 0.9 | 50.8 | 20.0 | 1.5 | 17.8 | 2.4 | 20.2 | 2.0 | 66.7 |
| 4 | 0.4 | 0.5 | 0.9 | 50.1 | 20.4 | 1.2 | 18.4 | 2.7 | 21.1 | 2.0 | 66.3 | |
| 8 | 0.3 | 0.4 | 0.9 | 51.6 | 19.6 | 1.7 | 15.7 | 3.0 | 18.7 | 2.1 | 67.6 | |
| 12 | 0.3 | 0.4 | 0.8 | 52.2 | 19.0 | 1.7 | 13.7 | 3.2 | 16.9 | 2.2 | 68.4 | |
| 16 | 0.3 | 0.4 | 0.8 | 54.5 | 17.3 | 2.5 | 13.2 | 3.4 | 16.7 | 2.3 | 69.3 | |
The ratio of S lignin monomers to G lignin monomers gradually increased in wild-type specimens over the duration of the incubation (Table 3), and did not change considerably in the transgenic lines.
DISCUSSION
Of two transgenic poplar tree lines with elevated syringyl content, only the line containing an extremely large amount of syringyl monomer (line 64) showed improved resistance against both brown and white rot fungi. Each fungus included in the study responded differently to genetically modified wood substrate: brown rot fungi primarily targeted carbohydrates, simultaneous white rot fungi degraded both lignin and sugars but did not alter the syringyl lignin content in line 64, and selective delignification by C. subvermispora resulted in the smallest weight losses and very little change in the chemical compositions of the wild-type and transgenic poplar tree lines.
Brown rot.
Microscopic examinations (Fig. 4 to 6) of the woody biomass revealed degradation patterns typical for brown rot fungi: extensive degradation of polysaccharides in cell walls of the fibers, leaving a fragile lignin framework. Fiber tracheids of the transgenic line 64 with the extreme syringyl monomer content exhibited enhanced resistance to degradation by all three brown rot fungi (Fig. 4C, 5C, and 6C). Both C. puteana and P. placenta induced comparable weight losses (ca. 35%) in the transgenic line 64 following a 16-week incubation, demonstrating that the degraded cell walls are alike (Fig. 4C and 5C).
Fig 4.

Transverse (A and C) and longitudinal (B) surfaces of wild-type (A and B) and transgenic line 64 (C) poplar wood incubated with Postia placenta for 16 weeks. Note the extensive degradation of cellulose, leaving a porous framework of residual lignin within cell walls (A and C).
Fig 6.
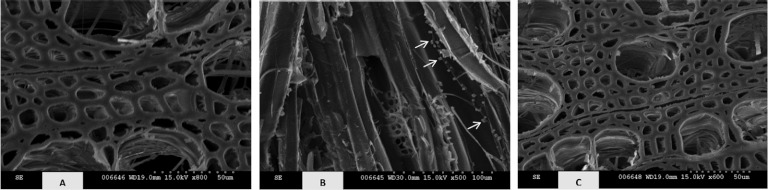
Transverse (A and C) and longitudinal (B) surfaces of wild-type (A and B) and transgenic line 64 (C) poplar incubated with Gloeophyllum trabeum for 16 weeks. Note the vessels of WT poplar wood colonized by mycelium (A and B) and intact fibers of transgenic poplar (C). Arrows indicate deposition of cube-shaped crystals along the mycelium (B).
Fig 5.

Transverse (A and C) and longitudinal (B) surfaces of wild-type (A, B) and transgenic line 64 (C) poplar incubated with Coniophora puteana for 16 weeks. Note vessels filled with mycelia (A and B) serving as gateways for colonization and later diffuse degradation of cellulose in fibers (B). Arrows indicate a white rot-type degradation of middle lamella (A). Note the formation of cavities in xylem ray parenchyma cells (B).
Brown rot fungi preferentially degrade cell wall polysaccharides and, according to recent findings, possess the ability to cause significant lignolysis (13). Consistent with this, in wood samples isolated from wild-type and transgenic trees, the brown rot colonization led to an overall decrease in the polysaccharide composition of the wood. During the initial stages of degradation, the carbohydrates most affected were sugars derived from the primary walls and middle lamella (galactose and arabinose), which are often attributed to the building blocks of hemicelluloses and/or arabinogalactan proteins (AGP) in the primary wall (2). Glucose content was not significantly altered by G. trabeum, whereas in the cases of C. puteana and P. placenta degradation gradually decreased, suggesting minor decomposition of cellulose. This is consistent with the ultrastructural changes observed in wood samples by SEM (Fig. 4 and 5). C. puteana is reported to be the only brown rot fungus possessing genes for glycoside hydrolase (GH) families GH6 and GH7 in its genome (8), which include cellobiohydrolase, an enzyme capable of eroding the crystalline portion of the cellulose to produce amorphous regions susceptible to endoglucanase-mediated cleavage (40), which are subsequently hydrolyzed to glucose by β-glucosidases. In contrast, P. placenta and G. trabeum lack GH6 and GH7 family enzymes. The unique patterns of degradation by C. puteana observed in the current study are consistent with recent reports (8, 41) confirming that C. puteana has the full battery of cellulases (endoglucanases, cellobiohydrolases, and β-glucosidases), which thus results in the highest degree of glucose degradation.
Given that high-molecular-weight lignocellulolytic enzymes are unable to penetrate into the complex cell wall structure during the early stages of the wood decay, it has been suggested that low-molecular-weight compounds act as the initiators of both cellulose and lignin solubilization (42, 43). Recent genomic studies investigating the mechanisms of decay of P. placenta showed that brown rot decay is facilitated by the extracellular generation of hydroxyl radicals and the participation of low-molecular-weight compounds (hydrogen peroxide, iron-reducing compounds, and oxalates) which diffuse into the cell wall (11, 42–45). It has been proposed that oxalic acid binds Fe3+ to form a stable Fe-oxalate complex diffusing into the cell wall together with hydrogen peroxide and iron-reducing compounds (46). Subsequently, Fe3+ is sequestered from the Fe-oxalate complex and is reduced by hydrogen peroxide to Fe2+, which then participates in Fenton chemistry to generate nonspecific hydroxyl radicals that can cleave the glucan chains of cellulose as well as demethylate, depolymerize, and repolymerize lignin (5, 45, 47, 48). A suite of cytochrome P450 genes was recently identified in all three brown rot fungi, even larger than that those previously identified in P. chrysosporium (8, 19). These cytochrome P450 monooxygenases have previously been hypothesized to be candidates for lignin biodegradation (49–51) and to drive lignin demethylation reactions and degrade extractives (52).
Although all three poplar lines demonstrated elevated relative quantities of total lignin after brown rot decomposition, a rather peculiar trend was observed with the monolignol composition. The enzymatic systems of C. puteana induced a relative increase in the syringyl content in wild-type poplar wood, indicating preferential degradation of guaiacyl units, owing presumably to the earlier reported presence of a lignin-degrading laccase (53) which makes the degradation pattern of C. puteana exhibit both brown- and white rot features: thinning of secondary walls and degradation of middle lamella (Fig. 5A). In contrast, in transgenic line 64 with an extremely high syringyl content, the S/G ratio decreased, indicating either preferential degradation of syringyl units or the transformation of syringyl units into guaiacyl as a result of demethoxylation.
Even though G. trabeum did not alter the ratio of monomers in the wild type, it exhibited a preference for the guaiacyl subunits in the transgenic line 64. A drop in the S/G ratio in the wild type and its increase in line 64 incubated with P. placenta suggests a preference for guaiacyl monomers in the transgenic syringyl-abundant substrate. Although in nature P. placenta is more frequently associated with gymnosperm degradation (5, 54) and cannot cope with high syringyl content, it also occurs naturally on aspen (55). Interestingly, a preference for syringyl subunits is evident on the naturally occurring substrate without shifting the S/G ratio. Moreover, the selective delignifier, C. subvermispora, exhibited a similar trend of wood substrate preference.
Simultaneous white rot.
Modifications in the chemical composition of poplar degraded by P. chrysosporium and T. versicolor are indicative of different decomposition strategies employed by these fungi compared to that of brown rot fungi (Fig. 7 and 8). The enzymatic systems of white rot fungi effectively depolymerize lignin, facilitating access to the cell wall carbohydrates (56), as was illustrated here by the significantly higher weight losses observed and the greater extent of lignocellulose decomposition (Table 2). Moreover, the white rot fungi secrete lignin-degrading enzymes such as lignin peroxidases, manganese peroxidases, and laccases externally into a mucilaginous sheath, composed largely of β-1,3-1,6-linked glucan, which is found closely associated both with the hyphae and with regions of the woody cell wall that are undergoing decay (57), and the depletion of lignin occurs in the immediate vicinity of wood substrate (58, 59). P. chrysosporium also has the capacity to alter the carbohydrate composition via oxidoreductive enzymes, such as cellobiose dehydrogenase (60). Syringyl units were preferentially degraded in the wild type; however, the absence of a preference for any of the monolignols is clearly demonstrated by the unchanging syringyl content in line 64 throughout the incubation.
Fig 7.

Transverse (A and C) and longitudinal (B) surfaces of wild-type (A and B) and transgenic line 64 (C) poplar incubated with Trametes versicolor for 16 weeks. Note the extensive colonization of vessels with mycelium (A and C) and formation of erosion troughs and cavities in wild type (B).
Fig 8.
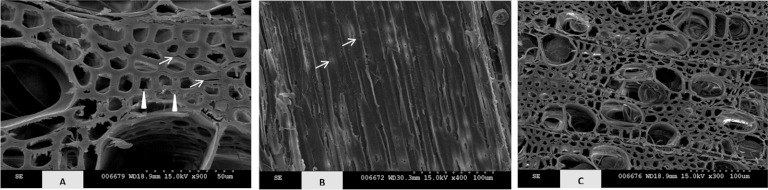
Transverse (A and C) and longitudinal (B) surfaces of wild-type (A and B) and transgenic line 64 (C) poplar incubated with Phanerochaete chrysosporium for 16 weeks. Note the sparse amount of mycelium at the advanced stage of degradation. Arrows indicate fiber delignification (A) and formation of bore holes (B), and arrowheads indicate formation of erosion holes in the secondary walls (A).
Selective delignification.
Our study demonstrated that C. subvermispora extensively degraded lignin without removing significant proportions of the available carbohydrates. Lignin compositional analysis indicated that the S/G ratios increased slightly as the fungal degradation advanced (Table 3). This implies that guaiacyl units are degraded preferentially relative to syringyl units in all three poplar lines colonized by C. subvermispora. Lower weight losses in both transgenic lines indicate a significantly improved decay resistance to selective delignification. In contrast, Choi et al. (61) observed a reduction in S/G ratio with the length of incubation, and they attributed this to demethoxylation reactions, which give rise to guaiacyl units from syringyl units. Another study showed that C. subvermispora is able to heavily depolymerize lignin as early as the first 4 weeks of degradation by cleaving α-β and β-O-4 linkages (62). Thereafter, the amount of aryl-ether linkages tended to stabilize, and the biodegradation was marked by a significant mineralization of the lignin macromolecule (63). These findings concur with our observations that insoluble lignin fractions increased with the length of incubation (Table 3).
Lignin monomer composition and wood decay resistance.
For decades scientists have made various attempts to explain the effect of lignin on wood decay. Generally, the concentration of lignin and type of lignin found in wood were considered to be the two major factors influencing wood decay (28, 64, 65). The composition and distribution of lignin in different cell layers and cell types appear to play a role as well (31, 66). Guaiacyl-derived lignin subunits are preferentially deposited in the walls of vessels (67–69). In fibers, the middle lamella is rich in guaiacyl-lignin and the ray secondary walls contain a high proportion of syringyl-lignin (70). Obst et al. (31) demonstrated that woods with fairly uniform syringyl content in the middle lamella and cell corners of fibers were more resistant to decay. Furthermore, they observed that in some wood species, syringyl units were preferentially degraded, whereas in others, syringyl content had no general correlation with the rate of decay. Tropical hardwoods containing guaiacyl-rich lignin have also been shown to be more resistant to decay than those containing syringyl-rich lignin (71). In poplar xylem, fiber tracheids make up to 56% of all cell types, leaving 34% to vessels and 10% to xylem ray parenchyma (72). Assuming that vessels tend to have an elevated concentration of guaiacyl lignin subunits, it is likely that in transgenic line 64 wood with an overall high S/G ratio, the ratio is even higher in fiber tracheids and lower in vessels, rendering lignin in middle lamella and cell walls of fibers more uniform than in the wild-type genotype.
It appears that the monolignol composition can influence the structure of the lignin (73) macromolecule, and several studies suggest that this affects the susceptibility of wood to degradation. For example, Faix et al. (65) demonstrated that syringyl-guaiacyl lignins are degraded more readily from macromolecules to fragments than purely guaiacyl lignins. Investigating the susceptibility of wood from 7 different species to degradation by T. versicolor, Obst et al., (31) observed the highest mass losses in sweetgum (Liquidambar stiraciflua L.) (S/G = 1.1), red maple (Acer rubrum L.) (S/G = 0.8), and white birch (Betula papyrifera Marsh.) (S/G = 1.2). These species represent the trees with S/G ratios close to 1 and uniform amounts of both monolignols. Two species that exhibited enhanced decay resistance were boxelder maple (Acer negundo L.) (S/G = 0.1) and Pacific madrone (Arbutus menziesii Pursh.) (S/G = 2.3). Both of these trees represent species with higher syringyl monolignol composition, and this fact might imply that the prevalence of either syringyl or guaiacyl renders the lignin molecule less susceptible to degradation. However, the densities of boxelder (513 kg m−3) and Pacific madrone (961 kg m−3) lie well above average values, which may render the wood less conducive to hyphal growth. This may be a more likely explanation for the improved durability observed, rather than the chemical features of the lignin polymers, highlighting the difficulty in drawing conclusions from such comparative studies in which there are many variables.
In contrast to previous studies, our chemical analyses of wood samples that were uniform in properties except S/G ratio and lignin structure and subjected to decay by wood rot fungi indicate differences in resistance to decay that can be directly attributed to the type and structure of lignin. Improved resistance of transgenic poplar against brown rot decay appears to be associated initially with the elevated amounts of syringyl lignin (Table 1; Fig. 2 and 3). It is, nevertheless, clear that each fungus employed a different strategy for cell wall degradation and that lignin solubilization is driven largely by the wood substrate composition. Lignin demethoxylation is considered a major initial degradation strategy employed by brown rot fungi (5). Previously, it was suggested that the demethylating system of brown rot fungi is not specific, and as such the same mechanism could target both C-3 and C-5 methoxyl groups of syringyl subunits (9). The remaining lignin is demethylated on aryl methoxy groups and contains a greater number of ring hydroxyl groups (74). However, it seems that G. trabeum and P. placenta possess different demethoxylation mechanisms. It was demonstrated previously (75) that G. trabeum has a greater capacity to demethoxylate dimeric and monomeric model lignin compounds than P. placenta, and this results in a higher degree of lignin modification (Fig. 6A).
Another restricting factor in lignin degradation is hypothesized to be the three-dimensional structure of the lignin polymer. In a recent study, Stewart et al. (73) suggested that the C4H::F5H overexpression influences the degree of component homogeneity in transgenic lines. The authors observed that lignin chains in these poplar lines with extremely high syringyl content are predominantly linear with prevailing β-O-4 linkages and a lower degree of polymerization. Guaiacyl units are required in lignin to facilitate branching, since they are methoxylated only at the 3 position of the aromatic ring, leaving the 5 position free to participate in branching reactions (73). A more linear structure presumably results in a more compact arrangement of lignin chains in a lignocellulosic complex, rendering it more recalcitrant to attack by oxidizing agents.
While our work indicates that lignin structure averaged over entire wood samples is important in affecting the rate of wood decay and cell wall deconstruction by both brown and white rot fungi, our studies do not address the issue of the potential for a difference in decay-mediated alterations of monomer content that may occur in different cell types and cell layers with different lignin subunit compositions and structures. Further investigations of anatomical and morphological traits of lignin derived from substrates with various monomer compositions are required to completely understand the role of monolignols in decay resistance.
Conclusions.
Transgenic poplar extremely rich in syringyl lignin exhibited improved resistance to degradation by an array of decay fungi, possessing a wide spectrum of lignocellulolytic activities and degradation mechanisms. Thus, lignin monomer composition and linearity of macromolecules are important parameters affecting wood durability. Low guaiacyl content did not render wood more susceptible to fungal decay, which is discordant with an existing theory about improved decay resistance accomplished by elevated guaiacyl content. The findings of this study reveal that transgenic poplar wood with an extremely high syringyl content is recalcitrant to degradation and therefore may afford improved performance where wood durability is a desired factor, especially in the long-term storage of feedstocks required for wood processing, such as pulping or bioenergy applications.
Footnotes
Published ahead of print 8 February 2013
REFERENCES
- 1. Geddes CC, Nieves IU, Ingram LO. 2011. Advances in ethanol production. Curr. Opin. Biotechnol. 22:312–319 [DOI] [PubMed] [Google Scholar]
- 2. Fengel D, Wegener G. 1984. Wood. Chemistry, ultrastructures, reactions. De Gruyter, New York, NY [Google Scholar]
- 3. Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, Boerjan W. 2004. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 3:29–60 [Google Scholar]
- 4. Campbell MM, Sederoff RR. 1996. Variation in lignin content and composition (mechanisms of control and implications for the genetic improvement of plants). Plant Physiol. 110:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eriksson K-E, Blanchette RA, Ander P. 1990. Microbial and enzymatic degradation of wood and wood components. Springer, Berlin, Germany [Google Scholar]
- 6. Taylor JW. 1995. Making the Deuteromycota redundant: a practical integration of mitosporic and meiosporic fungi. Can. J. Bot. 73:754–759 [Google Scholar]
- 7. Schwarze FWMR. 2007. Wood decay under the microscope. Fungal Biol. Rev. 21:133–170 [Google Scholar]
- 8. Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TKA, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. 2012. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719 [DOI] [PubMed] [Google Scholar]
- 9. Jin L, Nicholas DD, Kirk TK. 1990. Mineralization of the methoxyl carbon of isolated lignin by brown-rot fungi under solid substrate conditions. Wood Sci. Technol. 24:263–276 [Google Scholar]
- 10. Filley TR, Cody GD, Goodell B, Jellison J, Noser C, Ostrofsky A. 2002. Lignin demethylation and polysaccharide decomposition in spruce sapwood degraded by brown rot fungi. Org. Geochem. 33:111–124 [Google Scholar]
- 11. Goodell B. 2003. Brown-rot fungal degradation of wood: our evolving view, p 474 In Goodell B, Nicholas D, Schultz T. (ed), Wood deterioration and preservation. American Chemical Society, Washington, DC [Google Scholar]
- 12. Yelle DJ, Ralph J, Lu F, Hammel KE. 2008. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 10:1844–1849 [DOI] [PubMed] [Google Scholar]
- 13. Yelle DJ, Wei D, Ralph J, Hammel KE. 2011. Multidimensional NMR analysis reveals truncated lignin structures in wood decayed by the brown rot basidiomycete Postia placenta. Environ. Microbiol. 13:1091–1100 [DOI] [PubMed] [Google Scholar]
- 14. Goodell B, Qian Y, Jellison J. 2008. Development of commercial wood preservatives, p 9–31 American Chemical Society, Washington, DC [Google Scholar]
- 15. Shi J, Chinn MS, Sharma-Shivappa RR. 2008. Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour. Technol. 99:6556–6564 [DOI] [PubMed] [Google Scholar]
- 16. Ferraz A, Guerra A, Mendonça R, Masarin F, Vicentim MP, Aguiar A, Pavan PC. 2008. Technological advances and mechanistic basis for fungal biopulping. Enzyme Microb. Technol. 43:178–185 [Google Scholar]
- 17. Lee H, Choi Y, Kim M, Huh N, Kim G, Lim Y, Kang S, Cho S, Kim J. 2010. Degrading ability of oligocyclic aromates by Phanerochaete sordida screening of white rot fungi. Folia Microbiol. (Praha) 55:447–453 [DOI] [PubMed] [Google Scholar]
- 18. Dashtban M, Schraft H, Wensheng Q. 2009. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int. J. Biol. Sci. 5:578–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martínez AT, Ruiz-Dueñas FJ, Martínez MJ, del Río JC, Gutiérrez A. 2009. Enzymatic delignification of plant cell wall: from nature to mill. Curr. Opin. Biotechnol. 20:348–357 [DOI] [PubMed] [Google Scholar]
- 20. Gübitz GM, Mansfield SD, Böhm D, Saddler JN. 1998. Effect of endoglucanases and hemicellulases in magnetic and flotation deinking of xerographic and laser-printed papers. J. Biotechnol. 65:209–219 [Google Scholar]
- 21. Mansfield SD, Saddler JN, Gübitz GM. 1998. Characterization of endoglucanases from the brown rot fungi Gloeophyllum sepiarium and Gloeophyllum trabeum. Enzyme Microb. Technol. 23:133–140 [Google Scholar]
- 22. Schilling J, Tewalt J, Duncan S. 2009. Synergy between pretreatment lignocellulose modifications and saccharification efficiency in two brown-rot fungal systems. Appl. Microbiol. Biotechnol. 84:465–475 [DOI] [PubMed] [Google Scholar]
- 23. Lynd L, Elamder R, Wyman C. 1996. Likely features and costs of mature biomass ethanol technology. Appl. Biochem. Biotechnol. 57-58:741–761 [Google Scholar]
- 24. Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, Himmel M, Keller M, McMillan JD, Sheehan J, Wyman CE. 2008. How biotech can transform biofuels. Nat. Biotechnol. 26:169–172 [DOI] [PubMed] [Google Scholar]
- 25. Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Á Martínez. T, Martínez MJ. 2011. Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Bioresour. Technol. 102:7500–7506 [DOI] [PubMed] [Google Scholar]
- 26. Bailey PJ, Liese W, Roesch R. 1968. Some aspecst of cellulose degradation iin lignified cell walls, p 546–557 In Walters AH, Elphick JJ. (ed), Biodegradation of materials. Elsevier, New York, NY [Google Scholar]
- 27. Takahashi M. 1976. Removal of lignin from partially delignified softwoods by soft-rot and white-rot fungi. Wood Res. 61:1–10 [Google Scholar]
- 28. Highley TL. 1982. Influence of type and amount of lignin on decay by Coriolus versicolor. Can. J. For. Res. 12:435–438 [Google Scholar]
- 29. Mansfield SD, Mooney C, Saddler JN. 1999. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 15:804–816 [DOI] [PubMed] [Google Scholar]
- 30. Mansfield SD, Kang K-Y, Chapple C. 2012. Designed for deconstruction—poplar trees altered in cell wall lignification improve the efficacy of bioethanol production. New Phytol. 194:91–101 [DOI] [PubMed] [Google Scholar]
- 31. Obst J, Highley T, Miller RB. 1994. Influence of lignin type on the decay of woody angiosperms by Trametes versicolor, p 357–374 In Llewellyn GC, Dashek WV, O'Rear CE. (ed), Biodeterioration research. Plenium Press, New York, NY [Google Scholar]
- 32. Rubin EM. 2008. Genomics of cellulosic biofuels. Nature 454:841–845 [DOI] [PubMed] [Google Scholar]
- 33. Huntley SK, Ellis D, Gilbert M, Chapple C, Mansfield SD. 2003. Significant increases in pulping efficiency in C4H-F5H-transformed poplars: improved chemical savings and reduced environmental toxins. J. Agric. Food Chem. 51:6178–6183 [DOI] [PubMed] [Google Scholar]
- 34. Coleman HD, Park JY, Nair R, Chapple C, Mansfield SD. 2008. RNAi-mediated suppression of p-coumaroyl-CoA 3′-hydroxylase in hybrid poplar impacts lignin deposition and soluble secondary metabolism. Proc. Natl. Acad. Sci. U. S. A. 105:4501–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mansfield SD. 2009. Solutions for dissolution—engineering cell walls for deconstruction. Curr. Opin. Biotechnol. 20:286–294 [DOI] [PubMed] [Google Scholar]
- 36. Franke R, McMichael CM, Meyer K, Shirley AM, Cusumano JC, Chapple C. 2000. Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis encoding ferulate 5-hydroxylase. Plant J. 22:223–234 [DOI] [PubMed] [Google Scholar]
- 37. European committee for Standardization 1996. European Standard (EN 113). Wood preservatives. Test method for determining the protective effectiveness against wood destroying basidiomycetes. Determination of the toxic values. European Committee for Standardization, Brussels, Belgium [Google Scholar]
- 38. TAPPI 1985. UM 250. Acid soluble lignin in wood and pulp. TAPPI Press, Atlanta, GA [Google Scholar]
- 39. Robinson AR, Mansfield SD. 2009. Rapid analysis of poplar lignin monomer composition by a streamlined thioacidolysis procedure and near-infrared reflectance-based prediction modeling. Plant J. 58:706–714 [DOI] [PubMed] [Google Scholar]
- 40. Schmidhalter DR, Canevascini G. 1993. Purification and characterization of two exo-cellobiohydrolases from the brown-rot fungus Coniophora puteana (Schum ex Fr) Karst. Arch. Biochem. Biophys. 300:551–558 [DOI] [PubMed] [Google Scholar]
- 41. Kajisa T, Igarashi K, Samejima M. 2009. The genes encoding glycoside hydrolase family 6 and 7 cellulases from the brown-rot fungus Coniophora puteana. J. Wood Sci. 55:376–380 [Google Scholar]
- 42. Goodell B, Jellison J, Liu J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. 1997. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J. Biotechnol. 53:133–162 [Google Scholar]
- 43. Evans CS, Dutton MV, Guillén F, Veness RG. 1994. Enzymes and small molecular mass agents involved with lignocellulose degradation. FEMS Microbiol. Rev. 13:235–239 [Google Scholar]
- 44. Vanden Wymelenberg A, Gaskell J, Mozuch M, Sabat G, Ralph J, Skyba O, Mansfield SD, Blanchette RA, Martinez D, Grigoriev I, Kersten PJ, Cullen D. 2010. Comparative transcriptome and secretome analysis of wood decay fungi Postia placenta and Phanerochaete chrysosporium. Appl. Environ. Microbiol. 76:3599–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arantes V, Jellison J, Goodell B. 2012. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 94:323–338 [DOI] [PubMed] [Google Scholar]
- 46. Arantes V, Qian Y, Milagres AMF, Jellison J, Goodell B. 2009. Effect of pH and oxalic acid on the reduction of Fe3+ by a biomimetic chelator and on Fe3+ desorption/adsorption onto wood: Implications for brown-rot decay. Int. Biodeterior. Biodegradation. 63:478–483 [Google Scholar]
- 47. Martinez D, Challacombe J, Morgenstern I, Hibbett D, Schmoll M, Kubicek CP, Ferreira P, Ruiz-Duenas FJ, Martinez AT, Kersten P, Hammel KE, Vanden Wymelenberg A, Gaskell J, Lindquist E, Sabat G, Splinter BonDurant S, Larrondo LF, Canessa P, Vicuna R, Yadav J, Doddapaneni H, Subramanian V, Pisabarro AG, Lavin JL, Oguiza JA, Master E, Henrissat B, Coutinho PM, Harris P, Magnuson JK, Baker SE, Bruno K, Kenealy W, Hoegger PJ, Kües U, Ramaiya P, Lucas S, Salamov A, Shapiro H, Tu H, Chee CL, Misra M, Xie G, Teter S, Yaver D, James T, Mokrejs M, Pospisek M, Grigoriev IV, Brettin T, Rokhsar D, Berka R, Cullen D. 2009. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc. Natl. Acad. Sci. U. S. A. 106:1954–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eastwood DC, Floudas D, Binder M, Majcherczyk A, Schneider P, Aerts A, Asiegbu FO, Baker SE, Barry K, Bendiksby M, Blumentritt M, Coutinho PM, Cullen D, de Vries RP, Gathman A, Goodell B, Henrissat B, Ihrmark K, Kauserud H, Kohler A, LaButti K, Lapidus A, Lavin JL, Lee Y-H, Lindquist E, Lilly W, Lucas S, Morin E, Murat C, Oguiza JA, Park J, Pisabarro AG, Riley R, Rosling A, Salamov A, Schmidt O, Schmutz J, Skrede I, Stenlid J, Wiebenga A, Xie X, Kües U, Hibbett DS, Hoffmeister D, Högberg N, Martin F, Grigoriev IV, Watkinson SC. 2011. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 333:762–765 [DOI] [PubMed] [Google Scholar]
- 49. Shary S, Kapich AN, Panisko EA, Magnuson JK, Cullen D, Hammel KE. 2008. Differential expression in Phanerochaete chrysosporium of membrane-associated proteins relevant to lignin degradation. Appl. Environ. Microbiol. 74:7252–7257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kamada F, Abe S, Hiratsuka N, Wariishi H, Tanaka H. 2002. Mineralization of aromatic compounds by brown-rot basidiomycetes—mechanisms involved in initial attack on the aromatic ring. Microbiology 148:1939–1946 [DOI] [PubMed] [Google Scholar]
- 51. Moktali V, Park J, Fedorova-Abrams N, Park B, Choi J, Lee Y-H, Kang S. 2012. Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes. BMC Genomics 13:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suzuki H, MacDonald J, Syed K, Salamov A, Hori C, Aerts A, Henrissat B, Wiebenga A, vanKuyk P, Barry K, Lindquist E, LaButti K, Lapidus A, Lucas S, Coutinho P, Gong Y, Samejima M, Mahadevan R, Abou-Zaid M, de Vries R, Igarashi K, Yadav J, Grigoriev I, Master E. 2012. Comparative genomics of the white-rot fungi, Phanerochaete carnosa and P. chrysosporium, to elucidate the genetic basis of the distinct wood types they colonize. BMC Genomics 13:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee KH, Wi SG, Singh AP, Kim YS. 2004. Micromorphological characteristics of decayed wood and laccase produced by the brown-rot fungus Coniophora puteana. J. Wood Sci. 50:281–284 [Google Scholar]
- 54. Worrall JJ, Anagnost SE, Zabel RA. 1997. Comparison of wood decay among diverse lignicolous fungi. Mycologia 89:199–219 [Google Scholar]
- 55. Farr DF, Bills GF, Chamuris GP, Rossman AY. 1989. Fungi on plants and plant products in the United States. The American Phytopathological Society Press, St. Paul, MN [Google Scholar]
- 56. Vanden Wymelenberg A, Gaskell J, Mozuch M, Kersten P, Sabat G, Martinez D, Cullen D. 2009. Transcriptome and secretome analyses of Phanerochaete chrysosporium reveal complex patterns of gene expression. Appl. Environ. Microbiol. 75:4058–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruel K, Joseleau JP. 1991. Involvement of an extracellular glucan sheath during degradation of populus wood by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 57:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pointing SB, Pelling AL, Smith GJD, Hyde KD, Reddy CA. 2005. Screening of basidiomycetes and xylariaceous fungi for lignin peroxidase and laccase gene-specific sequences. Mycol. Res. 109:115–124 [DOI] [PubMed] [Google Scholar]
- 59. Martínez AT, Rencoret J, Nieto L, Jiménez-Barbero J, Gutiérrez A, del Río JC. 2011. Selective lignin and polysaccharide removal in natural fungal decay of wood as evidenced by in situ structural analyses. Environ. Microbiol. 13:96–107 [DOI] [PubMed] [Google Scholar]
- 60. Mansfield SD, De Jong E, Saddler JN. 1997. Cellobiose dehydrogenase, an active agent in cellulose depolymerization. Appl. Environ. Microbiol. 63:3804–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Choi J-W, Choi D-H, Ahn S-H, Lee S-S, Kim M-K, Meier D, Faix O, Scott G. 2006. Characterization of trembling aspen wood (Populus tremuloides L.) degraded with the white rot fungus Ceriporiopsis subvermispora and MWLs isolated thereof. Eur. J. Wood Wood Prod. 64:415–422 [Google Scholar]
- 62. Guerra A, Mendonça R, Ferraz A. 2002. Characterization of the residual lignins in pinus taeda biodegraded by Ceriporiopsis subvermispora by using in situ CuO oxidation and DFRC methods. Holzforschung. 56:157–160 [Google Scholar]
- 63. Fackler K, Gradinger C, Schmutzer M, Tavzes C, Burgert I, Schwanninger M, Hinterstoisser B, Watanabe T, Messner K. 2007. Biotechnological wood modification with selective white-rot fungi and its molecular mechanisms. Food Technol. Biotechnol. 45:269–276 [Google Scholar]
- 64. Blanchette RA. 1991. Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 29:381–403 [Google Scholar]
- 65. Faix O, Mozuch MD, Kirk TK. 1985. Degradation of gymnosperm (guaiacyl) vs. angiosperm (syringyl/guaiacyl) lignins by Phanerochaete chrysosporium. Holzforschung 39:203–208 [Google Scholar]
- 66. Srebotnik E, Messner K. 1994. A simple method that uses differential staining and light microscopy to sssess the selectivity of wood delignification by white rot fungi. Appl. Environ. Microbiol. 60:1383–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Walker JCF. 2006. Primary wood processing: principles and practice. Springer, Houten, Netherlands [Google Scholar]
- 68. Watanabe Y, Kojima Y, Ona T, Asada T, Sano Y, Fukazawa K, Funada R. 2004. Histochemical study on heterogeneity of lignin in Eucalyptus species II. The distribution of lignins and polyphenols in the walls of various cell types. IAWA J. 25:283–295 [Google Scholar]
- 69. Wu J, Fukuzawa K, Ohtani J. 1992. Distribution of syringyl and guaiacyl lignins in hardwood in relation to habitat and porosity form in wood. Holzforschung 46:181–185 [Google Scholar]
- 70. Fergus BJ, Goring DAI. 1970. The location of guaiacyl and syringyl lignins in birch xylem tissue. Holzforschung 24:113–117 [Google Scholar]
- 71. Syafii W, Yoshimoto T. 1991. Effect of lignin structure on decay resistance of some tropical woods. Indones. J. Trop. Agric. 3:32–37 [Google Scholar]
- 72. Wagenführ R. 1999. Anatomie des Holzes, 5th ed DRW, Stuttgart, Germany [Google Scholar]
- 73. Stewart JJ, Akiyama T, Chapple C, Ralph J, Mansfield SD. 2009. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol. 150:621–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kirk TK, Highley T. 1973. Quantitative changes in structural components of conifer woods during decay by white and brown-rot fungi. Phytopathology 63:1338–1342 [Google Scholar]
- 75. Niemenmaa O, Uusi-Rauva A, Hatakka A. 2008. Demethoxylation of [O14CH3]-labelled lignin model compounds by the brown-rot fungi Gloeophyllum trabeum and Poria (Postia) placenta. Biodegradation 19:555–565 [DOI] [PubMed] [Google Scholar]