Abstract
Nitrogen supplementation, which is widely used in winemaking to improve fermentation kinetics, also affects the products of fermentation, including volatile compounds. However, the mechanisms underlying the metabolic response of yeast to nitrogen additions remain unclear. We studied the consequences for Saccharomyces cerevisiae metabolism of valine and ammonium pulses during the stationary phase of four-stage continuous fermentation (FSCF). This culture technique provides cells at steady state similar to that of the stationary phase of batch wine fermentation. Thus, the FSCF device is an appropriate and reliable tool for individual analysis of the metabolic rerouting associated with nutrient additions, in isolation from the continuous evolution of the environment in batch processes. Nitrogen additions, irrespective of the nitrogen-containing compound added, substantially modified the formation of fermentation metabolites, including glycerol, succinate, isoamyl alcohol, propanol, and ethyl esters. This flux redistribution, fulfilling the requirements for precursors of amino acids, was consistent with increased protein synthesis resulting from increased nitrogen availability. Valine pulses, less efficient than ammonium addition in increasing the fermentation rate, were followed by a massive conversion of this amino acid in isobutanol and isobutyl acetate through the Ehrlich pathway. However, additional routes were involved in valine assimilation when added in stationary phase. Overall, we found that particular metabolic changes may be triggered according to the nature of the amino acid supplied, in addition to the common response. Both these shared and specific modifications should be considered when designing strategies to modulate the production of volatile compounds, a current challenge for winemakers.
INTRODUCTION
Nitrogen is the main limiting nutrient for yeasts during wine fermentation. It is generally believed that a minimum of about 140 mg/liter of nitrogen is necessary to complete fermentation of 200 g/liter sugar. However, the optimal nitrogen content of grape juice in terms of kinetics is predicted to be around 300 mg/liter of yeast assimilable nitrogen (YAN) (1–4). Low nitrogen levels in must are generally associated with low yeast biomass and low fermentation rates (5–7). To avoid fermentation problems in nitrogen-deficient musts, winemakers often add nitrogen at various stages of the winemaking process.
Nitrogen supplementation results in many changes in the course of fermentation (for reviews, see references 8 and 9), which may differ depending on the yeast strain used, the composition of the grape juice, the timing of the addition, and the type of nitrogen source. Adding nitrogen to the must before fermentation generally increases both the cell population and the fermentation rate, whereas its addition at the beginning of the stationary phase leads to a higher fermentation rate without influencing the cell population (6, 10, 11). However, for some strains, nitrogen addition at the beginning of fermentation, particularly in nitrogen-rich media, does not necessarily enhance growth (12). Addition of nitrogen also influences the organoleptic characteristics of wine, because it affects the formation of glycerol, organic acids, and volatile compounds (9, 10, 13–17). Unfortunately, much of the information available about the effects of nitrogen addition on metabolism is contradictory, probably due to the different conditions used in the relevant studies. For instance, ammonium supplementation of nitrogen-deficient musts always results in a decrease in volatile acidity (18, 19), whereas the production of acetate increases when ammonium is supplied to a nitrogen-rich medium (20) or as a consequence of adding a mixture of amino acids (10). Similarly, fusel alcohol formation is decreased more by the addition of large amounts of nitrogen at the beginning of fermentation than by later or smaller additions (19). The substantial discrepancies in literature data make it very difficult to draw any general conclusion about the effects of nitrogen supplementation on the synthesis of aromatic compounds (19). Further elucidation of the mechanisms underlying the metabolic response of yeast to nitrogen addition should provide a better understanding of the effects of nitrogen addition on the course of fermentation and yeast metabolism. This may eventually allow the design of strategies for the management of nitrogen that ensure an optimal supply throughout fermentation, a goal that remains currently a major challenge for winemakers (9).
Pulse experiments in chemostats have been widely used to study the dynamics of the metabolic response of microorganisms to modifications in their environment, as they result in a sudden relief from nutrient limitation (21–25). Over recent years, this type of approach combined with fast sampling methods has provided information about the adaptive mechanisms of microorganisms for their changing environments at metabolomic and transcriptomic levels (22, 26, 27). A particular advantage of chemostat over batch cultures is the stability of the physiological state of the cells, all environmental variables remaining constant at a steady state (28). Steady-state chemostat cultures are particularly useful for obtaining reproducible data (29, 30). However, this culture mode has limitations for studying yeast physiology under winemaking conditions. Winemaking is an industrial process, during which the growth phase covers only the first hours until nitrogen exhaustion. Thereafter, most of the sugar is consumed by resting cells, during the stationary phase. The metabolism of yeasts during this stage is of great importance for wine fermentation but cannot be investigated using chemostat cultures, which necessarily involve growing cells. Therefore, we developed a four-stage continuous bioreactor (four-stage continuous fermentation [FSCF]), an original device consisting of a sequential series of four steady states with the first stages continuously feeding the later ones with resting cells (31). The progressive exhaustion of YAN, which was entirely depleted in the third tank, allowed both the growth phase (first two stages, with residual YAN) and the stationary phase (resting cells in the last two stages) to be reproduced at steady state.
Here, we report the use of the FSCF system to study over time the metabolic response of Saccharomyces cerevisiae to additions of two nitrogen sources (ammonium and valine pulses) during the stationary phase of wine fermentation. This system, providing cells at steady state, appeared to be particularly suitable for investigating the direct effects of changes in the yeast environment, by avoiding the dynamic evolution of batch cultures. We compared batch and continuous cultures to assess the usefulness of the FSCF system to analyze the formation of metabolites, and in particular the synthesis of volatile compounds, during the stationary phase according to yeast nitrogen metabolism. We then focused on the third stage of FSCF, corresponding to the beginning of the stationary phase of batch fermentation, since in winemaking, nitrogen supplementation at this time of the process is widely used to prevent stuck fermentation. We compared the dynamics of the metabolic response of yeasts to additions of ammonium, which is commonly used in industry, and valine, as representative of the amino acids that are precursors for the synthesis of fusel alcohols. These analyses provide new insights into the metabolic reroutings in yeast to adapt to modifications in nutrient availability, and in particular to the type of added nitrogen source.
MATERIALS AND METHODS
Yeast strain and culture medium.
Fermentations were carried out with the commercial Saccharomyces cerevisiae strain EC1118 (Lallemand SA). The inoculum was grown aerobically in shaken flasks at 28°C, first in YPD medium (1% yeast extract, 2% peptone, 2% glucose) for 24 h and then in synthetic medium for 24 h; these precultures were used to inoculate the bioreactor at a density of 106 cells/ml. We used the synthetic medium SM100 described by Bely et al. (6), mimicking grape must, containing 200 g/liter glucose and 100 mg/liter of yeast assimilable nitrogen (YAN) as a mixture of amino acids (74 mg N/liter) and NH4Cl (26 mg N/liter), at pH 3.5.
Fermentation conditions. (i) Batch fermentation.
Batch fermentations were performed at 28°C, in 1-liter fermentors, stirred continuously (100 rpm) and linked to a mass flow meter that measured the CO2 release rate online.
(ii) FSCF.
The four-stage fermentation device was configured as described previously (31). The parameters of the FSCF were set to ensure progressive exhaustion of the nitrogen in the first two stages and thus the complete absence of any residual YAN in the third fermentor, modeling the beginning of the stationary phase.
After inoculation, fermentations were started in batch mode until the fermentative activity reached its maximum (about 17 h after inoculation); then, continuous fermentation was initiated. For a given dilution rate (D = Q/V [h−1], where Q is the input flow rate and V is the volume of medium in the tank), the system was considered to be stable after three residence times (Rt = 1/D) at steady state.
(iii) Nitrogen additions.
Once the steady state was established, the four tanks were sampled for determinations of dry weight, carbon metabolites, and volatile compounds. Nitrogen was added to 100 mg/liter (as 26.7 g/liter ammonium chloride or 58.6 g/liter l-valine) in the third stage (R3), corresponding to the beginning of the stationary phase (exhaustion of the assimilable nitrogen, maximum cell population).
The volume of stock solution added did not exceed 2% total volume, minimizing the perturbation of the dilution rate. Following this pulse of nitrogen, the medium was sampled hourly until complete exhaustion of assimilable nitrogen. Yields were calculated at the time of complete exhaustion of the added nitrogen.
(iv) Evolution of the medium composition in FSCF.
In FSCF, the evolution of the concentration of a component C can be expressed as the sum of three different elements (equation 1): the incoming flow rate of C, which depends on the concentration of C in the previous stage; the outflow rate, which is a function of the concentration of C in the stage considered; and the production (or consumption) rate, which depends on the synthesis (or uptake) activity of the microorganism. Equation 1 resumes the balance of C in stage Ri:
| (1) |
where Ci is the concentration of compound C in stage i (g/liter), Ci − 1 is the concentration of compound C in stage i − 1 (g/liter), Qei is inlet medium flow rate in stage i (liters/h), Qsi is outlet medium flow rate in stage i (liters/h), Vi is volume of medium in stage i (liters), and rci is production (or consumption) rate of compound C in stage i (g/liter/h).
At steady state, the composition of the medium is constant and dCi/dt = 0. Thus, rci can be easily deduced from the measurement of Ci and Ci + 1. In the case of a pulse of nutrient, equation 1 can be integrated assuming a constant rci in the time interval considered, as presented in equation 2:
| (2) |
where Cmi is the concentration in stage i, calculated as a logarithmic mean.
Thus, the production rate of compound C was calculated according to equation 3:
| (3) |
Analytical methods. (i) Measurement of cell population and dry weight—calculation of the specific growth rate.
The dry weight of yeasts (X) was determined gravimetrically using nitrocellulose filters (pore size, 0.45 μm; Millipore) from samples of 15 ml. Samples were dried for 48 h at 100°C.
The cell population and the cell size distribution were determined with a Coulter counter (model Z2; Beckman-Coulter, Margency, France) fitted with a 100-μm-aperture probe.
The specific growth rate was calculated after the measurement of the cell population in each stage. For stage i, μ is calculated as follows:
| (4) |
where X is the cell population in cells/ml.
(ii) Measurement of extracellular metabolites.
Glucose, ethanol, glycerol, and succinic acids in culture supernatants were determined by high-pressure liquid chromatography (HPLC) using an HPX-87H Aminex ion-exchange column (300 by 7.8 mm; Bio-Rad) at 45°C. The column was eluted with 8 mM H2SO4 at a flow rate of 0.6 ml/min. Acetic and pyruvic acids were determined with a Hewlett-Packard G1314A UV meter at 210 nm, and the other compounds were determined with an Agilent G1362A refractive index detector.
Volatile compounds were determined by gas chromatography-flame ionization detection (GC-FID) analysis, with a headspace autosampler and a BP20 column (SGE). To facilitate the extraction of volatile compounds to the gas phase, NaCl (1 g) was added to 3 ml of the fermentation sample in a 20-ml vial. To standardize the equilibrium conditions between the liquid and the headspace, the ethanol concentration in the vial was adjusted to 11% by adding 2 ml of a mixture of 12 g/liter tartaric acid solution diluted in either water or an ethanol-water mix (30%, vol/vol). Then, 50 μl of 4-methylpentan-2-ol at a concentration of 3 g/liter was added as an internal standard. The sample vial was heated at 50°C and agitated for 5 min in an HT200 headspace autosampler equipped with a gastight syringe, preheated to 60°C. One milliliter of headspace gas was analyzed with an HP6890 GC coupled to an FID. The injector temperature was 240°C. The GC oven was equipped with a BP20 column (30 m by 0.53 mm by 1.0 m; SGE). H2 was used as the carrier gas at a constant flow rate of 4.8 ml/min. The oven temperature program was 40°C for 3 min, 3°C/min to 80°C, and 15°C/min to 160°C, at which it was held for 1 min, and then 30°C/min to 220°C, at which it was held for 2 min. The detector was set at 250°C. Peak areas were acquired with Agilent Chemstation software.
The ammonium concentration was determined enzymatically (Enzytec Fluid 5390; Thermo). Free residual amino acids were quantified by cation-exchange chromatography followed by postcolumn derivatization with ninhydrin (Biochrom 30; Biochrom, Cambridge, United Kingdom).
RESULTS
Metabolite production during the stationary phase of FSCF and batch fermentations.
The value of FSCF is that it allows analysis of the effects of environmental modifications on yeast physiology at steady state, without the continuous evolution of the environment in batch processes. To determine the most appropriate stage of this culture system to investigate nitrogen addition during the stationary phase, three independent FSCFs were first carried out with the dilution rates reported in Table 1. Online monitoring of the rate of CO2 release, which reflected the fermentative activity of the cells (2), revealed that steady state was reached in the four stages after 30 h of culture and sustained over a minimum of 80 h (8 residence times [see Fig. S1 in the supplemental material]). Population and metabolite measurements over time confirmed the absence of alteration of the steady state during this period (see Table S1 in the supplemental material). Under these conditions, after 3 residence times at steady state, yeast assimilable nitrogen (YAN) was exhausted in the second stage (Table 1). The cell population reached a maximum in the third stage, and there was no significant growth in the last stage. Therefore, cells in the third stage were in a physiological state similar to the beginning of the stationary phase of batch wine fermentations. Consequently, this stage was selected for nitrogen additions.
Table 1.
Characteristics of the steady state in the four stages of the FSCFa
| Characteristic | Value in stage: |
|||
|---|---|---|---|---|
| R1 | R2 | R3 | R4 | |
| V (ml) | 500 | 500 | 700 | 700 |
| D (h−1) | 0.27 | 0.24 | 0.11 | 0.10 |
| dCO2/dt (mg/liter/h) | 0.37 ± 0.04 | 0.94 ± 0.09 | 0.54 ± 0.04 | 0.42 ± 0.02 |
| Residual YAN (mg/liter) | 1.63 ± 0.02 | 0.02 ± 0.02 | ND | ND |
| Residual glucose (g/liter) | 186 ± 7 | 178 ± 5 | 164 ± 5 | 151 ± 5 |
| Population (106 cells/ml) | 30 ± 3 | 58 ± 4 | 79 ± 4 | 82 ± 3 |
| Dry wt (g/liter) | 1.2 ± 0.3 | 1.3 ± 0.3 | 1.9 ± 0.3 | 2.3 ± 0.3 |
| μ (h−1) | 0.27 ± 0.01 | 0.12 ± 0.02 | 0.03 ± 0.01 | 0.003 ± 0.003 |
| pH | 3.30 ± 0.02 | 3.29 ± 0.04 | 3.31 ± 0.03 | 3.28 ± 0.02 |
Mean values and standard deviations were calculated from 3 independent FSCF experiments. For each culture, one sample was collected in the four stages, after three residence times at steady state. Abbreviations: V, volume; D, dilution rate; YAN, yeast assimilable nitrogen; ND, not detectable; μ, specific growth rate.
We further compared cell growth and metabolite production between the third stage of FSCF and batch fermentation (Table 2) after the same progression of fermentation (residual glucose of 164 ± 5 g/liter). In this stage, both ammonium and valine were entirely exhausted (see Table S2 in the supplemental material). The differences in biomass formation (cell population and dry weight) between the two fermentation processes were less than 5%. Also, the formation of intermediates of the central carbon metabolism (CCM) and higher alcohols by yeast during FSCF was similar to that during batch fermentation, with a difference of 4 to 8% (pyruvate being an exception). Ester synthesis by yeast grown in batch culture and that by yeast grown in FSCF cultures were comparable. For each FSCF, the carbon balance at steady state was between 94 and 98%.
Table 2.
Comparison of biomass and metabolite production at the beginning of the stationary phase of batch culture and FSCFa
| Characteristic or metabolite | WA | B |
|---|---|---|
| Characteristic | ||
| Dry wt (g/liter) | 1.91 ± 0.04 | 1.91 ± 0.05 |
| Cell population (106 cells/ml) | 79 ± 4 | 86 ± 5 |
| Metabolites measured in: | ||
| g/liter | ||
| Succinate | 0.220 ± 0.005 | 0.230 ± 0.005 |
| Glycerol | 1.78 ± 0.05 | 1.93 ± 0.09 |
| Acetate | 0.230 ± 0.004 | 0.210 ± 0.006 |
| Ethanol | 13.5 ± 0.4 | 15.1 ± 0.6 |
| Pyruvate | 0.078 ± 0.005 | 0.090 ± 0.005 |
| mg/liter | ||
| Isoamyl alcohol | 52 ± 2 | 54 ± 4 |
| Isobutanol | 15.0 ± 0.7 | 15.1 ± 0.7 |
| Propanol | 7.6 ± 0.3 | 8.4 ± 0.4 |
| Ethyl acetate | 3.10 ± 0.07 | 3.3 ± 0.1 |
| μg/liter | ||
| Ethyl hexanoate | 21 ± 2 | 22 ± 1 |
| Ethyl octanoate | 40 ± 5 | ND |
| Ethyl decanoate | 52.3 ± 0.5 | 11.1 ± 0.8 |
| Ethyl dodecanoate | 6.5 ± 0.5 | ND |
| Isoamyl acetate | 41 ± 1 | 44 ± 2 |
| Isobutyl acetate | 5 ± 1 | ND |
Concentrations of metabolites and growth parameters were compared at an equivalent progression of fermentation (residual glucose, 164 ± 5 g/liter), between the third stage of FSCF without addition at steady state (WA), sampled after 3 residence times at steady state, and batch culture (B). Mean values and standard deviations were calculated from 3 independent FSCF or batch experiments. ND, not determined.
Overall, this confirmed that FSCF satisfactorily mimics batch fermentations for the synthesis of both CCM intermediates and volatile compounds. Consequently, this system is appropriate for characterizing the physiological response of yeast at steady state to nitrogen addition, as is common in winemaking.
Metabolic response of S. cerevisiae to nitrogen pulses in the third stage of FSCF.
The metabolic response of the yeast EC1118 to nitrogen additions was analyzed using the FSCF device. Pulse experiments, carried out in triplicate, involved adding ammonium or valine to the third stage of FSCF fermentation at steady state (3 residence times) to give a nitrogen concentration of 100 mg/liter. In parallel, we added nitrogen to batch fermentations, at equivalent fermentation progress, as a control.
(i) Growth and fermentation rate.
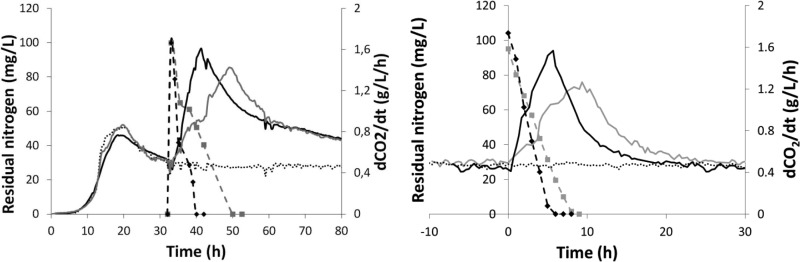
Following the pulse in FSCF, ammonium was consumed in 6 h and valine was consumed in 9 h (Fig. 1). This presumably reflects the differences in the rates of assimilation of ammonium and valine. Indeed, the specific uptake rate of ammonium, based on the consumption balance (equation 1), was twice as high as that of valine (0.61 mol N/g [dry weight]/h for ammonium and 0.29 mol N/g [dry weight]/h for valine). In batch fermentation also, the ammonium-specific consumption rate was twice as high as that of valine (0.40 mol N/g [dry weight]/h for ammonium and 0.21 mol N/g [dry weight]/h for valine). However, the specific consumption rates in batch fermentation were lower than those in FSCF. In batch fermentation, the accumulation of metabolites, in particular ethanol, was higher than that in FSCF, due to the dynamic process, and this may have been responsible for transporter deterioration and subsequent decrease of nitrogen uptake rates. The higher rates of consumption, in addition to the smaller amounts of assimilated nitrogen sources due to the washout in FSCF, resulted in a shorter phase of nitrogen assimilation under these conditions than that in batch fermentations (Fig. 1).
Fig 1.
Consequences of nitrogen pulses on fermentation performances and residual nitrogen concentration. CO2 production rate (solid lines) and residual nitrogen concentration (dashed lines) after a pulse of nitrogen (100 mg/liter) as ammonium (black lines) or valine (gray lines) were measured during batch fermentation (left) and in the third stage of FSCF (right). Evolutions during control fermentations, without nitrogen pulses, are reported as dotted lines. One representative experiment of three biological replicates is shown.
Consistent with previous data on batch fermentation (2), addition of nitrogen triggered a large increase of the CO2 production rate (2.8-fold for ammonium and 2.5-fold for valine). In both cases, the fermentation rate was maximum at the point of nitrogen exhaustion and decreased thereafter to its initial level.
No significant change in the population in FSCF was detected after the nitrogen pulse, for either nitrogen source. Conversely, in batch fermentation, the population increased by 10 to 20%. A small increase in cell population might have gone undetected in FSCF due to the dilution rate.
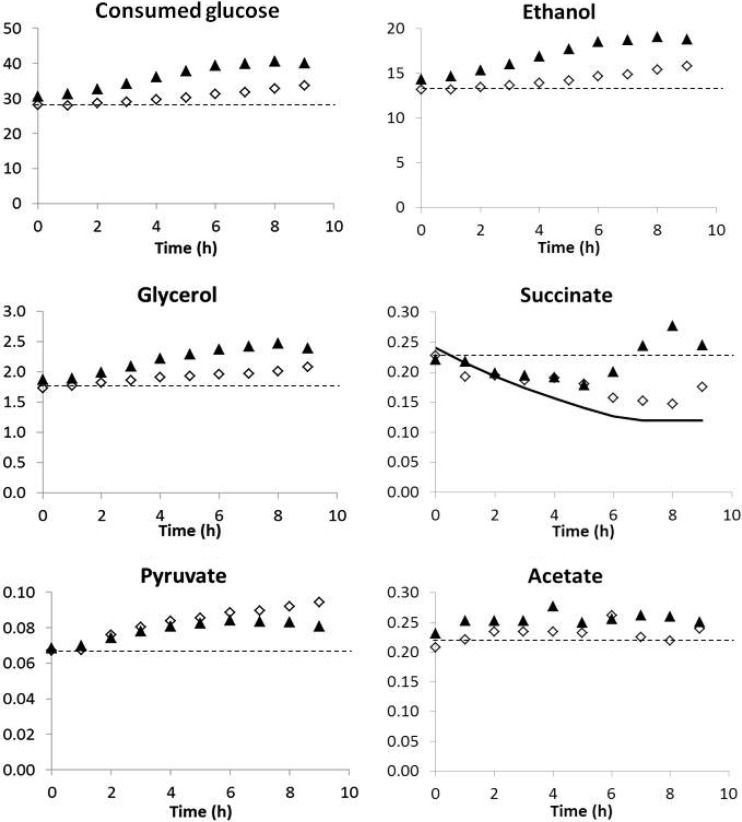
(ii) Production of central carbon metabolism intermediates.
The main metabolic response took place during the nitrogen consumption phase (Fig. 2 shows CCM metabolites). At steady state (i.e., before addition of nitrogen), metabolites did not accumulate in FSCF. The changes in metabolite concentrations in the medium during FSCF therefore reflected modifications in their production rate associated with the nitrogen pulse.
Fig 2.
Effect of nitrogen addition during the stationary phase of FSCF on metabolite concentrations. ▲, ammonium pulse in the third stage of FSCF; ♢, valine pulse in the third stage of FSCF; dashed line, concentration in the tank without nitrogen addition; the solid line (succinate) reports the evolution of metabolite concentration due only to washout of the tank (production rate = 0). Glucose, ethanol, glycerol, succinate, pyruvate, and acetate concentrations are expressed in g/liter. One representative experiment of three biological replicates is shown.
Consistent with the increase of the rate of CO2 production, nitrogen addition resulted in a transient increase in the glucose consumption and ethanol production rates (Fig. 2); these effects were more rapid with ammonium than with valine. The glycerol production rate followed the same pattern, with a larger increase for ammonium than for valine. The formation of pyruvate increased after the addition of valine and to a lesser extent after that of ammonium. The production of acetate increased slightly and similarly after the addition of the two nitrogen sources.
Unlike other metabolites, succinate synthesis decreased immediately after valine or ammonium pulse and throughout the nitrogen consumption phase (Fig. 2; see also Fig. S2 in the supplemental material). Its concentration in the medium was in line with the washout, indicating that the synthesis was almost stopped on nitrogen addition. Succinate synthesis restarted once the nitrogen was exhausted. This suggested that the changes in succinate synthesis were directly related to nitrogen metabolism.
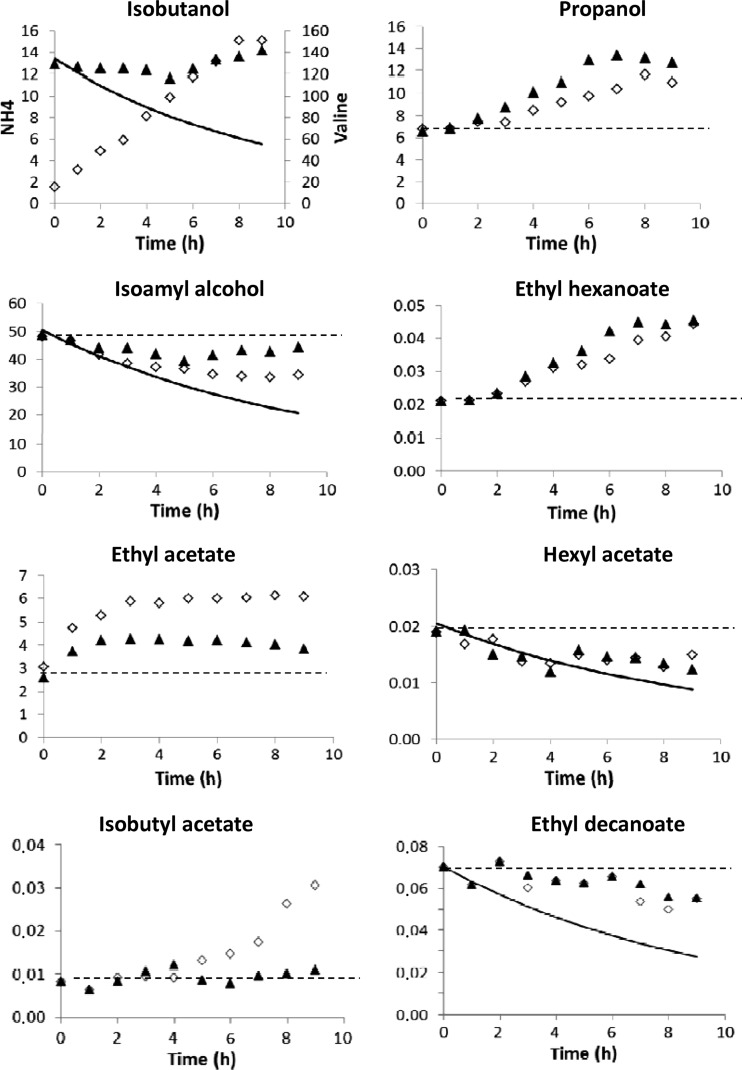
(iii) Metabolism of higher alcohols and esters.
The formation of fusel alcohols and esters in the third stage of FSCF was substantially modified by addition of valine or nitrogen (Fig. 3). The most obvious modifications concerned the synthesis of isobutanol and its derived ester, isobutyl acetate, which were increased 10-fold and 3.5-fold, respectively, after the addition of valine. The isobutyl acetate increase lagged 4 h behind that of isobutanol, suggesting a direct relationship between the formation of this acetate ester and the availability of its precursor (isobutanol). Ammonium addition was followed by slight decreases of the synthesis of isobutanol and isoamyl alcohol. Isoamyl alcohol production decreased after valine addition, probably due to the utilization of α-ketoisocaproate for the synthesis of leucine. Nevertheless, the synthetic activity continued to be expressed, as the concentration of isoamyl alcohol remained higher than what is predicted from washout.
Fig 3.
Volatile compound concentrations following nitrogen addition during the stationary phase of FSCF. ▲, ammonium pulse in the third stage of FSCF; ♢, valine pulse in the third stage of FSCF; dashed line, concentration in the tank without nitrogen addition; the solid line reports the evolution of metabolite concentration due only to washout of the tank (production rate = 0). Isobutanol, isoamyl alcohol, propanol, ethyl decanoate, ethyl hexanoate, ethyl acetate, hexyl acetate, and isobutyl acetate concentrations are expressed in mg/liter. One representative experiment of three biological replicates is shown.
Changes in the formation of propanol were independent of the added nitrogen source: in both cases, the propanol content increased 2-fold until the exhaustion of the nitrogen source and then slowly decreased.
Valine and ammonium pulses had similar effects on ethyl esters. The production rates of short-chain ethyl esters (ethyl acetate and ethyl hexanoate) increased with both nitrogen sources; ethyl acetate concentration increased directly after nitrogen addition to reach a plateau after 2 h, whereas ethyl hexanoate production increased 2 h after the pulse. The production rate of ethyl acetate was 50% higher with valine than with ammonium. Finally, the production of ethyl decanoate was reduced but not totally stopped.
The production of hexyl acetate stopped after addition of nitrogen, and its concentration followed the pattern expected from washing out.
(iv) Global metabolic rerouting associated with nitrogen pulses.
The yield of metabolite production as a function of glucose consumed was calculated for each nitrogen pulse experiment (Table 3). The repartition of the carbon was assessed to provide an overview of the effects of nitrogen on the carbon flux within the cells. The carbon flux through glycolysis and tricarboxylic acid (TCA) was modified by both ammonium and valine addition. In both cases, the flux through succinate synthesis was almost suppressed and the synthesis of acetate was reduced by 25 to 30%. The main difference between the two nitrogen sources was release of pyruvate, which increased by 25% after valine was added but was not affected by the addition of ammonium.
Table 3.
Yields of production of metabolites and volatile compounds according to glucose consumed in the third stage of FSCFa
| Metabolite | Yield |
||
|---|---|---|---|
| Without addition | Ammonium | Valine | |
| CO2 | 291 ± 6 | 300 ± 5 | 300 ± 6 |
| Ethanol | 605 ± 11 | 613 ± 12 | 602 ± 12 |
| Glycerol | 55 ± 2 | 58 ± 1 | 61 ± 3 |
| Acetate | 3.8 ± 0.6 | 2.9 ± 0.5 | 2.7 ± 0.6 |
| Pyruvate | 2.0 ± 0.5 | 2.0 ± 0.4 | 2.5 ± 0.5 |
| Succinate | 7.5 ± 1 | 1.5 ± 0.5 | 0.2 ± 0.1 |
| Isobutanol | 1.0 ± 0.2 | 0.4 ± 0.2 | 17.6 ± 0.9 |
| Isoamyl alcohol | 4.3 ± 0.2 | 1.2 ± 0.1 | 1. 2 ± 0.1 |
| Propanol | 0.21 ± 0.05 | 0.62 ± 0.05 | 0.8 ± 0.05 |
| Ethyl acetate | 0.09 ± 0.01 | 0.23 ± 0.03 | 0.45 ± 0.05 |
| Ethyl decanoate | 0.004 ± 0.001 | 0.002 ± 0.001 | 0.002 ± 0,001 |
| Ethyl hexanoate | 0.001 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 |
Yields in the third stage of FSCF, expressed as mCmol product/Cmol glucose (where Cmol is the number of mol of carbon coming from each metabolite), were compared between cultures without nitrogen addition and fermentations with ammonium and valine pulses. Mean values and standard deviations were calculated from 3 independent pulse experiments. Samples for the control without nitrogen addition were collected after three residence times at steady state, and those for ammonium or valine pulses were taken at the time of complete exhaustion of the added nitrogen source. Carbon balances were 97, 98, and 99% for the culture without nitrogen addition and the fermentations with ammonium and valine pulses, respectively.
The main difference between the effects of the two nitrogen sources on the production of volatile compounds (Table 3) involved isobutanol (which is synthesized from 2-ketoisovalerate originating from valine or CCM). Isobutanol production increased very substantially (10-fold) following valine but not ammonium addition. Therefore, the carbon skeleton required for the synthesis of this fusel alcohol was presumably provided by valine degradation rather than from glycolysis after the pulse. Nevertheless, the carbon balance between isobutanol and valine indicates that isobutanol produced following valine addition corresponded to 70% of the carbon consumed from valine.
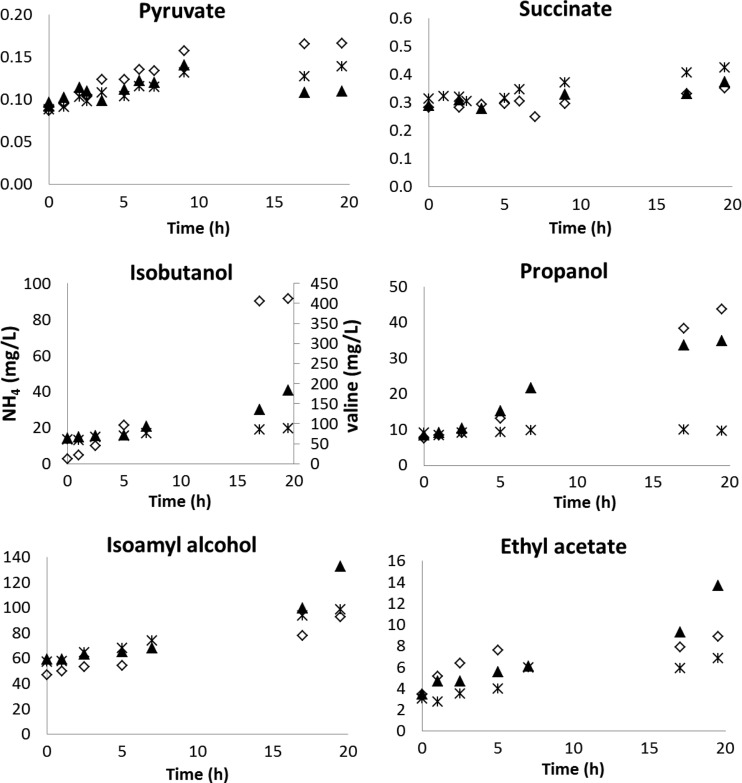
Ammonium and valine additions to batch fermentations were similarly investigated, and metabolite concentrations in control fermentations were compared to those following nitrogen supplementation (Fig. 4). Except for a 4-fold increase in propanol production triggered by nitrogen pulses (valine and ammonium) and a large increase in isobutanol formation as a result of valine addition, metabolite production during fermentations with nitrogen pulses was little different from that in control fermentations. In particular, nitrogen pulses had no detectable effect on the production of succinate, pyruvate, and ethyl esters, unlike observations in FSCF experiments. This illustrates how batch fermentation is not a reliable system for studying the response of yeast to singular perturbations of the environment, because the yeast physiology is never in stable equilibrium with the composition of the medium (17).
Fig 4.
Effects of nitrogen addition during the stationary phase of batch fermentation on metabolite concentrations. ▲, ammonium pulse; ♢, valine pulse;  , control batch fermentation, without nitrogen addition. Propanol, isoamyl alcohol, isobutanol, and ethyl acetate concentrations are expressed in mg/liter, and pyruvate and succinate concentrations are expressed in g/liter after nitrogen addition to batch fermentations. One representative experiment of three biological replicates is shown.
, control batch fermentation, without nitrogen addition. Propanol, isoamyl alcohol, isobutanol, and ethyl acetate concentrations are expressed in mg/liter, and pyruvate and succinate concentrations are expressed in g/liter after nitrogen addition to batch fermentations. One representative experiment of three biological replicates is shown.
DISCUSSION
We used an original device (the four-stage continuous fermentor [FSCF]) for a comprehensive analysis of the metabolic response of S. cerevisiae to nitrogen addition at the beginning of stationary phase of alcoholic fermentation. This system has previously been demonstrated to be relevant for simulating yeast growth and the formation of the main fermentation products, including ethanol, CO2, glycerol, and organic acids, at different stages of industrial wine fermentation (31). Here, we focused on the third stage of FSCF, where cells were in a physiological state equivalent to that of the first hours of the stationary phase in batch processes. The production levels of metabolites, in particular, fusel alcohols and esters—volatile compounds derived, at least in part, from nitrogen metabolism—were similar in this third stage of FSCF and batch cultures at the corresponding stage. These findings confirm that the FSCF device combines the advantages of continuous cultures, regarding steady state and reproducibility (30), and the ability to reproduce the physiological state of the stationary phase of wine fermentation. Consequently, FSCF is a suitable system for analyzing the time-dependent metabolic response of yeast to environmental changes, independently of changes resulting from the transformation of the medium by the fermentation process. It is therefore useful for elucidating how cells specifically adapt their metabolism to environmental modifications. This system was used to characterize the dynamics of the metabolic response to nitrogen additions during the stationary phase of wine fermentation. This issue has not been extensively studied. The few previous works focused exclusively on CCM metabolite formation (32) or on the production of esters (33) and are not informative about the kinetics of metabolite synthesis following nitrogen addition independently of the changes associated with the standard progression of batch fermentation. Nitrogen pulse experiments with the FSCF avoided the interfering effects on metabolic activity associated with the increase in inhibitory compounds, particularly ethanol, inevitable during batch processes. This culture mode also allowed the accurate observation of concentration variations of limited magnitude associated with nitrogen pulses, which are difficult to evidence in the context of the usual large changes in concentration during batch fermentation.
Nitrogen additions in the third stage of FSCF resulted in a substantial increase in the fermentation rate while biomass production was almost unaffected; this is consistent with previous observations during batch cultures (2, 11, 20). This recovery of an efficient fermentative activity has been explained by the increase of glucose uptake capacity, related to the de novo synthesis of glucose transporters (2, 34, 35, 59). More generally, previous works reported that one of the main consequences of nitrogen supplementation during wine fermentation was an enhanced de novo synthesis of proteins, which involves an increase in the anabolism of amino acids (36, 37). Torrea et al. (15) reported that changes in the rate of production of CO2 following nitrogen addition to chardonnay in batch fermentation must depend on the nitrogen availability but not on the nature (ammonium versus mixture of amino acids and ammonium) of the added source. Conversely, using precise online monitoring of the CO2 production rate, we observed that the effect of ammonium on fermentation performance, during both batch and FSCF cultures, was twice as high as that of valine, added at the same nitrogen equivalent concentration. Comparison of the kinetics of valine and ammonium assimilation with the profiles of CO2 production indicates that the greater effect of ammonium on the fermentation rate was related to an earlier and faster consumption of ammonium than of valine. In yeasts, ammonium and valine are assimilated via the transporters Mep1p, Mep2p, and Mep3p (40) and through the branched amino acid permeases (Bap2p and Bap3p) (41), respectively. Mep permeases are under nitrogen catabolic repression (NCR) control, which mediates the transcriptional repression of target genes by the four GATA-binding transcription factors in the presence of preferred nitrogen sources, as glutamine (38, 39, 41). Under nitrogen limitation conditions, as in the third stage of FSCF, the expression of these genes is derepressed. In contrast, BAP2 and BAP3 genes are under positive Ssy1p-mediated regulation, their expression being specifically induced by exogenous valine and leucine (41). Consequently, these genes were not expressed in the third stage of FSCF before nitrogen addition. In view of that, the lower efficiency of cells in degrading valine than in degrading ammonium after the pulse may be explained by a delay required for the induction of Bap permeases, in line with the regulation modes of the Mep and Bap transporters.
The FSCF cultures revealed a major rerouting of fluxes through the CCM and the biosynthesis of volatile compounds in response to valine and ammonium pulses. The most important modification was the specific increase in isobutanol and isobutyl acetate production as a consequence of valine pulses. The Ehrlich pathway has been described as being the main metabolic route for the catabolism of this amino acid (42, 43). The first step of this pathway consists of transamination resulting in the formation of glutamate, which is then used as a nitrogen donor for amino acid biosynthesis, and of 2-keto-isovalerate, which is the precursor keto acid of isobutanol. The yield of isobutanol from valine consumed, which was 0.7 Cmol isobutanol/Cmol valine, reflected the massive conversion of valine through this pathway (46). In line with a larger de novo synthesis of proteins as a consequence of nitrogen addition, the remaining valine (30% of assimilated valine) might be directly incorporated into proteins or used as precursor—via 2-keto-isovalerate—for synthesis of leucine (44), which is one of the most abundant proteinogenic amino acids (45). Similarly, the formation of pyruvate was specifically increased as a consequence of valine pulses. This compound is a central compound, being an intermediate in both glucose catabolism and TCA pathways. It also contributes to nitrogen metabolism, as a precursor for the synthesis of alanine and other branched-chain amino acids (leucine and isoleucine) (15, 47). The increased excretion of pyruvate after the addition of valine can be explained by a reduced requirement for pyruvate for the synthesis of branched-chain amino acids, since keto acid precursor may be provided through valine catabolism.
In addition to this specific response, there was also a common response to nitrogen pulses (valine or ammonium), which appeared to be driven mainly by the activation of the synthesis of amino acids as a consequence of the increase in nitrogen availability. The NAD consumption during the anabolism of amino acids largely contributed to the imbalance between the NAD and NADH pools related to biomass formation (48, 49). The excess of released NADH was for the most part regenerated by the formation of glycerol (49). Consistent with this, we found that adding nitrogen in FSCF resulted in an increase of the production of glycerol, likely due to the induction of de novo formation of amino acids. Moreover, the formation of propanol, reported to be directly associated with assimilation of nitrogen-containing compounds (14, 51; J. R. Mouret, personal communication), was considerably increased in the third stage of FSCF as a consequence of valine or ammonium pulses. Propanol is a product of the degradation of threonine by transamination followed by the spontaneous decarboxylation of the resulting 2-oxobutanoate and further reduction of propanol (50, 52). An excess of threonine synthesis under nitrogen supplementation may be responsible for the increase in propanol production.
Another important metabolic change associated with valine pulses was a massive decrease in the production of succinate, which was almost abolished during the nitrogen consumption phase, and in the formation of fusel alcohols, except isobutanol. Interestingly, the excretion of these metabolites resumed immediately after nitrogen exhaustion. This may be the consequence of a preferential use of α-keto acids derived from CCM for the formation of amino acids to fulfill requirements for protein synthesis (32) at the expense of the production of succinate and higher alcohols, including isoamyl alcohol and isobutanol. Consistent with this notion, the expression of LEU1, LEU2, BAT1, and BAT2, all genes involved in amino acid biosynthesis, was activated when ammonium was added to nitrogen-depleted medium (60). A decrease in succinate formation consecutive to nitrogen addition has been observed during wine batch fermentations (10, 15) but to a much lesser extent than in FSCF. Conversely, recent work reported a higher synthesis of isoamyl alcohol and 2-phenyl-ethanol when a mixture of amino acids was added during batch fermentation (15). This may be due to the increase of the intracellular availability of keto acids derived from the leucine and phenylalanine (present in the added amino acid mixture) assimilated and precursors of isoamyl alcohol and 2-phenyl-ethanol. During the growth phase of wine fermentation, succinate is mostly produced through the TCA cycle reductive branch (53). That succinate production decreases during the stationary phase, resulting in a redirection of the carbon flux toward glutamate formation, is in agreement with the synthesis of succinate via the TCA oxidative branch. This suggests that succinate may be produced by different metabolic routes, depending on the stage of fermentation.
It has been reported that acetate ester is mostly synthesized by one of two alcohol acetyltransferases, ATF1 and ATF2 (54, 55), depending on the environmental conditions. ATF1 expression is controlled by ethanol and heat stress and addition of glucose and nitrogen compounds (55). Changes in the formation of acetate esters following ammonium or valine pulses differed according to the ester and were more likely to be consequences of differing availabilities of precursors than of modulation in the activity of enzymes. Likewise, the ethyl acetate production rate increased rapidly after nitrogen addition, probably due to the increase in acetate and ethanol synthesis; the intracellular precursor concentration has been found to be the main limiting factor of ethyl ester production (56). Surprisingly, we found that the production of ethyl decanoate decreased, in contrast to that of other ethyl esters (acetate and hexanoate). This may be due to intracellular accumulation of short-chain ethyl esters, possibly as unsaturated fatty acid (UFA) analogues for membrane synthesis, as suggested by Saerens et al. (57) and Mason and Dufour (58).
This study, using an original experimental setup, provides new insights into the metabolic response of yeast cells to nitrogen pulses during the stationary phase of wine fermentation. In particular, we evidence the key role of the resynthesis of amino acids in the metabolic rerouting, independently of the added nitrogen source. In addition to this common response, specific modifications were induced depending on the nature of the nitrogen source added. These findings underline the complexity of modulating the production of volatile compounds, which is currently a major issue for winemakers. Our work clearly indicates that strategies to favor the formation of volatile compounds by nitrogen addition can be rationally developed only by integrating, at least, the definition of a target aroma compound, an understanding of the composition of grape juice, the nature of the nitrogen source added, and the time of supplementation.
ACKNOWLEDGMENTS
This work was supported by the European Community Seventh Framework Programme (FP7/2007-2013) under grant agreement CAFE no. KBBE-212754 (http://www.cafe-project.org), by the INRA Sys-Arômes program, and by BIOFLAVOUR Cost Action FA0907.
Footnotes
Published ahead of print 15 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02853-12.
REFERENCES
- 1. Agenbach WA. 1977. A study of must nitrogen content in relation to incomplete fermentations, yeast production and fermentation activity, p 66–88 In Proc. S. Afr. Soc. Enol. Vitic., Cape Town, South Africa South African Society of Enology and Viticulture, Stellenbosch, South Africa [Google Scholar]
- 2. Bely M, Sablayrolles JM, Barre P. 1990. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 70:246–252 [Google Scholar]
- 3. Jiranek V, Langridge P, Henschke PA. 1995. Amino acid and ammonium utilization by Saccharomyces cerevisiae wine yeasts from a chemically defined medium. Am. J. Enol. Vitic. 46:75–83 [Google Scholar]
- 4. Ough CS, Huang Z, An D, Stevens D. 1991. Amino acid uptake by four commercial yeasts at two different temperatures of growth and fermentation: effects on urea excretion and reabsorption. Am. J. Enol. Vitic. 42:26–40 [Google Scholar]
- 5. Alexandre H, Charpentier C. 1998. Biochemical aspects of stuck and sluggish fermentation in grape must. J. Ind. Microbiol. Biotechnol. 20:20–27 [Google Scholar]
- 6. Bely M, Rinaldi A, Dubourdieu D. 2003. Influence of assimilable nitrogen on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J. Biosci. Bioeng. 96:507–512 [DOI] [PubMed] [Google Scholar]
- 7. Monteiro FF, Bisson LF. 1991. Amino acid utilization and urea formation during vinification fermentations. Am. J. Enol. Vitic. 42:199–208 [Google Scholar]
- 8. Bell SJ, Henschke PA. 2005. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 11:242–295 [Google Scholar]
- 9. Ugliano M, Henschke PA, Herderich MJ, Pretorius IS. 2007. Nitrogen management is critical for wine flavour and style. Aust. N. Z. Wine Ind. J. 22:24–30 [Google Scholar]
- 10. Beltran G, Esteve-Zarzoso B, Rozès N, Mas A, Guillamón JM. 2005. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 53:996–1002 [DOI] [PubMed] [Google Scholar]
- 11. Blateyron L, Sablayrolles JM. 2001. Stuck and slow fermentations in enology: statistical study of causes and effectiveness of combined additions of oxygen and diammonium phosphate. J. Biosci. Bioeng. 91:184–189 [DOI] [PubMed] [Google Scholar]
- 12. Taillandier P, Ramon Portugal F, Fuster A, Strehaiano P. 2007. Effect of ammonium concentration on alcoholic fermentation kinetics by wine yeasts for high sugar content. Food Microbiol. 24:95–100 [DOI] [PubMed] [Google Scholar]
- 13. Carrau FM, Medina K, Farina L, Boido E, Henschke PA, Dellacassa E. 2008. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 8:1196–1207 [DOI] [PubMed] [Google Scholar]
- 14. González-Marco A, Jiménez-Moreno N, Ancín-Azpilicueta C. 2010. Influence of nutrients addition to nonlimited-in-nitrogen must on wine volatile composition. J. Food Sci. 75:206–211 [DOI] [PubMed] [Google Scholar]
- 15. Torrea D, Varela C, Ugliano M, Ancin-Azpilicueta C, Leigh Francis I, Henschke PA. 2011. Comparison of inorganic and organic nitrogen supplementation of grape juice—effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 127:1072–1083 [DOI] [PubMed] [Google Scholar]
- 16. Ugliano M, Travis B, Francis IL, Henschke PA. 2010. Volatile composition and sensory properties of Shiraz wines as affected by nitrogen supplementation and yeast species: rationalizing nitrogen modulation of wine aroma. J. Agric. Food Chem. 58:12417–12425 [DOI] [PubMed] [Google Scholar]
- 17. Vilanova M, Ugliano M, Varela C, Siebert T, Pretorius IS, Henschke PA. 2007. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 77:145–157 [DOI] [PubMed] [Google Scholar]
- 18. Barbosa C, Falco V, Mendes-Faia A, Mendes-Ferreira A. 2009. Nitrogen addition influences formation of aroma compounds, volatile acidity and ethanol in nitrogen deficient media fermented by Saccharomyces cerevisiae wine strains. J. Biosci. Bioeng. 108:99–104 [DOI] [PubMed] [Google Scholar]
- 19. Hernandez-Orte P, Bely M, Cacho J, Ferreira V. 2006. Impact of ammonium additions on volatile acidity, ethanol, and aromatic compound production by different Saccharomyces cerevisiae strains during fermentation in controlled synthetic media. Aust. J. Grape Wine Res. 12:150–160 [Google Scholar]
- 20. Bely M, Sablayrolles JM, Barre P. 1990. Description of alcoholic fermentation kinetics: its variability and significance. Am. J. Enol. Vitic. 41:319–324 [Google Scholar]
- 21. Kresnowati MTAP, Suarez-Mendez CM, van Winden WA, van Gulik WM, Heijnen JJ. 2008. Quantitative physiological study of the fast dynamics in the intracellular pH of Saccharomyces cerevisiae in response to glucose and ethanol pulses. Metab. Eng. 10:39–54 [DOI] [PubMed] [Google Scholar]
- 22. Lara AR, Taymaz-Nikerel H, Mashego MR, van Gulik WM, Heijnen JJ, Ramírez OT, van Winden WA. 2009. Fast dynamic response of the fermentative metabolism of Escherichia coli to aerobic and anaerobic glucose pulses. Biotechnol. Bioeng. 104:1153–1161 [DOI] [PubMed] [Google Scholar]
- 23. Sonnleitner B. 1998. Dynamic adaptation of microbes. J. Biotechnol. 65:47–60 [DOI] [PubMed] [Google Scholar]
- 24. Stanbury PF, Whitaker A, Hall SJ. 1999. Principles of fermentation technology. Butterworth-Heinemann, Oxford, United Kingdom [Google Scholar]
- 25. Taymaz-Nikerel H, van Gulik WM, Heijnen JJ. 2011. Escherichia coli responds with a rapid and large change in growth rate upon a shift from glucose-limited to glucose-excess conditions. Metab. Eng. 13:307–318 [DOI] [PubMed] [Google Scholar]
- 26. Kresnowati MTAP, van Winden WA, Almering MJH, ten Pierick A, Ras C, Knijnenburg TA, Daran-Lapujade P, Pronk JT, Heijnen JJ, Daran JM. 2006. When transcriptome meets metabolome: fast cellular responses of yeast to sudden relief of glucose limitation. Mol. Syst. Biol. 2:49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmitz M, Hirsch E, Bongaerts J, Takors R. 2002. Pulse experiments as a prerequisite for the quantification of in vivo enzyme kinetics in aromatic amino acid pathway of Escherichia coli. Biotechnol. Prog. 18:935–941 [DOI] [PubMed] [Google Scholar]
- 28. Mashego M, Rumbold K, De Mey M, Vandamme E, Soetaert W, Heijnen J. 2007. Microbial metabolomics: past, present and future methodologies. Biotechnol. Lett. 29:1–16 [DOI] [PubMed] [Google Scholar]
- 29. Bull AT. 2010. The renaissance of continuous culture in the post-genomics age. J. Ind. Microbiol. Biotechnol. 37:993–1021 [DOI] [PubMed] [Google Scholar]
- 30. Hoskisson PA, Hobbs G. 2005. Continuous culture—making a comeback? Microbiology 151:3153–3159 [DOI] [PubMed] [Google Scholar]
- 31. Clement T, Perez M, Mouret JR, Sablayrolles JM, Camarasa C. 2011. Use of a continuous multistage bioreactor to mimic winemaking fermentation. Int. J. Food Microbiol. 150:42–49 [DOI] [PubMed] [Google Scholar]
- 32. Schulze U, Lidén G, Villadsen J. 1996. Dynamics of ammonia uptake in nitrogen limited anaerobic cultures of Saccharomyces cerevisiae. J. Biotechnol. 46:33–42 [DOI] [PubMed] [Google Scholar]
- 33. Miller AC, Wolff SR, Bisson LF, Ebeler SE. 2007. Yeast strain and nitrogen supplementation: dynamics of volatile ester production in chardonnay juice fermentations. Am. J. Enol. Vitic. 58:470–483 [Google Scholar]
- 34. Palmieri L, Lasorsa FM, De Palma A, Palmieri F, Runswick MJ, Walker JE. 1997. Identification of the yeast ACR1 gene product as a succinate-fumarate transporter essential for growth on ethanol or acetate. FEBS Lett. 417:114–118 [DOI] [PubMed] [Google Scholar]
- 35. Jiménez-Martí E, Aranda A, Mendes-Ferreira A, Mendes-Faia A, del Olmo ML. 2007. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds. Antonie Van Leeuwenhoek 92:61–75 [DOI] [PubMed] [Google Scholar]
- 36. Theobald U, Mailinger W, Baltes M, Rizzi M, Reuss M. 1997. In vivo analysis of metabolic dynamics in Saccharomyces cerevisiae. I. Experimental observations. Biotechnol. Bioeng. 55:305–316 [DOI] [PubMed] [Google Scholar]
- 37. Marini AM, Soussi-Boudekou S, Vissers S, Andre B. 1997. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:4282–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cunningham TS, Cooper TG. 1991. Expression of the DAL80 gene, whose product is homologous to the GATA factors and is a negative regulator of multiple nitrogen catabolic genes in Saccharomyces cerevisiae, is sensitive to nitrogen catabolite repression. Mol. Cell. Biol. 11:6205–6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Georis I, Feller A, Vierendeels F, Dubois E. 2009. The yeast GATA factor Gat1 occupies a central position in nitrogen catabolite repression-sensitive gene activation. Mol. Cell. Biol. 29:3803–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magasanik B, Kaiser CA. 2002. Nitrogen regulation in Saccharomyces cerevisiae. Gene 290:1–18 [DOI] [PubMed] [Google Scholar]
- 41. Ljungdahl PO. 2009. Amino-acid-induced signalling via the SPS-sensing pathway in yeast. Biochem. Soc. Trans. 37:242–247 [DOI] [PubMed] [Google Scholar]
- 42. Hazelwood LA, Daran JM, van Maris AJA, Pronk JT, Dickinson JR. 2008. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 74:2259–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lilly M, Bauer F, Styger G, Lambrechts MG, Pretorius IS. 2006. The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res. 6:726–743 [DOI] [PubMed] [Google Scholar]
- 44. Marobbio CM, Giannuzzi G, Paradies E, Pierri CL, Palmieri F. 2008. Alpha-isopropylmalate, a leucine biosynthesis intermediate in yeast, is transported by the mitochondrial oxalacetate carrier. J. Biol. Chem. 283:28445–28453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruinenberg PM, van Dijken JP, Scheffers WA. 1983. A theoretical analysis of NADPH production and consumption in yeasts. J. Gen. Microbiol. 129:953–964 [DOI] [PubMed] [Google Scholar]
- 46. Dickinson JR, Harrison SJ, Hewlins MJE. 1998. An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 273:25751–25756 [DOI] [PubMed] [Google Scholar]
- 47. Albers E, Larsson C, Lidén G, Niklasson C, Gustafsson L. 1996. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 62:3187–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verduyn C, Postma E, Scheffers W, van Dijken J. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395–403 [DOI] [PubMed] [Google Scholar]
- 49. Ansell R, Granath K, Hohmann S, Thevelein JM, Adler L. 1997. The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 16:2179–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moreira N, de Pinho PG, Santos C, Vasconcelos I. 2011. Relationship between nitrogen content in grapes and volatiles, namely heavy sulphur compounds, in wines. Food Chem. 126:1599–1607 [DOI] [PubMed] [Google Scholar]
- 51. Van Der Sluis C, Rahardjo YSP, Smit BA, Kroon PJ, Hartmans S, Ter Schure EG, Tramper J, Wijffels R. 2002. Concomitant extracellular accumulation of alpha-keto acids and higher alcohols by Zygosaccharomyces rouxii. J. Biosci. Bioeng. 93:117–124 [DOI] [PubMed] [Google Scholar]
- 52. Yoshimoto H, Fukushige T, Yonezawa T, Sone H. 2002. Genetic and physiological analysis of branched-chain alcohols and isoamyl acetate production in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 59:501–508 [DOI] [PubMed] [Google Scholar]
- 53. Camarasa C, Grivet JP, Dequin S. 2003. Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways for succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology 149:2669–2678 [DOI] [PubMed] [Google Scholar]
- 54. Lilly M, Lambrechts MG, Pretorius IS. 2000. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl. Environ. Microbiol. 66:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Verstrepen KJ, Van Laere SDM, Vanderhaegen BMP, Derdelinckx G, Dufour JP, Pretorius IS, Winderickx J, Thevelein JM, Delvaux FR. 2003. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. 69:5228–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saerens SMG, Delvaux FR, Verstrepen KJ, Thevelein JM. 2010. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 3:165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saerens SM, Verstrepen KJ, Van Laere SD, Voet AR, Van Dijck P, Delvaux FR, Thevelein JM. 2006. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J. Biol. Chem. 281:4446–4456 [DOI] [PubMed] [Google Scholar]
- 58. Mason AB, Dufour JP. 2000. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 16:1287–1298 [DOI] [PubMed] [Google Scholar]
- 59. Lucero P, Moreno E, Lagunas R. 2002. Catabolite inactivation of the sugar transporters in Saccharomyces cerevisiae is inhibited by the presence of a nitrogen source. FEMS Yeast Res. 1:307–314 [DOI] [PubMed] [Google Scholar]
- 60. Marks VD, van der Merwe GK, van Vuuren HJ. 2003. Transcriptional profiling of wine yeast in fermenting grape juice: regulatory effect of diammonium phosphate. FEMS Yeast Res. 3:269–287 [DOI] [PubMed] [Google Scholar]