Abstract
Agromonas oligotrophica (Bradyrhizobium oligotrophicum) S58T is a nitrogen-fixing oligotrophic bacterium isolated from paddy field soil that is able to grow in extra-low-nutrient environments. Here, the complete genome sequence of S58 was determined. The S58 genome was found to comprise a circular chromosome of 8,264,165 bp with an average GC content of 65.1% lacking nodABC genes and the typical symbiosis island. The genome showed a high level of similarity to the genomes of Bradyrhizobium sp. ORS278 and Bradyrhizobium sp. BTAi1, including nitrogen fixation and photosynthesis gene clusters, which nodulate an aquatic legume plant, Aeschynomene indica, in a Nod factor-independent manner. Although nonsymbiotic (brady)rhizobia are significant components of rhizobial populations in soil, we found that most genes important for nodule development (ndv) and symbiotic nitrogen fixation (nif and fix) with A. indica were well conserved between the ORS278 and S58 genomes. Therefore, we performed inoculation experiments with five A. oligotrophica strains (S58, S42, S55, S72, and S80). Surprisingly, all five strains of A. oligotrophica formed effective nitrogen-fixing nodules on the roots and/or stems of A. indica, with differentiated bacteroids. Nonsymbiotic (brady)rhizobia are known to be significant components of rhizobial populations without a symbiosis island or symbiotic plasmids in soil, but the present results indicate that soil-dwelling A. oligotrophica generally possesses the ability to establish symbiosis with A. indica. Phylogenetic analyses suggest that Nod factor-independent symbiosis with A. indica is a common trait of nodABC- and symbiosis island-lacking strains within the members of the photosynthetic Bradyrhizobium clade, including A. oligotrophica.
INTRODUCTION
Agromonas oligotrophica (Bradyrhizobium oligotrophicum) S58T is a nitrogen-fixing oligotrophic bacterium that was isolated from paddy field soil (1). In 1983, a type strain of A. oligotrophica was proposed, based on comparisons of morphological and physiological characteristics with S58 (1), and this proposal was subsequently validated (2). In 2012, Ramírez-Bahena et al. (3), however, suggested a reclassification of Agromonas oligotrophica into Bradyrhizobium oligotrophicum, on the basis of phylogenetic analysis of housekeeping genes, phenotypic characterization, and DNA-DNA hybridization. Cells of A. oligotrophica proliferate by budding and are able to grow in extraoligotrophic environments such as 10,000-fold-diluted nutrient broth, and they are very sensitive to the supply of organic compounds (1). Agromonas oligotrophica likely plays an important role in the decomposition of organic matter and the recycling of other nutrients in paddy field soil (4). Agromonas oligotrophica is also abundant in the roots of rice, with 108 to 109 cells g−1 dry matter, but it is not known whether it can provide fixed nitrogen to the host plant (5). Many other soil oligotrophic bacteria in close proximity to A. oligotrophica S58 on model colony-forming curves have been isolated from paddy field soil by using a diluted nutrient broth (1).
Agromonas oligotrophica S58 is phylogenetically close to Bradyrhizobium sp. ORS278 and BTAi1 (6). These strains nodulate the stem and root of an aquatic legume, Aeschynomene indica, although they lack the canonical nodABC genes required for synthesis of the core structures of Nod factors (NF) on a symbiosis island or a symbiosis plasmid (7, 8). This NF-independent nodulation system is hypothesized to be primitive, because infection with the NF-independent symbiont occurs through epidermal fissures generated by the emergence of lateral roots (7, 8). Random Tn5 mutagenesis of Bradyrhizobium sp. ORS278 identified some genes relevant to nodule development and symbiotic nitrogen fixation, but no complete nodulation-deficient mutants have been identified (7, 9). Thus, the NF-independent nodulation mechanism remains to be elucidated. However, it has been hypothesized that a purine derivative, such as cytokinin, might play a role in triggering nodule formation instead of a Nod factor (7, 10).
Generally, symbiotic gene clusters of (brady)rhizobia are acquired horizontally as a symbiosis island or a symbiotic plasmid, as is strongly suggested by the genome structures of Bradyrhizobium japonicum USDA110 (31) and USDA6T (11) and of Mesorhizobium loti MAFF303099 (12). Nonsymbiotic (brady)rhizobia lacking the symbiotic gene clusters are often found among these species, including Bradyrhizobium sp. strain S23321 (13), and rhizobia have been isolated from the rhizosphere of Lotus corniculatus (14). Recent ecological studies (15, 16) have revealed that host legumes select symbiosis islands among Bradyrhizobium populations, which suggests that lateral transfer of the symbiosis genes into nonsymbiotic bradyrhizobia in soil is an evolutionary process producing legume symbionts.
Originally, we hypothesized that A. oligotrophica would be a nonsymbiotic bradyrhizobium, because it was isolated from field soils (1, 2). If this hypothesis were true, then a genome comparison between symbiotic (e.g., ORS278) and nonsymbiotic (S58) bradyrhizobia would allow us to identify the gene repertory relevant to symbiotic interactions with A. indica (8). In the present study, we therefore determined the complete genome sequence of A. oligotrophica S58 and compared it with known bradyrhizobial genome sequences, and then we examined phenotypes of A. oligotrophica and its close relatives symbiotic on A. indica.
MATERIALS AND METHODS
Bacterial strains, media, and DNA preparation.
The bacterial strains and plasmids studied are listed in Table 1. Agromonas oligotrophica and Bradyrhizobium spp. were cultured to the stationary phase at 30°C in HM salt medium (17) containing 0.1% arabinose and 0.025% yeast extract. The cells were harvested by centrifugation, and total DNA of S58 was prepared by using a blood genomic DNA extraction Maxiprep system (Viogene, Sunnyvale, CA). Total DNA of strains other than S58 was prepared as previously described (18).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Agromonas oligotrophica strains | ||
| S58T | Wild-type strain derived from paddy soil | 1 |
| S58::dsRed | This study | |
| S58::gusA | This study | |
| S42 | Wild-type strain derived from paddy soil | 1 |
| S55 | Wild-type strain derived from paddy soil | 1 |
| S72 | Wild-type strain derived from paddy soil | 1 |
| S80 | Wild-type strain derived from paddy soil | 1 |
| Bradyrhizobium sp. strains | ||
| ORS278 | Wild-type strain derived from stem nodule of A. indica | 7 |
| BTAi1 | Wild-type strain derived from stem nodule of A. sensitiva | 7 |
| G14130 | Slow-growing oligotrophic bacterium derived from grassland soil | 32 |
| HWK12 | 2,4-d-degrading bacterium derived from Hawaiian soil | 44 |
| HW13 | 2,4-d-degrading bacterium derived from Hawaiian soil | 44 |
| Bradyrhizobium japonicum strains | ||
| USDA110 | Soybean bradyrhizobium | 31 |
| USDA122 | Soybean bradyrhizobium | 6, 45 |
| USDA6 | Soybean bradyrhizobium; type strain of B. japonicum | 11 |
| NC4 | Soybean bradyrhizobium | 6 |
| NC6 | Soybean bradyrhizobium | 6 |
| NK2 | Soybean bradyrhizobium | 6 |
| T7 | Soybean bradyrhizobium | 6 |
| T9 | Soybean bradyrhizobium | 6 |
| Bradyrhizobium elkanii strain | ||
| USDA76 | Soybean bradyrhizobium; type strain of B. elkanii | 45 |
| Rhodopseudomonas palustris strain | ||
| CGA009 | Photosynthetic bacterium | 46 |
| Escherichia coli strain | ||
| DH5α | recA; cloning strain | Toyobo Inc. |
| Plasmids | ||
| pmTn5SSgusA20 | Plasmid used for transposon insertion; gusA, Apr, Smr, Spr | 47 |
| pBjGroEL4::dsRed2 | Plasmid used for transposon insertion; dsRed, Apr, Smr, Spr | See Fig. S1 in the supplemental material |
| pRK2013 | ColE replicon carrying RK2 transfer genes; Kmr | 48 |
Escherichia coli was grown at 37°C in Luria-Bertani medium (19). Antibiotics were added to the medium at the following concentrations: for B. japonicum, 100 μg tetracycline (Tc) ml−1, 100 μg spectinomycin (Sp) ml−1, 100 μg streptomycin (Sm) ml−1, 100 μg kanamycin (Km) ml−1, and 100 μg polymyxin B (Pm) ml−1; for E. coli, 50 μg Tc ml−1, 50 μg Sp ml−1, 50 μg Sm ml−1, 50 μg Km ml−1, and 50 μg ampicillin (Ap) ml−1.
Tagging with DsRed and GusA.
Agromonas oligotrophica S58 was tagged with pmTn5SSgusA20 (Table 1) and pBjGroEL4::dsRed2 (see Fig. S1 in the supplemental material) by triparental mating on HM agar plates and using pRK2013 as a helper plasmid (48). Transconjugants were selected by using HM agar plates containing 100 μg Sp ml−1, 100 μg Sm ml−1, and 50 μg Pm ml−1.
Southern hybridization analysis for detecting pufBALM.
Total DNA from each strain was digested with BamHI and then electrophoresed on a 0.8% agarose gel in Tris-acetate-EDTA buffer. DNA from the gel was transferred onto a nylon membrane (Biodyne-B; Japan Pall Co. Ltd., Tokyo, Japan). The pufBALM gene was amplified by PCR from total DNA of Rhodopseudomonas palustris CGA009 using the primer pair 5′-CTTCGTGACCAGCTTCTTCC and 5′-GTTGTTGGTCCAGTCCAGGT and 30 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 2 min followed by incubation for 7 min at 72°C. A PCR fragment (1.9 kb) of the pufBALM gene was labeled with a digoxigenin (DIG) labeling and detection kit (Roche Diagnostics, Indianapolis, IN) for use as a probe. DNA hybridization was carried out as described previously (21) except at a hybridization temperature of 55°C.
Phylogeny.
A phylogenetic analysis was performed by comparing the 16S rRNA gene sequences (genome coordinates 3,187,406 to 3,188,898 and 7,419,987 to 7,421,479 bp) and the internal transcribed spacer (ITS) sequences between the 16S and 23S rRNA genes (coordinates 3,188,899 to 3,189,972 and 7,418,915 to 7,419,986 bp) of the S58 genome with the corresponding sequences of other Bradyrhizobiaceae (see Table S1 in the supplemental material). The sequences were aligned by using the CLUSTAL W program, and neighbor-joining trees were constructed by using MEGA version 5.02 software (20). One thousand bootstrap replicates were used to generate a consensus tree.
Genome sequencing and assembly and gap closing.
The genome sequence of A. oligotrophica S58 was determined by the whole-genome shotgun strategy by using Sanger and 454 pyrosequencing. For Sanger sequencing with a 3730xl sequencer (Applied Biosystems, Foster City, CA), about 20 μg of DNA was sheared with a HydroShear (Gene Machines, San Carlos, CA) for a short-insert genomic library; another 80 μg was sheared for construction of a long-insert library. DNA fragments of 3 kb (for the short-insert library) and 10 kb (for the long-insert library) were subcloned into the plasmid pTS1 vector (Nippon Gene, Tokyo, Japan) to construct shotgun libraries. Template DNA was prepared by amplifying the inserted DNA of each clone by PCR of an aliquot of the bacterial culture. We generated 53,760 reads by sequencing both ends of the clones, giving 3.9-fold genome coverage from the Sanger sequencing. For pyrosequencing with a GS FLX Titanium system (Roche Applied Science, Mannheim, Germany), 5 μg of genomic DNA was sheared by nebulization to obtain fragments ranging from 300 to 800 bp. Template DNA was prepared according to the supplier's protocol. The pyrosequencing data, giving 11.9-fold genome coverage, were assembled by using Newbler assembly software, and 524 contigs were generated. The GS FLX contig sequence data were then imported as “pseudoreads” of the Sanger data into a Phred/Phrap/Consed system (22–24). The hybrid assembly of the Sanger and 454 pyrosequencing data eventually generated 34 contigs. Gap closing and resequencing of low-quality regions of the assembled data were performed by PCR, primer walking, and direct sequencing of appropriate plasmid clones. The finished sequence was estimated by the Phrap software to have an error rate of less than 1 per 10,000 bases (Phrap score ≥ 40).
Gene assignment and annotation.
tRNA regions were predicted by using the tRNA scan-SE 1.23 program (25). Protein-coding regions were predicted by using MetaGeneAnnotator software with default parameters (26). The functions of predicted protein-coding regions were annotated through comparisons with the NCBI nr database.
Genome comparison.
Putative orthologous genes between A. oligotrophica S58 and two photosynthetic bradyrhizobial strains (Bradyrhizobium ORS278 and BTAi1) were identified by using bidirectional BLASTp comparisons with an E value cutoff of 10−20. Orthologous relationships are depicted by a Venn diagram. In addition, whole-genome comparisons between S58 and Bradyrhizobium ORS278 and BTAi1 were performed by using GenomeMatcher v.1.68 (27) with default parameters.
Nodulation tests on A. indica.
Seeds of A. indica were immersed and shacked in concentrated sulfuric acid for 25 min, washed three times with sterile distilled water, and then immersed in sterile distilled water overnight. The seeds were then immersed in 0.5% (vol/vol) sodium hypochlorite for 1 min and washed 10 times with sterile distilled water. Finally, the seeds were placed in sterilized plates containing tissue paper wetted with sterile distilled water for 2 days at 30°C in the dark and then transplanted to a Leonard jar (CUL-JAR300; Asahi Glass Co., Ltd., Tokyo, Japan) containing sterile vermiculite and nitrogen-free nutrient solution (28). After transplantation, the seedlings were inoculated with bacteria at 1 × 108 cells per plant. The inocula were grown to the stationary phase in HM broth medium containing 0.1% arabinose and 0.025% yeast extract at 30°C with shaking at 300 rpm. They were then centrifuged, washed twice with sterile distilled water, and resuspended in sterile distilled water (1 × 108 cells ml−1). Plants were grown in a growth chamber (Biotron LH-300; Nippon Medical & Chemical Instrument, Tokyo, Japan) for 31 days (A. indica) at 25°C under conditions of a 16-h/8-h light/dark cycle. The tested strains of A. oligotrophica are listed in Table 1. Bradyrhizobium sp. ORS278 and Bradyrhizobium sp. BTAi1 were used as positive controls.
Light microscopy.
For microscopic observation of root nodules, A. indica was inoculated with 1 × 108 cells of DsRed- or gusA-tagged S58. At 16 days after inoculation (DAI), 40-μm-thick sections of root nodules were prepared with a Vibratome sectioning system (Vibratome 3000; Vibratome Co., St. Louis, MO) and observed with a confocal laser scanning microscope (LSM 710; Carl Zeiss, Jena, Germany). The emission signals were collected on two channels: channel 1 (555 to 700 nm) was used to visualize the DsRed emission, and channel 2 (410 to 550 nm) was used for visualization of the plant autofluorescence. To test S58 colonization on root surfaces, A. indica inoculated with gusA-tagged S58 was sampled at 7 DAI. The nodulated roots were immersed in a GUS assay solution (50 mg liter−1 of 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid [X-Gluc], 2 g liter−1 of SDS, 20% methanol, 20 mM sodium phosphate buffer, pH 7.0) in vacuo for 30 min and incubated at 30°C overnight. GUS expression was observed under a stereoscopic microscope (SZX12; Olympus Corporation, Tokyo, Japan). For stem nodule observation, the cultured cells of gusA-tagged S58 were adjusted to an optical density of 1.0 at 660 nm with sterilized water. The cells were carefully painted onto the stem of A. indica by using a sterilized brush (ZBS1-8; Pentel Co., Ltd., Tokyo, Japan) 14 days after transplanting. The sectioning, GUS staining, and stereomicroscopic observation of the stem nodules (27 DAI) were performed as described above.
TEM observation.
Root nodules of A. indica inoculated with S58 (33 DAI) were cut into 1-mm3 cubes and fixed with 1% glutaraldehyde in 100 mM sodium phosphate buffer (pH 7.0) overnight, postfixed in 5% osmium tetroxide for 1 h at room temperature, dehydrated in a graded acetone series (60% to 100%), and embedded in resin consisting of 6.6 g Epon 812 (TAAB Laboratories Equipment Ltd., Aldermaston, England), 3 g dodecenyl succinic anhydride, 4 g methyl nadic anhydride, and 0.17 g 2,4,6-tri(dimethylaminomethyl)phenol. Ultrathin sections were prepared by using a microtome and mounted on collodion carbon-coated copper grids. The sections were examined with a transmission electron microscope (TEM) (JEOL 1210; JEOL Ltd., Tokyo, Japan).
Rice inoculation experiments.
Seeds of Oryza sativa cv. Nipponbare were surface sterilized with 70% ethanol for 1 min, immersed in 2.4% (wt/vol) sodium hypochlorite solution for 15 min, and then rinsed three times with sterilized distilled water (for 15 min each time). The seeds were transferred onto sterile 0.8% water agar plates and germinated overnight at 30°C in the dark. Two-day-old seedlings were aseptically transferred to perforated 1.5-ml microtubes (131-415C; Watson Co., Ltd., Tokyo, Japan) hanging at the liquid surface in 27-ml glass test tubes (TEST18NP; Asahi Glass Co., Ltd.) containing 25 ml of nitrogen-free nutrient solution (29) inoculated with gusA-tagged S58 (final concentration, 109 cells ml−1). The seedlings were grown for 12 days in a growth chamber (Biotron LH-300) at 25°C under conditions of a 16-h/8-h light/dark cycle. Root samples were GUS stained and observed as described above.
Nucleotide sequence accession number.
The complete nucleotide sequence of A. oligotrophica S58 was submitted to DDBJ under accession number AP012603.
RESULTS AND DISCUSSION
Phylogeny of strain S58.
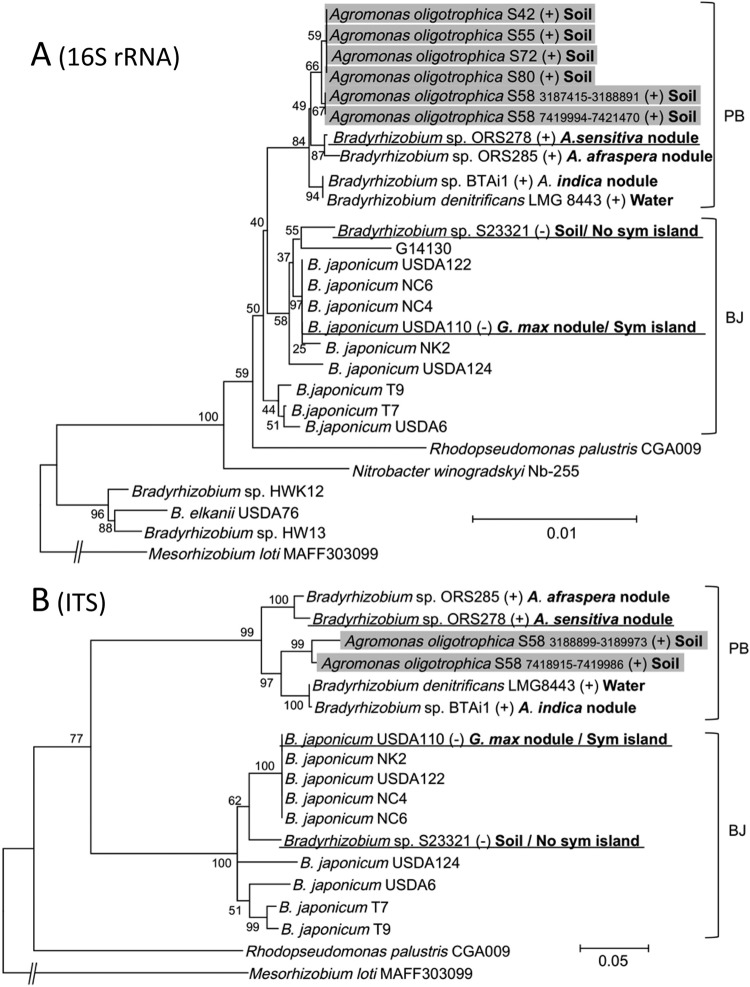
To examine the phylogenetic relationships between S58 and other members of the Bradyrhizobiaceae (Table 1), a phylogenetic tree was constructed based on 16S rRNA sequences (Fig. 1A). Two copies of the 16S rRNA gene in S58 were clustered within a group of photosynthetic bradyrhizobia, including ORS278 and BTAi1, which are A. indica symbionts (Fig. 1A). To increase the phylogenetic resolution, the ITS region was also analyzed (Fig. 1B). Again, two copies of the S58 ITS region were clustered with those of photosynthetic bradyrhizobia, separately from those of Bradyrhizobium japonicum strains. These results suggest that the S58 genome may resemble the genomes of the A. indica symbionts ORS278 and BTAi1.
Fig 1.
Phylogenetic relationships of Agromonas oligotrophica S58 and other members of the Bradyrhizobiaceae determined on the basis of 16S rRNA gene sequences (A) and internal transcribed spacer sequences (B). For all trees, Mesorhizobium loti MAFF303099 was used as an outgroup. Numbers at the nodes are the percentages of 1,000 bootstrap replications supporting that partition. Branches corresponding to partitions reproduced in less than 50% of the bootstrap replicates are not shown. Isolation sources are shown following strain names. Positive and negative data representing Nod factor-independent nodulation on A. indica are marked with (+) and (−), respectively. Bradyrhizobium sp. S23321 isolates from soil lacked symbiosis (sym) island-containing nodABC genes and do not nodulate soybean (Glycine max) and sitratro (13), whereas B. japonicum USDA110 with symbiosis islands nodulates soybean and siratro in a Nod factor-dependent manner (13, 31). Abbreviations: PB, photosynthetic Bradyrhizobium clade; BJ, Bradyrhizobium japonicum clade. Although both soil-dwelling A. oligotrophica S58 (PB) and Bradyrhizobium sp. S23321 (BJ) lacked symbiosis islands, S58 (PB) exclusively nodulates A. indica in a NF-independent manner.
Photosynthetic genes of strain S58.
A. oligotrophica S58 produced pink pigmentation on HM agar medium, suggesting that it is a photosynthetic bacterium. Southern hybridization was performed using pufBALM of Rhodopseudomonas palustris CGA009 as a probe (Table 1). pufBALM encodes light-harvesting complexes (pufBA) and a reaction center (pufLM). Hybridization signals were detected in S58 (lane 9), ORS278 (lane 11), and BTAi1 (lane 12) (see Fig. S2 in the supplemental material). Other bradyrhizobial strains, including B. japonicum, did not show pufBALM hybridization signals. These results suggest that strain S58 has photosynthetic genes, and prompted us to determine the genome sequence of S58.
General descriptions of the S58 genome.
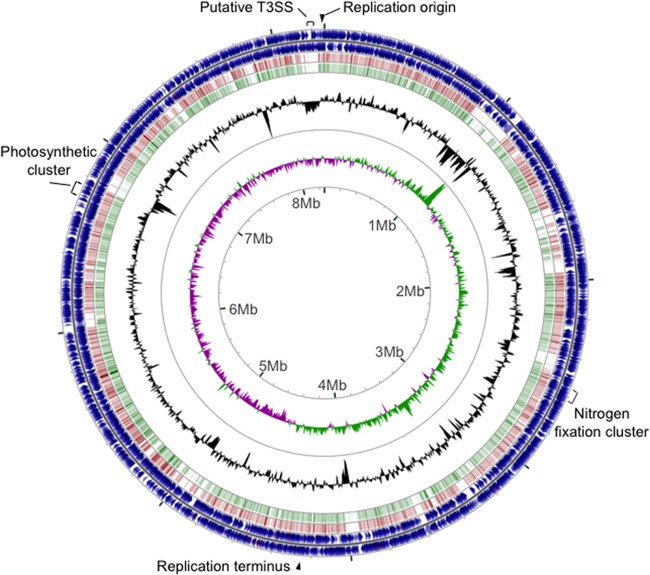
The complete genome determination of A. oligotrophica S58 showed a single circular chromosome of 8,264,165 bp with an average GC content of 65.1% (Table 2). The S58 genome contained two copies of the rRNA gene cluster, located at the coordinates 3,187,406 to 3,193,035 and 7,415,855 to 7,421,479. Fifty-one tRNA genes, corresponding to all 20 of the standard amino acids, are scattered throughout the S58 genome. In total, 7,228 protein-coding genes were predicted by using MetaGeneAnnotator software (26). A GC skew analysis was performed to predict the locations of the origin and terminator of DNA replication (30). Shifts of the GC skew were observed in two regions of the genome, at coordinates 0.2 Mb and 4.4 Mb (Fig. 2, innermost circle). The putative replication origin, determined by comparison with the genome ORS278, is about 1,000 bp upstream from dnaA. The conserved sequence pattern required to convert a dimer chromosome to a monomer after aberrant DNA duplication led to the designation of dif (31). The genome sequence of S58 revealed that dif is located at coordinates 4,414,012 to 4,413,986, near one side of a GC skew shift (Fig. 2). It is likely that DNA replication terminates in this region.
Table 2.
Features of the genomes of Agromonas oligotrophica S58 and two closely related bradyrhizobial strains, ORS278 and BTAi1
| Strain | Value(s) |
||
|---|---|---|---|
| S58 | ORS278 | BTAi1 | |
| Genome size (bp) | 8,264,165 | 7,456,587 | 8,493,515 |
| G+C content (%) | 65.1 | 65.5 | 64.9 |
| No. of rRNA genes | 2 | 2 | 2 |
| No. of tRNA genes | 51 | 50 | 52 |
| No. of protein-coding genes | 7,228 | 6,752 | 7,729 |
| No. of plasmids (size in bp) | 0 | 0 | 1 (228,826) |
Fig 2.
Circular view of whole-genome alignments of the chromosome of Agromonas oligotrophica S58. The outermost circle and the second circle show the positions of the putative protein-coding genes in clockwise and counterclockwise directions, respectively. The third and fourth circles from the outside represent BLASTn comparisons with Bradyrhizobium sp. BTAi1 (red) and Bradyrhizobium sp. ORS278 (green), respectively (E-value < 10−10). The innermost and second-innermost circles show the GC (guanine-cytosine) skew (green and purple) and the GC content (black), respectively. The GC skew circle shows the deviation from the average GC content of the entire sequence (higher-than-average GC content is shown in green; lower-than-average GC content is shown in purple). The marking inside the innermost circle represents genome positions in megabases. The positions of the putative replication origin, putative replication terminus, nitrogen fixation genes, and photosynthetic genes are shown outside the outermost circle. T3SS, type III secretion system.
Comparative genomics among S58, ORS278, and BTAi1.
The number of protein-coding genes and the chromosome size of the S58 genome were similar to those of BTAi1 and larger than those of ORS278 (Table 2). A dot plot analysis comparing the S58 genome with those of ORS278 and BTAi1 showed high levels of similarity overall, although many genomic rearrangements were observed (see Fig. S3 in the supplemental material). A BiBlast comparison, performed to compare the gene contents among the three strains, revealed that 5,015 genes (48.6%) are conserved among all three strains (Fig. 3); in the case of S58, 69.4% of its genes (5,015/7,228) are conserved among the three strains. These results indicate that the S58 genome is very similar to the genomes of ORS278 and BTAi1.
Fig 3.
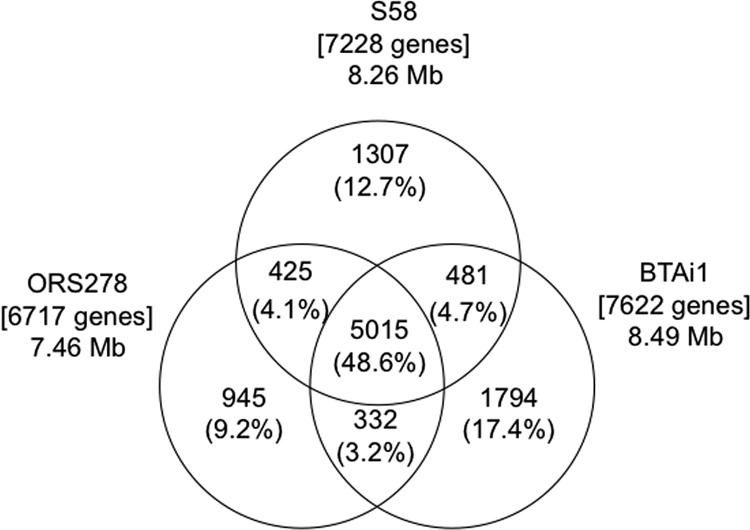
Comparative genomics analysis among Agromonas oligotrophica S58, Bradyrhizobium sp. ORS278, and Bradyrhizobium sp. BTAi1. Each genome is represented by a circle, and the numbers of shared and unique genes are shown by the overlapping and nonoverlapping parts of the circles. The proportion of total genes represented by each area of the diagram is shown in parentheses. The total number of genes in each genome is shown in square brackets.
Nodulation and symbiotic genes of the S58 genome.
Saito et al. (32) reported that strain S58 does not possess nodABC and does not nodulate siratro (Macroptililium atropurpureum). nodA, nodB, and nodC encode N-acyltransferase (EC 2.3.1), chitooligosaccharide deacetylase (EC 3.5.1), and N-acetylglucosaminyltransferase (EC 2.4.1), respectively. These three enzymes are required for synthesis of the basic backbone of lipochitooligosaccharides (Nod factors), which induce rhizobial infection and nodule organogenesis in compatible leguminous plants (33). BLAST analysis revealed that the S58 genome lacks nodABC genes, although it possesses homologous genes corresponding to low levels of amino acid identity (23% to 31%) (see Table S2 in the supplemental material). Several protein-coding genes on the S58 genome showed high similarity to nodGMNPQ (67 to 97%) (see Table S2 in the supplemental material). The S58 genome also possesses nodD1 (S58_55840), which has 69% similarity to that of USDA110 but which is not found in the ORS278 and BTAi1 genomes. Aeschynomene indica symbionts ORS278 and BTAi1 lacking nodABC genes should have symbiotic mechanisms other than Nod factors to interact with their host legumes. A large-scale transposon mutagenesis study of strain ORS278 identified 29 ndv genes that, when mutated, resulted in severely impaired nodule development by ORS278 (7, 9). Unexpectedly, the BiBlast comparison revealed that most of the ndv genes (27 of the 29 genes in ORS278), including purine biosynthesis genes (34), are conserved in the S58 genome (see Table S3 in the supplemental material).
Nitrogen fixation gene cluster.
The nitrogen fixation cluster (fixR, nifA, sufBCDSE, nifHDKENX, nifHV, and fixABC) was highly conserved among the S58, ORS278, and BTAi1 genomes (see Fig. S4 in the supplemental material). In a previous mutagenesis study, 87 genes in ORS278 were identified as important for symbiotic nitrogen fixation (7, 9). The BLASTp comparison analyses revealed that the S58 genome conserves 84 of the 87 genes, including genes for nitrogenase, nif gene regulation, electron transfers, and central energy metabolisms. The S58 genome lacks only cbbL2 (Calvin-Benson-Bassham L2) (BRADO2274) and the genes coding for a putative ABC transporter (BRADO3096) and a hypothetical protein (BRADO6923) (see Table S4 in the supplemental material). ORS278 houses two RubisCO (ribulose 1,5-bisphosphate carboxylase/oxygenase) clusters called RubisCO 1 and 2 and encoded by cbbL1 and cbbS1 (BRADO1659 and BRADO1660) and cbbL2 and cbbS2 (BRADO2274 and BRADO2275), respectively, whereas S58 houses only one RubisCO cluster (S58_58780 to S58_58840), which shows a high level of similarity to RubisCO 1 on the ORS278 genome (see Fig. S5 in the supplemental material). Gourion et al. (35) reported that only cbbL1 is expressed in the nodules formed with ORS278.
Photosynthetic gene cluster.
The photosynthetic gene cluster of A. oligotrophica S58 was also compared with the clusters of R. palustris CGH009 and Bradyrhizobium sp. ORS278, BTAi1, and S23321 (see Fig. S6 in the supplemental material). Photosynthetic gene clusters were highly conserved among S58, ORS278, and BTAi1. Although pufBALM genes were conserved between S58 and CGH009, as indicated by the Southern hybridization result (see Fig. S2 in the supplemental material), the similarity between S58 and CGH009 was not as high as the similarities among S58, ORS278, and BTAi1 (see Fig. S6 in the supplemental material). Photosynthesis is considered to play an important role in the formation of stem nodules (36). It has been proposed that during the early steps of symbiosis, the energy provided by the photosynthetic apparatus facilitates ex planta survival and infectivity, whereas during the later steps this energy can be used by the bacteria to fix nitrogen (36).
The genome comparison results presented above showed that almost all important genes for nodulation, symbiotic nitrogen fixation, and photosynthesis are well conserved among S58, ORS278, and BTAi1, raising the possibility that S58 forms stem and root nodules on A. indica.
Nodulation test of S58 on A. indica.
We originally expected that A. oligotrophica S58 would be one of the nonsymbiotic bradyrhizobia on A. indica, because nonsymbiotic rhizobia are common in soil environments (13–16). However, as reported above, most genes important for nodule development, symbiotic nitrogen fixation, and photosynthesis are highly conserved among the genomes of S58, ORS278, and BTAi1, which prompted us to conduct a nodulation experiment with strain S58 on A. indica. When seedlings of A. indica were inoculated with A. oligotrophica S58, apparent nodules were induced on the root and stem (Fig. 4A and D). To verify that the nodules were induced by S58, DsRed- and gusA-tagged S58 cells were constructed (Table 1) and used to inoculate A. indica. The labeled S58 cells were observed in the infected zones of root (Fig. 4B) and stem (Fig. 4E) nodules, demonstrating that the nodules were induced by A. oligotrophica S58.
Fig 4.
Aeschynomene indica nodules induced by Agromonas oligotrophica S58. (A) Root nodules observed with a stereomicroscope (41 days after inoculation [DAI]). (B) Confocal microscopic three-dimensional (3D) image showing a cross section of a root nodule (16 DAI). The emission signal was collected on two channels: channel 1 (555 to 700 nm) was used to visualize the DsRed emission, and channel 2 (410 to 550 nm) was used for visualization of the plant tissue. (C) Transmission electron microscopy image showing infected cells in a root nodule (33 DAI). Black and white arrowheads show peribacteroid membranes and poly-β-hydroxybutylate granules, respectively. (D) Stem nodule observed with a stereomicroscope (27 DAI). (E) Light microscopy image of gusA-tagged S58 cells (blue) in stem nodule (27 DAI). (F) Root systems at 14 DAI. At the sites of emergence of lateral roots, the intense blue coloration reveals dense S58 colonization of the cracks, observed by stereomicroscopy.
As in the case of ORS278 (37), infection of A. indica (cross-inoculation [CI] group 3) with strain S58 was intercellular and occurred via the epidermal fissures generated by the emergence of lateral roots (Fig. 4F). TEM analysis of mature nodules induced by S58 (33 DAI) showed that the S58 bacteroid is spherical and enclosed by a peribacteroid membrane that originates from the plasma membrane of the host plant (black arrowheads in Fig. 4C). Electron-transparent areas, which were most probably poly-β-hydroxybutyrate granules, were also observed in the bacteroid (white arrowheads in Fig. 4C). These features are similar to those of nodules induced by Achynomene nodule isolate ORS278 (37).
Nitrogen fixation by A. indica inoculated with strain S58 was evident by the increased fresh weight of whole plants grown in a nitrogen-free nutrient solution compared with that of uninoculated controls (P < 0.01) (Fig. 5A and B). The symbiotic response of S58 appeared to be similar to that of ORS278 and superior to that of BTAi1 (Fig. 5A and B). Although S58 was isolated from paddy field soil, it showed a high level of symbiotic nitrogen fixation on A. indica.
Fig 5.
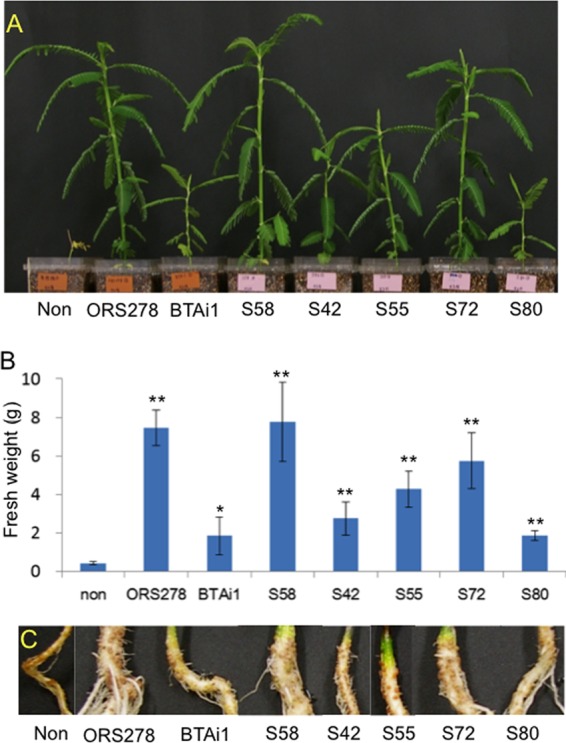
Experiments using inoculation into Aeschynomene indica performed with Agromonas oligotrophica S58, Bradyrhizobium sp. ORS278, Bradyrhizobium sp. BTAi1, and other soil isolates (A. oligotrophica S42, S55, S72, and S80) (31 DAI). Plants were grown under nitrogen-free conditions. (A) Photographs showing plant phenotypes. (B) Whole-plant fresh weights determined from three replicates. Statistical significance was calculated with Student's t test for differences between inoculated and uninoculated plant results. *, P < 0.05; **, P < 0.01. (C) Photographs showing root nodules. non, uninoculated controls.
Nodulation tests of other soil isolates close to strain S58.
To examine whether other A. oligotrophica strains are, like strain S58, capable of symbiotic nitrogen fixation, A. indica plants were inoculated with A. oligotrophica strains S42, S55, S72, and S80 (Fig. 1), all of which were isolated from paddy field soil (Table 1). Surprisingly, all tested strains formed effective nodules on the roots of A. indica (Fig. 5C), and the fresh weights of the host plants were significantly increased by the inoculation (P < 0.01) (Fig. 5A and B). Symbiotic nitrogen fixation occurred in A. indica inoculated with each of the five strains of A. oligotrophica, because the growth of the inoculated plants under nitrogen-free conditions significantly increased compared with that of uninoculated plants (Fig. 5A and B).
Type III secretion system.
To form functional nodules, some rhizobia utilize a type III secretion system to transport bacterial effector proteins into the cytoplasm of target eukaryotic cells (38). In B. japonicum, the rhc genes that encode core components of a type III secretion systems are found on a symbiotic island (11, 31). There were homologous genes in the S58 genome showing low (less than 60%) levels of amino acid identity (see Table S5 in the supplemental material); these genes were probably acquired by lateral gene transfer, because the GC content of this region of the S58 genome is lower (Fig. 3). However, it is unclear whether these genes play important roles in the infection of A. indica, because they are absent in the genomes of ORS278, BTAi1, and other strains lacking the nod genes (39).
Inoculation test of strain S58 on rice plant.
The colonization ability of strain S58 on rice was surveyed, because Kennedy (5) reported that A. oligotrophica was abundant in rice roots. A gusA-tagged S58 mutant was inoculated into a rice plant, Oryza sativa L. cv. Nipponbare, to test the ability of strain S58 to form colonies in and on the tissues of rice roots. S58 cells had colonized the root surface and intercellular spaces by 8 DAI (Fig. 6). These results suggest an endophytic life style of A. oligotrophica S58 in rice roots in paddy fields as well as endosymbiosis with A. indica roots.
Fig 6.
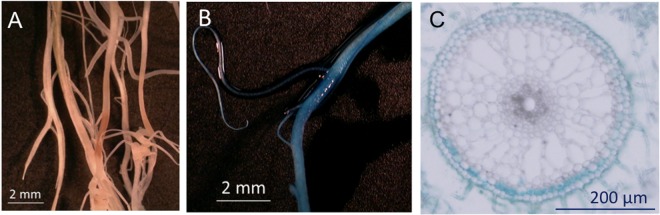
Stereo- and light microscopy observations, showing the distribution of gusA-tagged Agromonas oligotrophica S58 in roots of Oryza sativa cv. Nipponbare 8 days after inoculation. (A and B) Stereomicroscopy image of the lateral root zone left uninoculated (A) and inoculated with gusA-tagged S58 (B). The intense blue coloration on the root surface indicates dense bacterial colonization. (C) Cross section of a root inoculated with gusA-tagged S58, showing the proliferation of bacteria in the intercellular space between epidermal cells observed by bright-field microscopy.
Evolutionary and ecological considerations.
The establishment of symbiosis between legumes and nitrogen-fixing (brady)rhizobial bacteria generally starts with the exchange of chemical signals, that is, flavonoids and Nod factors, between the partners (33). In this symbiotic system, the sets of bacterial gene clusters for symbiosis (e.g., nod and nif) are located on a symbiotic plasmid or in a horizontally acquired chromosomal region (symbiosis island) (11, 12, 31). Therefore, the transfer of symbiotic gene clusters is generally considered to turn nonrhizobia into rhizobia (i.e., nonrhizobial strains that acquire a symbiotic gene cluster become rhizobia) (10, 13). However, our results suggest that a similar transfer may not have occurred in the case of NF-independent symbiosis with A. indica.
Both soil-dwelling A. oligotrophica S58 and Bradyrhizobium sp. S23321 (13) lacked a typical symbiosis island. However, A. oligotrophica S58 in a photosynthetic Bradyrhizobium (PB) clade exclusively nodulates A. indica in a NF-independent manner (Fig. 1). In addition, van Berkum et al. (40, 41) reported that LMG 8443, which is the type strain of Bradyrhizobium denitrificans (PB clade in Fig. 1) and was isolated from lake water in Germany, has the ability to form nodules on A. indica. These facts strongly suggest that NF-independent symbiosis with A. indica is a common characteristic of most (maybe all) strains within this taxonomic group (PB clade), a finding that is apparently inconsistent with the observation that rhizobia and nonrhizobia are intermixed in the same taxonomic group.
As for the life style of PB clade members, it is interesting that nonleguminous rice plants accommodated A. oligotrophica S58 in the root tissues (Fig. 6). In this regard, A. oligotrophica decomposes aromatic compounds, including ferulic acid, p-coumaric acid, and p-anisic acid (1, 5, 42). In particular, ferulic acid is abundant in rice straw and its decayed products as lignin-related phenolics, which A. oligotrophica is able to catabolize (42). Living and dead tissues of rice plants might support the survival of PB clade members in the soil environments of paddy fields.
The present study results present an interesting issue with regard to how soil-dwelling A. oligotrophica strains generally possess the ability to induce effective nodules on A. indica. One possible explanation is that key molecules for NF-independent symbiosis play important roles in the maintenance of some basic cellular function in A. oligotrophica. In this regard, bradyrhizobial purine derivatives, such as cytokinin, might play a key role in triggering nodule formation (10).
Taxonomy.
NF-independent symbiosis with A. indica is a shared characteristic of most PB clade members, including Agromonas oligotrophica (Bradyrhizobium oligotrophicum), Bradyrhizobium denitrificans LMG 8443T, and Bradyrhizobium sp. ORS278 and BTAi1. In addition, these members have photosynthetic genes, and (at least Agromonas oligotrophica and Bradyrhizobium denitrificans) propagate by budding (1, 40, 41, 43). The present study revealed that the S58 genome shows an overall high level of similarity to the genomes of ORS278 and BTAi1 (see Fig. S3 in the supplemental material). Elucidation of the taxonomy of Bradyrhizobium species is in a state of flux so far. Ramírez-Bahena et al. reported that experiments employing DNA-DNA hybridization between A. oligotrophica S58 and B. denitrificans LMG 8443T showed DNA-DNA relatedness as low as 46% (3), indicating that strain S58 belongs to a species different from B. denitrificans. The NF-independent symbiosis, cell division, and genome structure within the genus Bradyrhizobium may become crucial traits from a taxonomic point of view in the future.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the Nonprofit Organization Attic Research Center, from the Ministry of Agriculture, Forestry and Fisheries of Japan (Development of Mitigation and Adaptation Techniques to Global Warming, and Genomics for Agricultural Innovation; PMI-0002), to K.M., by Grants-in-Aid for Scientific Research (A) 23248052 and for Challenging Exploratory Research 23658057 from the Ministry of Education, Culture, Sports, Science and Technology of Japan to K.M., and by the Japan Society for the Promotion of Science (JSPS) [grant number H2247071] to T.O.
We thank E. Giraud (IRD) for providing the seeds of A. indica; H. Kouchi (National Institute of Agrobiological Sciences) for providing pBjGroEL4::dsRed2; T. Sato (Tohoku University, Japan) for providing the seeds of Oryza sativa cv. Nipponbare; H. Ohta (Ibaraki University) for constructive suggestions; and K. Ohki (Fukui Prefectural University) for generous support of the TEM observations.
Footnotes
Published ahead of print 8 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00009-13.
REFERENCES
- 1. Ohta H, Hattori T. 1983. Agromonas oligotrophica gen. nov., sp. nov., a nitrogen-fixing oligotrophic bacterium. Antonie Van Leeuwenhoek 49:429–446 [DOI] [PubMed] [Google Scholar]
- 2. Ohta H, Hattori T. 1985. Agromonas oligotrophica gen. nov., sp. nov. in validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int. J. Syst. Bacteriol. 35:223–225 [Google Scholar]
- 3. Ramírez-Bahena MH, Chahboune R, Peix A, Velázquez E. 8 June 2012. Reclassification of Agromonas oligotrophica into genus Bradyrhizobium as Bradyrhizobium oligotrophicum comb. nov. Int. J. Syst. Evol. Microbiol. [Epub ahead of print.] doi:10.1099/ijs.0.041897-0 [DOI] [PubMed] [Google Scholar]
- 4. Ryuda N, Hashimoto T, Ueno D, Inoue K, Someya T. 2011. Visualization and direct counting of individual denitrifying bacterial cells in soil by nirK-targeted direct in situ PCR. Microbes Environ. 26:74–80 [DOI] [PubMed] [Google Scholar]
- 5. Kennedy C. 2005. Genus III Agromonas, p 448–452 In Brenner DJ, Krieg NR, Stanley JT. (ed), Bergey's manual of systematic bacteriology, vol. 2 The Proteobacteria part C, alpha-, beta-, delta-, and epsilonproteobacteria. Springer, New York, NY [Google Scholar]
- 6. Itakura M, Saeki K, Omori H, Yokoyama T, Kaneko T, Tabata S, Ohwada T, Tajima S, Uchiumi T, Honnma K, Fujita K, Iwata H, Saeki Y, Hara Y, Ikeda S, Eda S, Mitsui H, Minamisawa K. 2009. Genomic comparison of Bradyrhizobium japonicum strains with different symbiotic nitrogen-fixing capabilities and other Bradyrhizobiaceae members. ISME J. 3:326–339 [DOI] [PubMed] [Google Scholar]
- 7. Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, Avarre JC, Jaubert M, Simon D, Cartieaux F, Prin Y, Bena G, Hannibal L, Fardoux J, Kojadinovic M, Vuillet L, Lajus A, Cruveiller S, Rouy Z, Mangenot S, Segurens B, Dossat C, Franck WL, Chang WS, Saunders E, Bruce D, Richardson P, Normand P, Dreyfus B, Pignol D, Stacey G, Emerich D, Verméglio A, Médigue C, Sadowsky M. 2007. Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316:1307–1312 [DOI] [PubMed] [Google Scholar]
- 8. Okubo T, Fukushima F, Minamisawa K. 2012. Evolution of Bradyrhizobium-Aeschynomene mutualism: living testimony of the ancient world or highly evolved state? Plant Cell Physiol. 53:2000–2007 doi:10.1093/pcp/pcs150 [DOI] [PubMed] [Google Scholar]
- 9. Bonaldi K, Gourion B, Fardoux J, Hannibal L, Cartieaux F, Boursot M, Vallenet D, Chaintreuil C, Prin Y, Nouwen N, Giraud E. 2010. Large-scale transposon mutagenesis of photosynthetic Bradyrhizobium sp. strain ORS278 reveals new genetic loci putatively important for nod-independent symbiosis with Aeschynomene indica. Mol. Plant Microbe Interact. 23:760–770 [DOI] [PubMed] [Google Scholar]
- 10. Masson-Boivin C, Giraud E, Perret X, Batut J. 2009. Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trend Microbiol. 17:458–466 [DOI] [PubMed] [Google Scholar]
- 11. Kaneko T, Maita H, Hirakawa H, Uchiike N, Minamisawa K, Watanabe A, Sato S. 2011. Complete genome sequence of the soybean symbiont Bradyrhizobium japonicum strain USDA6T. Genes 2:763–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, Sasamoto S, Watanabe A, Idesawa K, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kiyokawa C, Kohara M, Matsumoto M, Matsuno A, Mochizuki Y, Nakayama S, Nakazaki N, Shimpo S, Sugimoto M, Takeuchi C, Yamada M, Tabata S. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331–338 [DOI] [PubMed] [Google Scholar]
- 13. Okubo T, Tsukui T, Maita H, Okamoto S, Oshima K, Fujisawa T, Saito A, Futamata H, Hattori R, Shimomura Y, Haruta S, Morimoto S, Wang Y, Sakai Y, Hattori M, Aizawa S, Nagashima KV, Masuda S, Hattori T, Yamashita A, Bao Z, Hayatsu M, Kajiya-Kanegae H, Yoshinaga I, Sakamoto K, Toyota K, Nakao M, Kohara M, Anda M, Niwa R, Jung-Hwan P, Sameshima-Saito R, Tokuda S, Yamamoto S, Yamamoto S, Yokoyama T, Akutsu T, Nakamura Y, Nakahira-Yanaka Y, Takada Hoshino Y, Hirakawa H, Mitsui H, Terasawa K, Itakura M, Sato S, Ikeda-Ohtsubo W, Sakakura N, Kaminuma E, Minamisawa K. 2012. Complete genome sequence of Bradyrhizobium sp. S23321: insights into symbiosis evolution in soil oligotrophs. Microbes Environ. 27:306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sullivan JT, Eardly BD, van Berkum P, Ronson CW. 1996. Four unnamed species of nonsymbiotic rhizobia isolated from the rhizosphere of Lotus corniculatus. Appl. Environ. Microbiol. 62:2818–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Menna P, Hungria M. 2011. Phylogeny of nodulation and nitrogen-fixation genes in Bradyrhizobium: supporting evidence for the theory of monophyletic origin, and spread and maintenance by both horizontal and vertical transfer. Int. J. Syst. Evol. Microbiol. 61:3052–3067 [DOI] [PubMed] [Google Scholar]
- 16. Parker MA. 2012. Legumes select symbiosis island sequence variants in Bradyrhizobium. Mol. Ecol. 21:1769–1778 [DOI] [PubMed] [Google Scholar]
- 17. Cole MA, Elkan GH. 1973. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 4:248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sameshima-Saito R, Chiba K, Hirayama J, Itakura M, Mitsui H, Eda S, Minamisawa K. 2006. Symbiotic Bradyrhizobium japonicum reduces N2O surrounding the soybean root system via nitrous oxide reductase. Appl. Environ. Microbiol. 72:2526–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 20. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sameshima-Saito R, Chiba K, Minamisawa K. 2006. Correlation of denitrifying capability with the existence of nap, nir, nor and nos genes in diverse strains of soybean bradyrhizobia. Microbes Environ. 21:174–184 [Google Scholar]
- 22. Ewing B, Green P. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8:186–194 [PubMed] [Google Scholar]
- 23. Ewing B, Hillier L, Wendl MC, Green P. 1998. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 8:175–185 [DOI] [PubMed] [Google Scholar]
- 24. Gordon D, Abajian C, Green P. 1998. Consed: a graphical tool for sequence finishing. Genome Res. 8:195–202 [DOI] [PubMed] [Google Scholar]
- 25. Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25:955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noguchi H, Taniguchi T, Itoh T. 2008. MetaGeneAnnotator: detecting species-specific patterns of ribosomal binding site for precise gene prediction in anonymous prokaryotic and phage genomes. DNA Res. 15:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohtsubo Y, Ikeda-Ohtsubo W, Nagata Y, Tsuda M. 2008. GenomeMatcher: a graphical user interface for DNA sequence comparison. BMC Bioinformatics 9:376 doi:10.1186/1471-2105-9-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minamisawa K, Itakura M, Suzuki M, Ichige K, Isawa T, Yuhashi K, Mitsui H. 2002. Horizontal transfer of nodulation genes in soil and microcosms from Bradyrhizobium japonicum to B. elkanii. Microbes Environ. 17:82–90 [Google Scholar]
- 29. Mae T, Ohira K. 1981. The remobilisation of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol. 22:1067–1074 [Google Scholar]
- 30. Grant JR, Stothard P. 2008. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 36:W181–W184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaneko T, Nakamura Y, Sato S, Minamisawa K, Uchiumi T, Sasamoto S, Watanabe A, Idesawa K, Iriguchi M, Kawashima K, Kohara M, Matsumoto M, Shimpo S, Tsuruoka H, Wada T, Yamada M, Tabata S. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189–197 [DOI] [PubMed] [Google Scholar]
- 32. Saito A, Mitsui H, Hattori R, Minamisawa K, Hattori T. 1998. Slow-growing and oligotrophic soil bacteria phylogenetically close to Bradyrhizobium japonicum. FEMS Microb. Ecol. 25:277–286 [Google Scholar]
- 33. Kouchi H, Imaizumi-Anraku H, Hayashi M, Hakoyama T, Nakagawa T, Umehara Y, Suganuma N, Kawaguchi M. 2010. How many peas in a pod? Legume genes responsible for mutualistic symbioses underground. Plant Cell Physiol. 51:1381–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borjigin N, Furukawa K, Shimoda Y, Tabata S, Sato S, Eda S, Minamisawa K, Mitsui H. 2011. Identification of Mesorhizobium loti genes relevant to symbiosis by using signature-tagged mutants. Microbes Environ. 26:165–171 [DOI] [PubMed] [Google Scholar]
- 35. Gourion B, Delmotte N, Bonaldi K, Nouwen N, Vorholt JA, Giraud E. 2011. Bacterial RuBisCO is required for efficient Bradyrhizobium/Aeschynomene symbiosis. PLoS One 6:e21900 doi:10.1371/journal.pone.0021900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giraud E, Hannibal L, Fardoux J, Vermeglio A, Dreyfus B. 2000. Effect of Bradyrhizobium photosynthesis on stem nodulation of Aeschynomene sensitiva. Proc. Natl. Acad. Sci. U. S. A. 97:14795–14800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonaldi K, Gargani D, Prin Y, Fardoux J, Gully D, Nouwen N, Goormachtig S, Giraud E. 2011. Nodulation of Aeschynomene afraspera and A. indica by photosynthetic Bradyrhizobium sp. strain ORS285: the nod-dependent versus the nod-independent symbiotic interaction. Mol. Plant Microbe Interact. 24:1359–1371 [DOI] [PubMed] [Google Scholar]
- 38. Tsukui T, Eda S, Kaneko T, Sato S, Okazaki S, Kakizaki-Chiba K, Itakura M, Mitsui H, Yamashita A, Terasawa K, Minamisawa K. 2013. The type III secretion system of Bradyrhizobium japonicum USDA122 mediates symbiotic incompatibility with Rj2 soybean. Appl. Environ. Microbiol. 79:1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mornico D, Miche L, Bena G, Nouwen N, Vermeglio A, Vallenet D, Smith AAT, Giraud E, Médigue C, Moulin L. 2012. Comparative genomics of Aeschynomene symbionts: insights into the ecological lifestyle of Nod-independent photosynthetic Bradyrhizobia. Genes 3:35–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Berkum P, Eardly BD. 2002. The aquatic budding bacterium Blastobacter denitrificans is a nitrogen-fixing symbiont of Aeschynomene indica. Appl. Environ. Microbiol. 68:1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Berkum P, Leibold JM, Eardly BD. 2006. Proposal for combining Bradyrhizobium spp. (Aeschynomene indica) with Blastobacter denitrificans and to transfer Blastobacter denitrificans (Hirsch and Muller, 1985) to the genus Bradyrhizobium as Bradyrhizobium denitrificans (comb. nov.). Syst. Appl. Microbiol. 29:207–215 [DOI] [PubMed] [Google Scholar]
- 42. Ohta H. 2000. Growth characteristics of Agromonas oligotrophica on ferulic acid. Microbes Environ. 15:133–142 [Google Scholar]
- 43. Hattori R, Wanatane H, Tonosaki A, Hattori T. 1995. Unusual morphology of Agromonas oligotrophica and the effect of NaCl and organic nutrient on its fine structure. J. Gen. Appl. Microbiol. 41:23–30 [Google Scholar]
- 44. Kamagata Y, Fulthorpe RR, Tamura K, Takami H, Forney LJ, Tiedje JM. 1997. Pristine environments harbor a new group of oligotrophic 2,4-dichlorophenoxyacetic acid-degrading bacteria. Appl. Environ. Microbiol. 63:2266–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Berkum P, Fuhrmann JJ. 2000. Evolutionary relationships among the soybean bradyrhizobia reconstructed from 16S rRNA gene and internally transcribed spacer region sequence divergence. Int. J. Syst. Evol. Microbiol. 50:2165–2172 [DOI] [PubMed] [Google Scholar]
- 46. Oda Y, Larimer FW, Chain PS, Malfatti S, Shin MV, Vergez LM, Hauser L, Land ML, Braatsch S, Beatty JT, Pelletier DA, Schaefer AL, Harwood CS. 2008. Multiple genome sequences reveal adaptations of a phototrophic bacterium to sediment microenvironments. Proc. Natl. Acad. Sci. U. S. A. 105:18543–18548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson KJ, Sessitsch A, Corbo JC, Giller KE, Akkermans AD, Jefferson RA. 1995. Beta-glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other gram-negative bacteria. Microbiology 141:1691–1705 [DOI] [PubMed] [Google Scholar]
- 48. Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]