Abstract
Bacteria are rapidly killed on copper surfaces, and copper ions released from the surface have been proposed to play a major role in the killing process. However, it has remained unclear whether contact of the bacteria with the copper surface is also an important factor. Using laser interference lithography, we engineered copper surfaces which were covered with a grid of an inert polymer which prevented contact of the bacteria with the surface. Using Enterococcus hirae as a model organism, we showed that the release of ionic copper from these modified surfaces was not significantly reduced. In contrast, killing of bacteria was strongly attenuated. When E. hirae cells were exposed to a solid iron surface, the loss of cell viability was the same as on glass. However, exposing cells to iron in the presence of 4 mM CuSO4 led to complete killing in 100 min. These experiments suggest that contact killing proceeds by a mechanism whereby the metal-bacterial contact damages the cell envelope, which, in turn, makes the cells susceptible to further damage by copper ions.
INTRODUCTION
The rapid killing of bacteria by solid copper surfaces has recently received much attention. In laboratory experiments, it had been shown that many bacterial species, such as Escherichia coli O157, Staphylococcus aureus, Salmonella enterica, Campylobacter jejuni, Clostridium difficile, Listeria monocytogenes, and Mycobacterium tuberculosis, are efficiently killed on copper or copper alloy surfaces (1–8). In contrast, on stainless steel, living cells could be recovered even after days. Copper and many copper alloys have consequently been registered at the U.S. Environmental Protection Agency as the first solid antimicrobial material. This has moved copper into the focus of infection control. Nosocomial infections are an increasing problem throughout the world and cost many lives (9). A number of hospital trials in which rooms have been fitted with copper alloy door handles, bathroom fixtures, tabletops, etc., have been conducted or are ongoing (10–14). They have shown that on copper surfaces, there is a substantial reduction of the microbial burden on a continuous basis. While further data are needed, it is clear that copper-containing materials can contribute to hospital hygiene, but they also lower the bacterial burden in other facilities where clean or aseptic working procedures are required (15).
With the use of copper in hospitals and other facilities, it has become important to understand the mechanism of the so-called “contact killing” of bacteria, as it may bear on the possibility of the emergence of resistant organisms, on cleaning procedures, and on material and object engineering. From laboratory studies, it has emerged that bacteria on copper surfaces suffer rapid membrane damage and DNA degradation, in addition to other, less well-defined cell damage (16–21). The order in which these processes take place and which one is the primary killing mechanism remain issues of debate. In fact, the sequence of events may depend on the type of microorganism (18). It is also clear that copper ions released from the surface play a role in contact killing, but bacterial copper resistance systems are not able to cope with the released copper (2, 22, 23).
A question which has not yet been addressed in detail is the role of physical contact of bacteria with the copper surface in contact killing. We thus engineered special copper surfaces, so-called “contact arrays”: copper surfaces were covered by an inert polymer into which arrays of holes less than 1 μm in diameter were etched by a photolithographic process, using laser interference (24). Enterococcus hirae was used as a model organism because Gram-positive organisms are frequent pathogens and the contact killing behavior of E. hirae had previously been studied (23). Also, its robust cell wall helped to preserve the shape of the bacteria during electron microscopy. The holes in the contact arrays were smaller than E. hirae, so the grid effectively prevented contact of the bacteria with the copper surface. It was found that contact killing on these contact arrays was reduced by 7 orders of magnitude compared to copper coupons, while the release of ionic copper was not significantly altered. Metallic iron did not appear to be active in contact killing, unless copper ions were also present. These experiments demonstrate the importance of both copper ions and bacterial-metal contact for efficient contact killing.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type E. hirae ATCC 9790 was grown anaerobically (air-saturated medium was transferred to sealed tubes; these cultures became anaerobic after approximately 1 h) to stationary phase at 37°C in 10 ml of N medium (25). Cells were centrifuged for 5 min at 5,000 × g, washed twice with 20 ml of 100 mM Tris-Cl (pH 7), and resuspended in 10 ml of the same buffer. The average cell density was 2 × 108 to 8 × 108 CFU/ml. All handling of cells was performed aerobically.
Coupons and contact arrays.
Control C1 copper coupons were 15- by 15-mm squares of highly polished (root mean square roughness < 50 nm), 99.99% pure copper and were cleaned by ultrasonication in chloroform and ethanol for 10 min each, followed by air drying. CA contact arrays were prepared by spin coating C1 coupons with cresol resin AZ 1518 (MicroChemicals GmbH, Ulm, Germany), diluted 1:4 with 2-methoxy-1-methylethylacetate, under clean-room conditions. Spin coating was conducted at 3,500 rpm for 60 s with a ramp of 3,000 rpm/s, resulting in an average film thickness of 1.74 ± 0.05 μm. Coated samples were immediately dried on a hot plate at 100°C for 60 s. Laser interference patterning (26) was accomplished by illumination with an Nd:YAG nanosecond laser (Quanta-Ray PRO210; frequency of 10 Hz) with one pulse (10 ns) at an average fluency of 33 mJ/cm2. Exposed coupons were developed with a boric acid-based solution (AZ 351 B; MicroChemicals GmbH) for 60 s, followed by rinsing with distilled water and drying in an ambient atmosphere. Control coupons (CL1) were developed similarly but without prior exposure. Iron coupons were of polished 98.3% Fe, 1.4% Mn, 0.17% C (DIN 5512-1) and were cleaned like the copper coupons. Following preparation and/or cleaning, all coupons used in this study were stored under nitrogen until use.
Measurement of contact killing.
To assess contact killing, a wet plating technique was used essentially as described in reference 23. Briefly, 25-μl volumes of cells suspended in 100 mM Tris-Cl were applied to plain or modified copper or iron coupons. Following incubation for various times in a water-saturated atmosphere, 20-μl samples were withdrawn and serial dilutions in phosphate-buffered saline (PBS) were spread on N agar plates. Following growth for 24 h, survival was calculated from the CFU. Contact arrays with a porous surface were subjected to a reduced pressure of 2 kPa for 5 s right after application of cells to remove air from the pores.
Copper and iron determinations.
Copper or iron release from coupons during wet-plating incubations was assessed by removing 20-μl aliquots, diluting them 50-fold with 0.065% HNO3, and measuring the copper content by inductively coupled plasma atomic emission (ICP-AE) on a Jobin Yvon JY 24 instrument (HORIBA Jobin Yvon GmbH, Munich, Germany) at 324.754 nm.
SEM.
Cells suspended in water at approximately 2 × 108 cells/ml were applied to copper coupons or contact arrays and air dried. Scanning electron microscopy (SEM) images were acquired on a high-resolution dual-beam microscope (FE Strata DB 235) at 5 kV using the secondary electron detector (SED) mode.
RESULTS
Design of contact arrays.
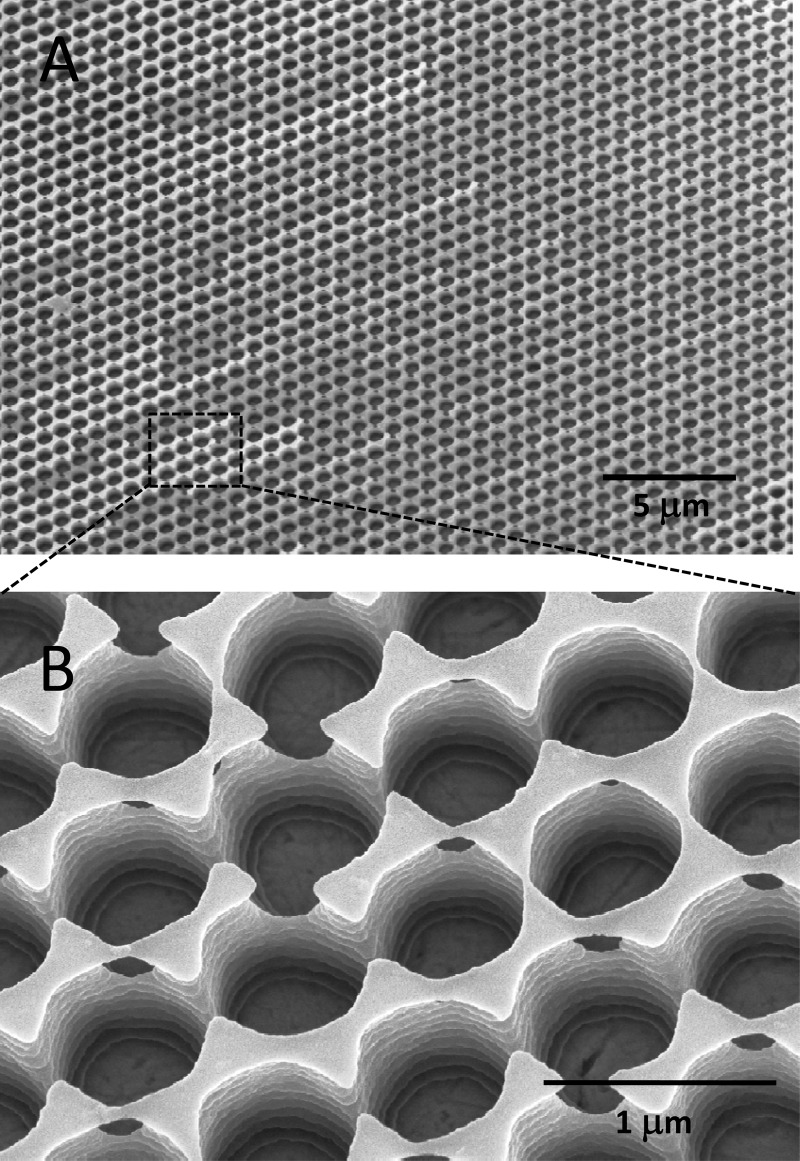
Laser interference patterning is a tool to generate topographic surface patterns on micrometer and submicrometer scales (26). We thus designed a microstructured polymer grid on top of a copper surface which would allow E. hirae cells to come close to the copper surface (<2 μm) yet prevent direct bacterial-metal contact. To prepare such contact arrays, copper coupons were spin coated with a positive photoresist, which was then exposed by a short laser pulse to a specific light intensity distribution with a lateral spacing parameter of 770 nm. The utilized, unique intensity distribution was designed by splitting and recombining the initial laser beam in a three-beam laser interference setup. Highly exposed areas of the photoresist were removed by photographic development, resulting in a honeycomb-like pattern of holes which extended down to the copper surface (Fig. 1). Preparation of the contact arrays required the optimization of a number of parameters, such as resist viscosity, spin coating parameters, interference pattern, laser fluency, etc. These engineering aspects of the work are not further discussed here.
Fig 1.
SEM images (tilted views) of the honeycomb-like structures on CA contact arrays at low magnification (A) and at high magnification (B). The bottom of the wells is formed by the copper surface. The lateral spacing of the holes was 770 nm.
SEM analysis of bacteria on copper coupons and contact arrays.
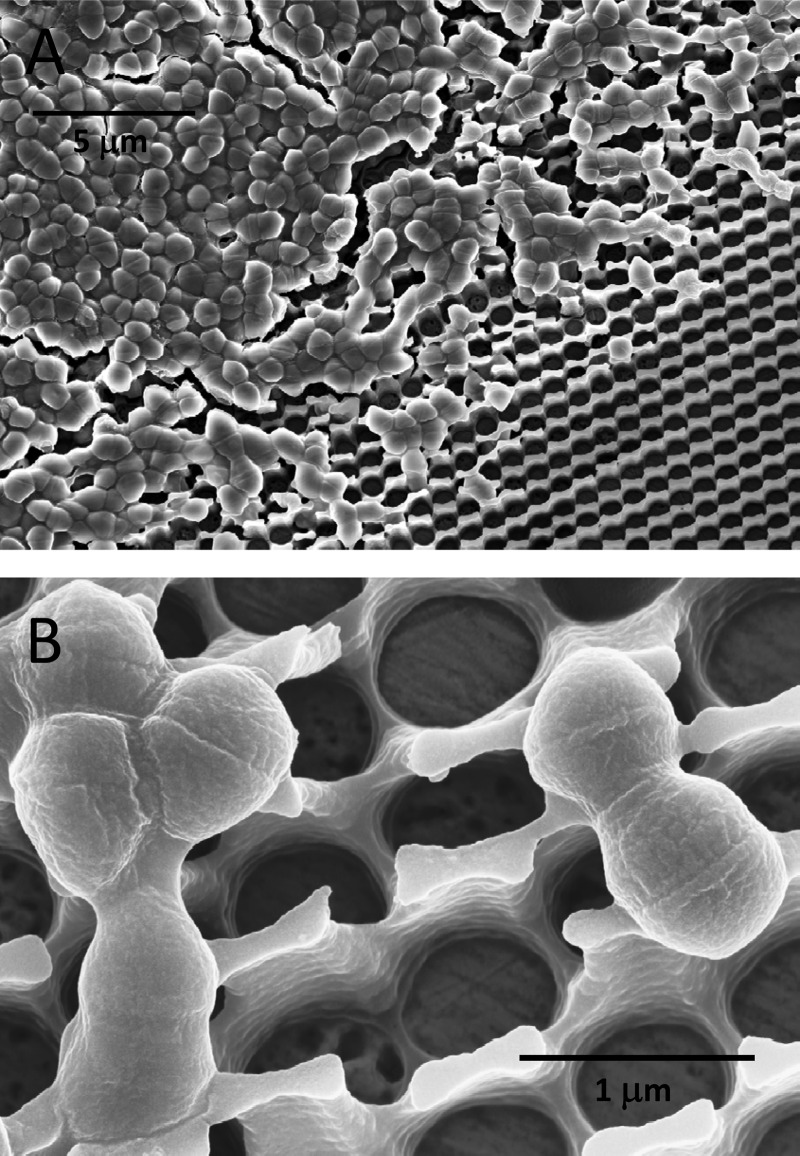
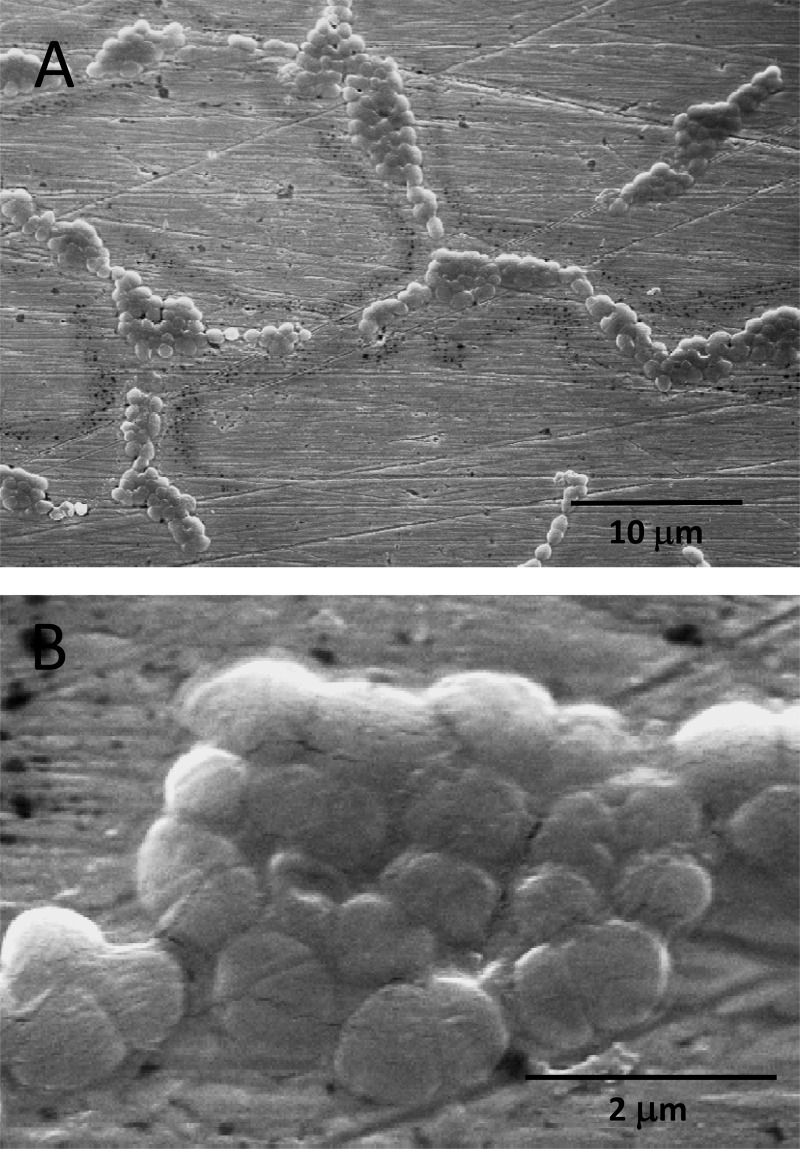
How bacteria are killed on copper surfaces is poorly understood. In particular, it remains unclear if contact killing mainly proceeds via dissolved copper ions and subsequent cellular damage or whether contact of bacteria with the copper surface is an important factor in the process. To discriminate between these two mechanistic aspects, the contact arrays described above were employed. As can be seen on the SEM pictures of Fig. 2, E. hirae cells placed on CA contact arrays stayed largely on the polymer grid. Only a few cells found their way into the holes of the grid and were potentially able to make contact with the copper surface. The retention of cells by the grid was aided by cell dimers, which were predominantly present in the type of cultures used. For comparison, Fig. 3 shows SEM images of E. hirae spread on regular C1 copper coupons. On all coupons, the cells were often aggregated, but it remains unclear to what extent this was an artifact of air drying of the coupons before SEM. No chemical fixing or dehydration procedures were employed for SEM sample preparation due to the chemical sensitivity of the photoresist coatings of the contact arrays.
Fig 2.
SEM image (tilted view) of bacteria on a contact array. E. hirae cells were wet plated on a CA contact array. After air drying, the sample was processed for electron microscopy as described in Materials and Methods. (A) Image at low magnification; (B) image at high magnification.
Fig 3.
SEM images of bacteria on a polished copper coupon. E. hirae cells were wet plated on a C1 copper coupon. After air drying, the sample was processed for electron microscopy as described in Materials and Methods. (A) Image at low magnification; (B) image at high magnification.
Contact killing on copper coupons and contact arrays.
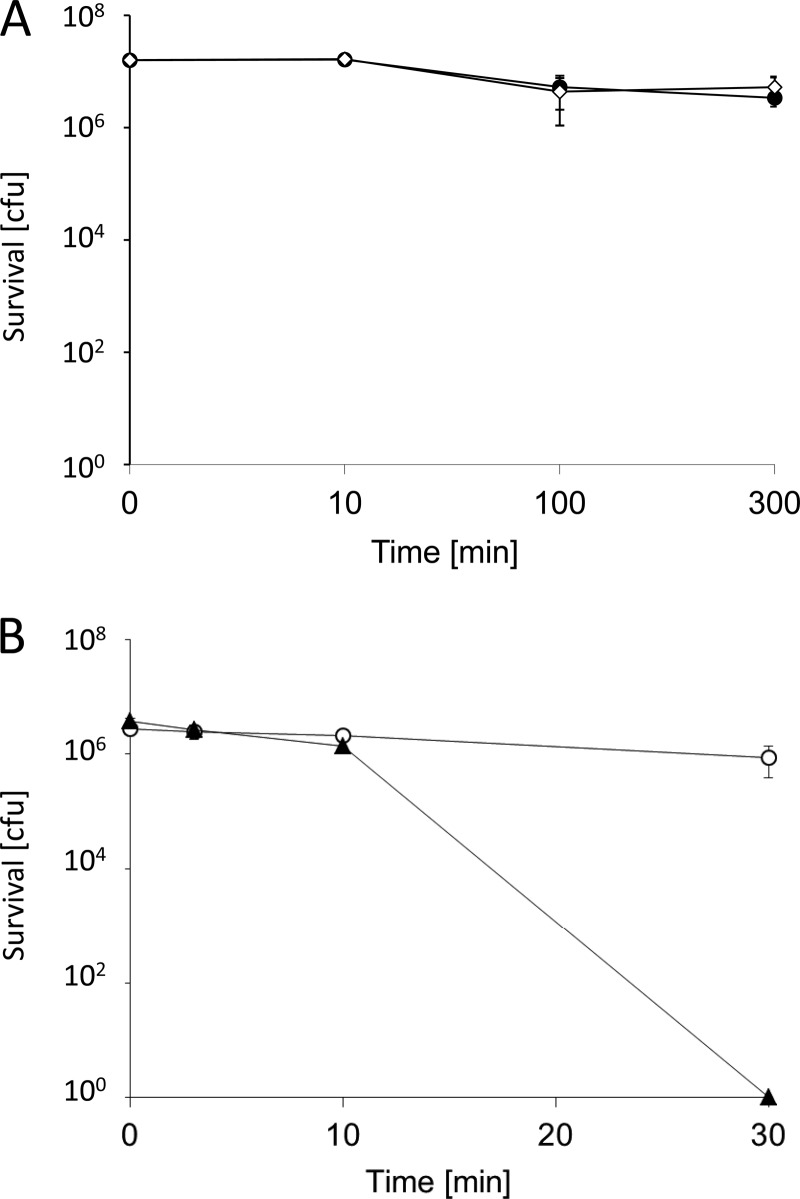
To assess contact killing of bacteria on C1 copper coupons and CA contact arrays, cell suspensions of E. hirae cells were used. Dry plating as first described by Espírito Santo et al. (22) on contact arrays was not feasible, as the spreading of cells with a cotton swab destroyed the fragile polymer structure. Thus, the wet-plating procedure of Molteni et al. (23) was employed. Control platings showed that there was essentially no contact killing by copper coupons coated with a continuous layer of photoresist, the starting material for the fabrication of the CA contact arrays; in fact, they were as inert as glass (Fig. 4A). When the photoresist-coated copper coupons were photolithographically processed to generate the honeycomb-like grids with holes extending down to the copper surface, constituting the CA contact arrays, a surprising result was obtained: there was no significant contact killing (Fig. 4B). Even after 3.5 h, cell survival was reduced only by 1 log (data not shown). In contrast, uncoated C1 copper coupons completely killed >106 bacteria in 30 min, in line with previous reports on contact killing of various bacterial species (e.g., see reference 19). This suggested that curtailing bacterial-metal contact on the CA arrays effectively prevented contact killing. However, it remained to be shown that copper release was not impaired on the CA contact arrays.
Fig 4.
Survival of E. hirae on different surfaces. (A) Survival of E. hirae was assessed by wet plating on either glass (♢) or CL1 resin-coated, unexposed but developed copper coupons used for the fabrication of contact arrays (●). (B) Survival of E. hirae under the same conditions as in panel A on C1 copper coupons (▲) or CA contact arrays (○). CFU are given for the whole sample volume which was applied to the coupons. The error bars show the standard deviations of three independent experiments.
Copper release by copper coupons and contact arrays.
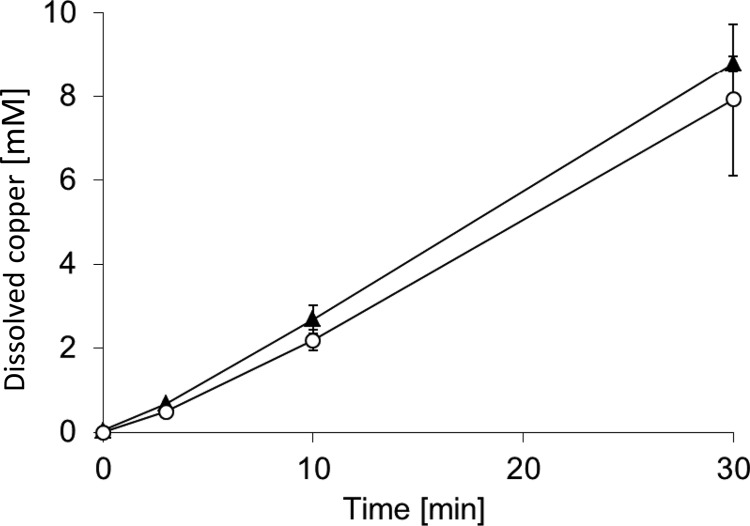
To test whether the lack of killing by CA contact arrays was due to reduced release of copper ions, copper release was measured. Figure 5 shows that the release of copper ions was not significantly different between C1 copper coupons and CA contact arrays. The concentrations of released copper in the aqueous phase after 30 min were 8.8 ± 0.2 mM for C1 copper coupons and 7.9 ± 1.8 mM for CA contact arrays; the difference between these values was not statistically significant. This clearly shows that the failure of CA contact arrays to support efficient contact killing was not due to reduced copper release; rather, it appeared that the inability of the bacteria to make contact with the copper surface prevented contact killing.
Fig 5.
Copper release by different surfaces. Copper release into the aqueous phase was measured by ICP-AE as a function of time of exposure to C1 copper coupons (▲) and CA contact arrays (○). The error bars show the standard deviations of three independent experiments.
Contact killing on iron coupons.
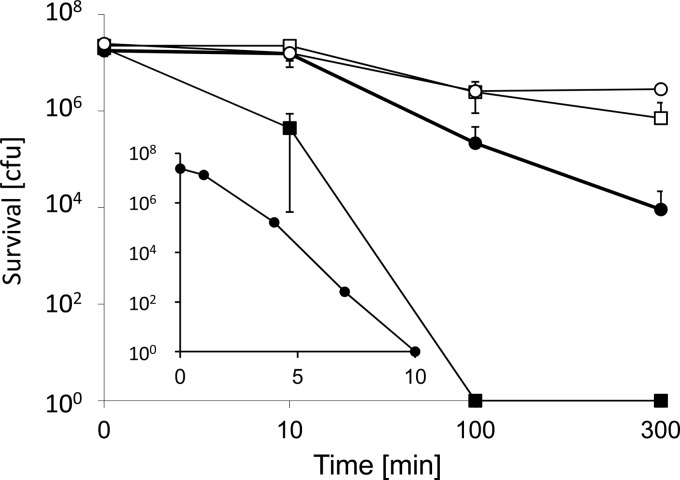
Iron and copper have similar redox potentials for the most oxidized ion couples, and both elements can catalyze Fenton chemistry (see Discussion). Thus, they both have the potential to damage cells, and it was of interest to test the activity of iron in contact killing. Surprisingly, contact killing by iron has never been investigated as far as we know. Figure 6 shows that there was insignificant contact killing of E. hirae over 300 min on iron. However, adding 4 mM CuSO4 to the cells before plating on iron led to complete killing of 2 × 107 cells in 100 min. The same copper concentration on glass also led to some killing, but this was orders of magnitude less than on iron: after 300 min, 104 CFU could still be recovered. The concentration dependence of the Cu-induced contact killing on iron could best be observed at 10 min, where survival was intermediate: survival decreased exponentially with increasing copper concentrations and reached zero at 10 mM CuSO4 (Fig. 6, inset). Under these conditions, there was still 73% survival on glass (data not shown). Thus, ionic copper and metallic iron acted synergistically to effect efficient contact killing of E. hirae. There was no adherence of bacteria to the iron coupons, as mechanical removal by vortexing with glass beads yielded the same results (not shown). Iron release from the coupons into the aqueous phase in the presence of cells at 100 min amounted to 5.5 ± 2.8 mM in the absence of copper ions and 12.6 ± 0.4 mM in the presence of 4 mM CuSO4. After 300 min, the respective values were 15.6 ± 4.4 mM and 22.4 ± 1.0 mM. However, iron is not very soluble under aerobic conditions at pH 7.5, and it must be assumed that most of the iron was present in the hydroxide form and that free Fe3+ remained very low. In fact, the formation of a visible film on the surface of the aqueous phase could be observed. So iron release is unlikely to play a role in contact killing by metallic iron. Rather, our results suggest a mechanism of contact killing whereby the contact of the bacteria with solid iron or copper weakens the bacterial cell wall and/or membrane, which, in turn, renders the cells susceptible to damage by copper ions. In the absence of the toxic effects of ionic copper, damage to the cell envelope can presumably be repaired when the cells are returned to growth conditions.
Fig 6.
Contact killing by iron coupons. Survival of E. hirae was assessed by wet plating on iron coupons as described in Materials and Methods, suspended either in Tris-Cl (pH 7) only (□) or in Tris-Cl (pH 7) plus 4 mM CuSO4 (■). Control experiments were conducted similarly on glass with cells in Tris-Cl (pH 7) (○) or in Tris-Cl (pH 7) plus 4 mM CuSO4 (●). CFU are given for the whole sample volume which was applied to the coupons. The error bars show the standard deviations of three independent experiments. (Inset) Survival at 10 min as a function of the copper concentration. Experimental conditions, abscissa, and ordinate are as in the larger graph.
DISCUSSION
We here report the following novel observations: (i) contact arrays with a polymer grid which prevents contact of the bacteria with the copper surface did not exhibit contact killing, (ii) the release of copper ions by these contact arrays was not impeded, and (iii) ionic copper elicited efficient contact killing on metallic iron. It has been shown in previous studies with Gram-positive and Gram-negative bacteria and yeast that membrane damage is a key event in contact killing (16, 17, 27–30). These findings combined with our observations support a hypothesis whereby bacterial-metal contact permeabilizes the cells, thus facilitating access of copper ions to cellular components. Intracellular copper ions, in turn, cause irreversible damage and cell death. This concept is strongly supported by the surprising observation reported here that E. hirae is efficiently killed by copper ions in the presence of metallic iron (Fig. 6). Iron has not been reported as a metal with contact killing activity.
Contact killing on copper is slower if bacteria are applied by the so-called wet inoculation technique, whereby a few microliters of a bacterial suspension are applied to metal coupons as a droplet (23). In the alternative “dry” procedure, a bacterial suspension is mechanically spread on the metal surface, which allows the water to evaporate in a few seconds (22). Of course there will still be cellular water and probably a significant film of water between the bacteria and the copper surface, allowing copper ions to migrate. But the forced, continuous contact of the bacteria with the copper surface may accelerate membrane damage as well as access of copper ions to the cell interior, which could explain the more rapid contact killing by dry versus wet inoculation. By either technique, the rate of release of copper ions from the metal surface is an important parameter, and a slower release reduces contact killing (23, 31).
It is currently widely accepted that the mechanism of contact killing involves the following key steps: damage of the outer and/or inner bacterial membrane, accumulation of copper ions in the cell, and degradation of the bacterial DNA (32). Inhibition of the respiratory chain by copper has been proposed as the primary event in contact killing of Staphylococcus aureus (21). While this may be a factor in contact killing of respiring cells, such a mechanism obviously does not apply to nonrespiring bacteria, like E. hirae used in this study. The sequence of events leading to cell death may vary depending on the organism, but generally, DNA degradation is probably not the primary event (16–18, 20, 30).
Two important questions in contact killing have so far remained open: (i) how do metals actively damage cells during contact killing, and (ii) what is the toxicity mechanism of copper ions in contact killing? Membrane damage has been reported repeatedly as a key initial event in contact killing. This was shown for several organisms and by a variety of techniques, including direct microscopic examination of cells (16, 30) using redox dyes like 5-cyano-2,3-ditolyl tetrazolium chloride or rhodamine 123 staining of respiring cells (4, 19, 20), or by the Live/Dead staining technique, which combines the green fluorescent SYTO9 DNA dye, which is membrane permeative and stains all cells, with the red fluorescent dye propidium iodide, which enters only damaged cells and stains cellular DNA with an affinity higher than that of SYTO9, thereby changing the fluorescence of dead cells from green to red (20, 21, 28, 29). However, how membrane damage occurs and how outer and/or inner membranes of bacteria are affected remains unclear. Our studies show that contact of bacteria with the metal surface may be a key event, and future studies should be aimed specifically at this process.
The toxic effect of copper ions on cells remains unclear. That the influx of copper ions into the cytoplasm is a key effector in contact killing is undisputed and is supported by several observations. First, it was found that cells preadapted to ionic copper and thus endowed with upregulated copper resistance mechanisms are more resistant to contact killing, while bacteria deficient in copper resistance systems are more readily killed on metallic copper (2, 22, 23). Second, using various techniques, it was demonstrated for several bacterial and fungal species that large amounts of copper ions enter the cytoplasm (17, 29, 30). With a cytoplasmic copper indicator, Coppersensor-1, Espírito Santo et al. estimated the cellular copper content in Staphylococcus at 2.6 × 1010 copper ions per cell after 5 min of contact with a dry copper surface (17). Finally, copper chelators like bicinchoninic acid, bathocuproine disulfonate, and EDTA significantly inhibit or even prevent contact killing (20, 22).
The toxicity of copper ions has generally been ascribed to its ability to catalyze Fenton chemistry according to reaction 1.
| (1) |
Combined with the Haber-Weiss cycle (reactions 2 and 3), this reaction can provide a rich source of reactive oxygen species (ROS), particularly in lactic acid bacteria like E. hirae, which can produce large amounts of hydrogen peroxide (33, 34). [Reaction 3 by itself has a negligible rate constant but is catalyzed by Cu(II) or Fe(III) complexes.]
| (2) |
| (3) |
ROS generated by these reactions can lead to irreversible damage of cellular components. ROS can also inhibit the respiratory chain or divert electrons from it, which leads to further ROS production. ROS generation and lipid peroxidation as a consequence of damage by copper ions were shown to occur in E. coli and Salmonella exposed to solid copper (18, 28). A mutant strain with higher levels of unsaturated fatty acids and thus more sensitive to ROS exhibited an earlier rise in lipid peroxidation, higher sensitivity to contact killing, and an earlier onset of DNA degradation (28). Evidence for oxidative damage was also apparent from the proteome of E. coli exposed to metallic copper by the increased presence of oxidatively modified proteins (35).
The cytotoxic mechanism of copper ions in contact killing, which involves massive deposition of copper in cells, may be different from that in cultured bacteria, where only moderate amounts of copper enter the cells. It was shown that in culture, the primary toxic effect of copper on E. coli was the displacement of [4Fe-4S] clusters of dehydratases by copper ions (36). Thus, the toxic principle under these conditions was intracellular Cu(I), rather than ROS and oxidative damage (37). Attack of [4Fe-4S] clusters was also demonstrated for Ag(I), Hg(II), Cd(II), and Zn(II) at concentrations which were barely toxic to cell growth (38). In line with a cytotoxicity mechanism based on iron-sulfur cluster attack, the cytotoxicity of ions followed their thiophilicity. To what extent iron-sulfur cluster attack plays a role in contact killing is not clear, but it is unlikely to be a key mechanism. For one, zinc, which has a high thiophilicity, displayed a death rate constant of contact killing which was less than 1/20 of that of copper or silver (39), conceivably because it is not redox active (see below); mercury and cadmium were never tested for contact killing.
Of 21 metallic elements tested, copper and silver exhibited similar rates of contact killing, which were 5- to 10-fold higher than those of the other metals tested (iron was not tested [39]). In fact, silver is well recognized as an antibacterial metal and is already in widespread use as an antibacterial agent, mainly in colloidal form (40). What makes copper and silver so unique? Like copper, silver leads to the production of ROS in E. coli and Staphylococcus aureus, and the toxicity could be completely suppressed by the antioxidant N-acetylcysteine (41). Gene expression analysis revealed upregulation of several antioxidant genes and the genes of a number of ion transporters, including the CopA copper/silver export ATPase (42). Membrane damage in contact killing by silver remains to be demonstrated but is most likely to occur.
Based on current knowledge, it appears that two conditions must be met for a metal to be antimicrobial: (i) to have a redox-active surface under ambient conditions and (ii) to release ions toxic to cells. Silver has a standard reduction potential of −0.8 V for the Ag/Ag+ couple, and copper has reduction potentials of −0.52 and −0.35 V for the redox couples Cu/Cu+ and Cu/Cu2+, respectively. These reduction potentials are in the range of biological reduction potentials, and it can be speculated that the redox-active metals disturb the cellular redox chemistry or catalyze destructive reactions at the cell surface. Iron covers a range of redox potentials, namely, 0.41 V for Fe/Fe2+, 0.04 V for Fe/Fe3+, and −0.77 V for the Fe2+/Fe3+ redox couple. The Fe2+/Fe3+ redox potential is in the range of those of silver and copper, and it appears feasible that it is this redox chemistry that causes membrane damage. If copper ions are also present, they will effect the intracellular damage leading to contact killing.
Quantitative proteomic profiling lends support to membrane damage as a key event in contact killing. E. coli exposed to metallic copper had upregulated cell envelope and capsule polysaccharide biogenesis proteins (35), indicative of stress on the microorganism's envelope (35). Proteins involved in translation, ribosomal structure, and biogenesis functions, on the other hand, were downregulated. The changes typical of copper stress in solution, namely, upregulation of proteins involved in secondary metabolite biosynthesis, catabolism, and transport, including efflux proteins and multidrug resistance proteins, were not observed under contact killing conditions. Using atomic force microscopy, Nan et al. (43) showed that antibacterial steel (stainless steel containing 3.8% copper) destroyed the cell membrane and/or cell wall of bacteria and increased the permeability of the cells, while control steel left cells intact. They also observed loss of fluid and K+ from the cells on antibacterial steel, further supporting severe membrane damage. Interestingly, leakage was found to occur primarily at the cell poles, and the authors speculated that the preponderance of negatively charged cardiolipin at these locations enhanced copper binding (43). They also demonstrated that the force of adhesion of bacteria to antibacterial stainless steel was considerably greater than to control steel, which could have been an effect of released Cu2+ ions. These findings highlight novel aspects of contact killing which conceivably also apply to other antimicrobial surfaces.
The importance of bacterial-metal contact in contact killing is further supported by the observation that heavy soiling of a copper surface substantially reduced contact killing (44). Furthermore, it was found that 76% of the bacteria isolated from Euro coins (25% Ni and 75% Cu, an alloy with pronounced antibacterial activity) were still susceptible to contact killing when retested on copper (45). Presumably, these bacteria had survived on the coins due to soiling. Taken together, these and our findings lend strong support to a contact killing mechanism whereby bacteria initially suffer severe damage to the cell envelope upon contact with an antimicrobial metal surface, which, in turn, allows access of copper (or silver) ions to cellular components, where further damage ensues.
ACKNOWLEDGMENTS
This work was supported by grant CR32I3E-136073 from the Swiss National Science Foundation and by grant MU 959/26-1 from the German Research Foundation. The copper materials used were kindly provided by KME Germany AG & Co. KG.
We thank Thomas Weber and Natalie Pagel for expert technical assistance.
Footnotes
Published ahead of print 8 February 2013
REFERENCES
- 1. Mehtar S, Wiid I, Todorov SD. 2008. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: an in-vitro study. J. Hosp. Infect. 68:45–51 [DOI] [PubMed] [Google Scholar]
- 2. Elguindi J, Wagner J, Rensing C. 2009. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J. Appl. Microbiol. 106:1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faúndez G, Troncoso M, Navarrete P, Figueroa G. 2004. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 4:19 doi:10.1186/1471-2180-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noyce JO, Michels H, Keevil CW. 2006. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 63:289–297 [DOI] [PubMed] [Google Scholar]
- 5. Noyce JO, Michels H, Keevil CW. 2006. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 72:4239–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weaver L, Michels HT, Keevil CW. 2008. Survival of Clostridium difficile on copper and steel: futuristic options for hospital hygiene. J. Hosp. Infect. 68:145–151 [DOI] [PubMed] [Google Scholar]
- 7. Wilks SA, Michels H, Keevil CW. 2005. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 105:445–454 [DOI] [PubMed] [Google Scholar]
- 8. Wilks SA, Michels HT, Keevil CW. 2006. Survival of Listeria monocytogenes Scott A on metal surfaces: implications for cross-contamination. Int. J. Food Microbiol. 111:93–98 [DOI] [PubMed] [Google Scholar]
- 9. Anonymous 2010. Annual epidemiological report on communicable diseases in Europe. European Centre for Disease Prevention and Control, Stockholm, Sweden: http://ecdc.europa.eu/en/publications/Publications/1111_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf [Google Scholar]
- 10. Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Shillam R, Christian P, Elliott TS. 2010. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 74:72–77 [DOI] [PubMed] [Google Scholar]
- 11. Laitinen K, Voutilainen P, Santala L. 2010. Clinical trial on using copper and brass surfaces in a hospital in West-Finland using microbiological assessment. Internal report. http://www.scda.com/doc/uploaded/file/Result_report_Pori_veteran_home_clinical_trial.pdf
- 12. Mikolay A, Huggett S, Tikana L, Grass G, Braun J, Nies DH. 2010. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 87:1875–1879 [DOI] [PubMed] [Google Scholar]
- 13. Rai S, Hirsch BE, Attaway HH, Nadan R, Fairey S, Hardy J, Miller G, Armellino D, Moran WR, Sharpe P, Estelle A, Michel JH, Michels HT, Schmidt MG. 2012. Evaluation of the antimicrobial properties of copper surfaces in an outpatient infectious disease practice. Infect. Control Hosp. Epidemiol. 33:200–201 [DOI] [PubMed] [Google Scholar]
- 14. Schmidt MG, Attaway HH, Sharpe PA, John J, Jr, Sepkowitz KA, Morgan A, Fairey SE, Singh S, Steed LL, Cantey JR, Freeman KD, Michels HT, Salgado CD. 2012. Sustained reduction of microbial burden on common hospital surfaces through the introduction of copper. J. Clin. Microbiol. 50:2217–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Gorman J, Humphreys H. 2012. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 81:217–223 [DOI] [PubMed] [Google Scholar]
- 16. Espírito Santo C, Lam EW, Elowsky CG, Quaranta D, Domaille DW, Chang CJ, Grass G. 2011. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 77:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Espírito Santo C, Quaranta D, Grass G. 2012. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen 1:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warnes SL, Caves V, Keevil CW. 2012. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ. Microbiol. 14:1730–1743 [DOI] [PubMed] [Google Scholar]
- 19. Warnes SL, Green SM, Michels HT, Keevil CW. 2010. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNA. Appl. Environ. Microbiol. 76:5390–5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Warnes SL, Keevil CW. 2011. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Appl. Environ. Microbiol. 77:6049–6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weaver L, Noyce JO, Michels HT, Keevil CW. 2010. Potential action of copper surfaces on meticillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 109:2200–2205 [DOI] [PubMed] [Google Scholar]
- 22. Espírito Santo C, Taudte N, Nies DH, Grass G. 2008. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 74:977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molteni C, Abicht HK, Solioz M. 2010. Killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 76:4099–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lasagni AF, Menéndez-Ormaza BS. 2010. Two- and three-dimensional micro- and sub-micrometer periodic structures using two-beam laser interference lithography. Adv. Eng. Mater. 12:54–60 [Google Scholar]
- 25. Terzaghi BE, Sandine WE. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mücklich F, Lasagni A, Daniel C. 2006. Laser interference metallurgy—using interference as a tool for micro/nano structuring. Int. J. Mater. Res. 97:1337–1344 [Google Scholar]
- 27. Gutierrez H, Portman T, Pershin V, Ringuette M. 2012. Evaluation of biocidal efficacy of copper alloy coatings in comparison with solid metal surfaces: generation of organic copper phosphate nanoflowers. J. Appl. Microbiol. [DOI] [PubMed] [Google Scholar]
- 28. Hong R, Kang TY, Michels CA, Gadura N. 2012. Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl. Environ. Microbiol. 78:1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quaranta D, Krans T, Espírito Santo C, Elowsky CG, Domaille DW, Chang CJ, Grass G. 2011. Mechanisms of yeast contact-killing on dry metallic copper surfaces. Appl. Environ. Microbiol. 77:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian WX, Yu S, Ibrahim M, Almonaofy AW, He L, Hui Q, Bo Z, Li B, Xie GL. 2012. Copper as an antimicrobial agent against opportunistic pathogenic and multidrug resistant Enterobacter bacteria. J. Microbiol. 50:586–593 [DOI] [PubMed] [Google Scholar]
- 31. Elguindi J, Moffitt S, Hasman H, Andrade C, Raghavan S, Rensing C. 2011. Metallic copper corrosion rates, moisture content, and growth medium influence survival of copper ion-resistant bacteria. Appl. Microbiol. Biotechnol. 89:1963–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grass G, Rensing C, Solioz M. 2011. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 77:1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baureder M, Reimann R, Hederstedt L. 2012. Contribution of catalase to hydrogen peroxide resistance in Enterococcus faecalis. FEMS Microbiol. Lett. 331:160–164 [DOI] [PubMed] [Google Scholar]
- 34. van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E. 2002. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187–216 [PubMed] [Google Scholar]
- 35. Nandakumar R, Espírito Santo C, Madayiputhiya N, Grass G. 2011. Quantitative proteomic profiling of the Escherichia coli response to metallic copper surfaces. Biometals 24:429–444 [DOI] [PubMed] [Google Scholar]
- 36. Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 106:8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park HJ, Nguyen TT, Yoon J, Lee C. 2012. Role of reactive oxygen species in Escherichia coli inactivation by cupric ion. Environ. Sci. Technol. 46:11299–11304 [DOI] [PubMed] [Google Scholar]
- 38. Xu FF, Imlay JA. 2012. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl. Environ. Microbiol. 78:3614–3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawakami H, Yoshida K, Nishida Y, Kikuchi Y, Sato Y. 2008. Antibacterial properties of metallic elements for alloying evaluated with application of JIS Z 2801:2000. ISIJ Int. 48:1299–1304 [Google Scholar]
- 40. Diaz-Visurraga J, Gutiérrez C, von Plessing C, Garcia A. 2012. Metal nanostructures as antibacterial agents, p 210–218 In Méndez-Vilas A. (ed), Science against microbial pathogens: communicating current research and technological advances. Formatex, Badajoz, Spain [Google Scholar]
- 41. Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, Kim Y-K, Lee YS, Jeong DH, Cho M-H. 2007. Antimicrobial effects of silver nanoparticles. Nanomedicine 3:95–101 [DOI] [PubMed] [Google Scholar]
- 42. Nagy A, Harrison A, Sabbani S, Munson RS, Jr, Dutta PK, Waldman WJ. 2011. Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 6:1833–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nan L, Liu Y, Lu M, Yang K. 2008. Study on antibacterial mechanism of copper-bearing austenitic antibacterial stainless steel by atomic force microscopy. J. Mater. Sci. Mater. Med. 19:3057–3062 [DOI] [PubMed] [Google Scholar]
- 44. Airey P, Verran J. 2007. Potential use of copper as a hygienic surface; problems associated with cumulative soiling and cleaning. J. Hosp. Infect. 67:272–278 [DOI] [PubMed] [Google Scholar]
- 45. Espírito Santo C, Morais PV, Grass G. 2010. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 76:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]