Abstract
Currently, nitritation-anammox (anaerobic ammonium oxidation) bioreactors are designed to treat wastewaters with high ammonium concentrations at mesophilic temperatures (25 to 40°C). The implementation of this technology at ambient temperatures for nitrogen removal from municipal wastewater following carbon removal may lead to more-sustainable technology with energy and cost savings. However, the application of nitritation-anammox bioreactors at low temperatures (characteristic of municipal wastewaters except in tropical and subtropical regions) has not yet been explored. To this end, a laboratory-scale (5-liter) nitritation-anammox sequencing batch reactor was adapted to 12°C in 10 days and operated for more than 300 days to investigate the feasibility of nitrogen removal from synthetic pretreated municipal wastewater by the combination of aerobic ammonium-oxidizing bacteria (AOB) and anammox. The activities of both anammox and AOB were high enough to remove more than 90% of the supplied nitrogen. Multiple aspects, including the presence and activity of anammox, AOB, and aerobic nitrite oxidizers (NOB) and nitrous oxide (N2O) emission, were monitored to evaluate the stability of the bioreactor at 12°C. There was no nitrite accumulation throughout the operational period, indicating that anammox bacteria were active at 12°C and that AOB and anammox bacteria outcompeted NOB. Moreover, our results showed that sludge from wastewater treatment plants designed for treating high-ammonium-load wastewaters can be used as seeding sludge for wastewater treatment plants aimed at treating municipal wastewater that has a low temperature and low ammonium concentrations.
INTRODUCTION
Nitrogen removal from wastewater treatment is necessary because of the significant adverse environmental impact of ammonia/ammonium, such as eutrophication and toxicity to aquatic life, on the receiving bodies. Generally, carbonaceous waste is removed in the first stage of wastewater treatment, which is followed by nitrogen removal systems. Conventionally, the removal of nitrogen (ammonium) is accomplished by the combination of nitrification and denitrification processes. Both of these are energy consuming and are associated with high costs. Moreover, these processes have an additional environmental impact due to high biomass production and greenhouse gas (CO2, N2O, etc.) emission, which promote global warming.
Anaerobic ammonium-oxidizing (anammox) bacteria convert ammonium and nitrite directly to dinitrogen gas (N2) under anoxic conditions. Since they were first detected in a denitrifying pilot plant by Mulder et al. in 1995 (1), anammox bacteria have been found in various oxygen-limited natural (2–4) and manmade ecosystems. The application of the anammox process in wastewater treatment results in significant energy reduction (60%) and greenhouse gas emission (90%) compared to those of traditional biological nitrogen removal processes (5–7). In full-scale nitritation-anammox wastewater treatment plants, ammonium-oxidizing bacteria (AOB) convert approximately half of the supplied ammonium to nitrite under O2 limitation, and in turn, nitrite, together with the remaining ammonium, is converted to N2 by anammox bacteria (8, 9). These systems are already applied for the treatment of warm and high-strength wastewaters, such as digester effluents and anaerobically treated industrial effluents (10, 11).
A more sustainable municipal wastewater treatment can be achieved with a first step in which available organic carbonaceous compounds are concentrated and converted to CH4 in an anaerobic digester (6). The remaining wastewater would have only ammonium left as the major pollutant, and it would be possible to use the nitritation-anammox process for nitrogen removal. However, municipal wastewater has a lower temperature (∼10 to 15°C, apart from tropical and subtropical regions) and a relatively low ammonium concentration (12). This may lead to lower specific activities and growth rates for both anammox bacteria and AOB. Indeed, it was reported that the activities of anammox bacteria and AOB both decreased at 15 to 20°C (13, 14) and that partial nitrification was difficult to achieve in winter because of the varying temperature of municipal wastewater (15). Nevertheless, several studies showed that nitrogen removal at a lower temperature by an anammox process can work (14, 16, 17); still, in none of these studies was it possible to maintain a stable anammox-AOB culture (nitritation-anammox) at temperatures lower than 20°C. On the other hand, in natural ecosystems, such as Northern European soils and marine sediments, anammox bacteria thrive under much colder temperatures and very low ammonium concentrations (μM range) (18, 19). Therefore, it should be possible to adapt a nitritation-anammox bioreactor to low ammonium concentrations to treat cold municipal wastewater using appropriate strategies.
To this end, a laboratory-scale (5-liter) sequencing batch reactor (SBR) was inoculated with anammox biomass from an SBR operating at 30°C. Oxygen was supplied gradually to allow the growth of AOB. The enriched nitritation-anammox biomass was adapted to 12°C. Synthetic wastewater simulating pretreated municipal wastewater was used as influent for the bioreactor. Activities of anammox, AOB, and aerobic nitrite oxidizers (NOB) were monitored by offline batch tests, and the microbial community composition was investigated by molecular analyses, including fluorescence in situ hybridization (FISH) and clone libraries. The responses of specific activity of each functional community to temperature change and low ammonium concentrations were discussed.
MATERIALS AND METHODS
Reactor setup and operation.
Two sequencing batch reactors (SBR; working volume, 5 liters) were used for the cultivation of anaerobic ammonium-oxidizing bacteria (anammox) and nitritation-anammox biomass.
A previously described anammox enrichment culture (20) was used to grow anammox biomass at different temperatures as follows. The anammox SBR was stirred at 200 rpm with a six-bladed turbine stirrer. Each cycle consisted of 11 h of filling, 20 min of biomass settling, and 40 min of drawing of the liquid. During each filling period, 1 liter of mineral medium (21) containing 630 mg N/liter (45 mM) nitrite and ammonium was supplied to the anammox SBR. To maintain anoxic conditions, the reactor and the medium vessel were flushed continuously with Ar/CO2 (95%/5%; 10 ml · min−1). CO2 present in the supplied gas and bicarbonate in the mineral medium was sufficient to buffer the solution and keep the pH in the reactor at ∼7.3. The temperature of the reactor was maintained at 30°C with a water bath. The temperature of the anammox SBR was decreased from 30°C stepwise (5°C steps) in 2 months to 15°C. At each step, activity tests were performed to determine the anammox activity as described below (“Activity tests”).
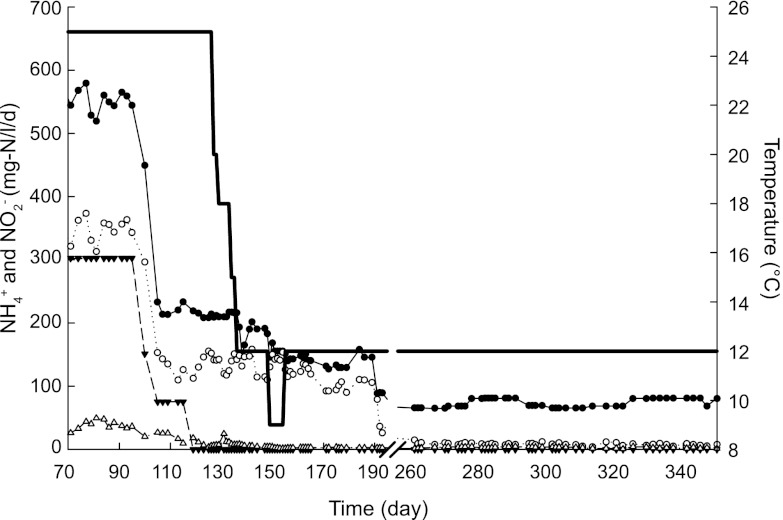
When the anammox SBR described above was operated at 30°C, one liter of this culture (70 to 80% enriched) was used as seed sludge to inoculate another SBR to enrich nitritation-anammox biomass as follows. At the time of the inoculation, the temperature was decreased in one step from 30°C to 25°C. The nitritation-anammox SBR was operated as described above with respect to SBR cycle, pH, and anoxia. During each filling period of the SBR cycle, 1 liter of mineral medium (21) containing 420 mg N/liter (30 mM) nitrite and 588 mg N/liter (42 mM) ammonium was supplied for 93 days. Oxygen (7.54 mg/day) was introduced to the reactor after 2 weeks of the inoculation to facilitate the growth of AOB. To mimic municipal wastewater, ammonium and nitrite concentrations were gradually decreased after day 94. The final concentrations of ammonium and nitrite in the influent were 70 mg N/liter (5 mM; day 191) and 0 mg N/liter (day 119), respectively (Fig. 1). Between days 125 and 136, the temperature of the reactor was stepwise decreased to 12°C (Fig. 1). Biomass (1.5 ml) was collected every 2 days for DNA extraction and every week for FISH analysis. When the system was operated at 12°C, over a period of 15 days (at days 330, 338, and 345), triplicate gas samples (100 μl) were taken for N2O measurements from both SBRs.
Fig 1.
Operation of the laboratory-scale nitritation-anammox SBR under different conditions. Filled and empty circles indicate ammonium concentrations in the influent and effluent, respectively, and filled triangles indicate nitrite concentrations in the influent. Nitrite concentrations in the effluent were always below the detection limit. Empty triangles indicate nitrate concentrations in the effluent.
Activity tests.
Specific activity of each functional community (Anammox, AOB, and NOB) was tested at days 13, 113, 137, and 268, which represent different operational stages (different oxygen supply and temperature). For anammox activity tests, 10 ml biomass was taken directly from the anammox SBR and the nitritation-anammox SBR. The samples were transferred to separate 30-ml serum bottles. After the addition of 1 to 2 mM (14 to 28 mg N) nitrite and ammonium and adjustment of the pH to 7.3, the bottles were capped with butyl rubber stoppers and sealed with aluminum crimp caps. The sealed bottles were then made anoxic by alternatively applying vacuum and supplying Ar/CO2 (95%/5%) at least 7 times. The bottles were incubated in shaking incubators (200 rpm) at 10 to 50°C with 5°C increments. Liquid samples (0.5 ml) were taken every 20 to 60 min (depending on actual activity) for ammonium and nitrite measurements.
For AOB and NOB activity tests, 10 ml biomass from the nitritation-anammox SBR was transferred to a 50-ml Erlenmeyer flask under atmospheric conditions. Biomass used for the NOB activity test was first washed 3 times with HEPES buffer (20 mM, pH 7.4) to remove all ammonium. Incubation and sampling procedures were the same as those for the anammox activity tests.
Analytical methods.
Collected liquid samples were centrifuged (5 min, 10,000 × g), and the supernatants were kept at −20°C until further analyses. Ammonium, nitrite, and nitrate were measured colorimetrically as previously described (22). Protein concentrations were measured using the Biuret method as previously described (23). N2O was measured with an Agilent 6890 Series GC (Agilent Technologies) equipped with a Porapack Q column and an electron capture detector (ECD).
FISH.
Fluorescence in situ hybridization (FISH) was performed on the nitritation-anammox SBR as described previously (24) with probes specific for Kuenenia and Brocadia-like anammox bacteria (AMX820 [25]), Kuenenia stuttgartiensis (KST1273 [24]), AOB (NEU653 [26]), and NOB (NTSPA0712 [27] and NIT1035 [28]). DAPI (4′,6-diamidino-2-phenylindole) was used to stain the whole community DNA.
DNA extraction, PCR amplification, cloning, and phylogenetic analyses.
Genomic DNA was extracted from 1 ml of centrifuged sample from the nitritation-anammox SBR, as described previously (29), and was stored at −20°C until further analyses. DNA concentrations were determined by the NanoDrop 1100 spectrophotometer (Thermo Scientific).
The 16S rRNA gene of anammox bacteria was amplified by PCR using the primer combination pla46F (28) and 1529R (30). For quantitative PCR (qPCR), the primer combinations hzsA526F/hzsA1829R (31) and amoA-1F/amoA-2R (32) were used for anammox bacteria and AOB, respectively, using the MyiQ single-color real-time PCR detection system (Bio-Rad). Each qPCR was performed in triplicate. To construct standard curves, plasmids containing the target gene were quantified using a NanoDrop 1100 spectrophotometer and then serially diluted in 10-fold steps before qPCR was performed. For all PCR amplifications, a 25-μl reaction mixture containing 12.5 μl GoTaq Green master mix (Promega Benelux BV, Leiden, the Netherlands), 10 pmol of primers, and 10 to 20 ng DNA of template DNA was used. PCR products were ligated to pGEM-T easy vector (Promega Benelux BV, Leiden, the Netherlands) according to the manufacturer's instructions. Plasmid DNA was extracted using the GeneJET plasmid miniprep kit (Fermentas GMBH, St. Leon-Rot, Germany), and the insert was sequenced by the M13 Forward primer (5′ GTAAAACGACGGCCAGT 3′). Phylogenetic trees were constructed using MEGA 5 software (33).
RESULTS
Enrichment of the aerobic ammonium-oxidizing bacteria.
The anammox biomass (∼80% enriched) from the sequencing batch reactor (SBR) operated at 30°C was used as an inoculum for the nitritation-anammox SBR. After inoculation, the bioreactor was operated at 25°C and anaerobically for 2 weeks with 420 mg N/liter (30 mM) nitrite and 588 mg N/liter (42 mM) ammonium as the substrates in the influent (flow rate, 1.8 liters/day). Within this period, anammox bacteria were responsible for all ammonium and nitrite consumption. To enrich an anammox-AOB coculture, O2 was supplied to the reactor continuously after day 14. To avoid the inhibition of anammox bacteria, O2 was supplied in such a way that it was always below the detection limit (0.05 mg O2/liter) due to the activity of AOB. At day 34, Nitrosomonas-like microorganisms were detected by FISH, and aerobic ammonium-oxidizing activity was detectable, indicating that an AOB community had already developed within 20 days. On day 119, the concentration of ammonium was decreased to 210 mg N/liter (15 mM) and nitrite was no longer supplied to the reactor. After this point, the nitrite required for the anammox reaction was completely produced by the AOB. To mimic pretreated municipal nitrogenous wastewater, the ammonium concentration was lowered to 70 mg N/liter (5 mM) on day 191 (Fig. 1) and was kept at this value for the rest of the experimental period. Ammonium supplied to the nitritation-anammox SBR was partially oxidized by AOB to nitrite, which was subsequently consumed by the anammox bacteria together with the remaining ammonium. Within just 10 days (between days 125 and 136), the temperature of the reactor could be gradually lowered from 25°C to 12°C without nitrite accumulation. On day 150, the temperature of the reactor was lowered to 9°C, which resulted in a gradual nitrite accumulation (up to 14 mg N/liter). Increasing the temperature back to 12°C on day 152 alleviated this accumulation.
Changes of community composition in response to temperature and oxygen.
The results of FISH analysis of the biomass from the nitritation-anammox SBR showed a clear increase of AOB abundance after the air supply started. At day 20, 1 week after the start of O2 addition, still only anammox bacteria were able to be detected by FISH (see Fig. S1 in the supplemental material) and the abundance of AOB was apparently below the detection limit (10,000 cells/ml, <1% of the microbial population). With increasing O2 supply, the abundance of AOB increased to about 50% of the total population (day 135) (see Fig. S1 in the supplemental material) and anammox bacteria and AOB comprised approximately 90% of the population as detected by DAPI (data not shown). Clone libraries (see Fig. S2 in the supplemental material) and FISH analyses revealed that after the introduction of oxygen, the dominant anammox species in the bioreactor was a “Candidatus Brocadia fulgida”-like strain and it did not change throughout the operation of the nitritation-anammox SBR. It was not possible to detect NOB with the most general probes during the experimental period.
Functional gene abundance of anammox and AOB.
Anammox bacteria and AOB in the nitritation-anammox SBR were quantified by real-time qPCR performed on the hzsA and amoA genes, respectively. At day 35, 20 days after the introduction of oxygen and when the temperature was 25°C, copy numbers of hzsA and amoA genes were ∼7 × 109 copies · ml−1 and ∼4 × 107 copies · ml−1, respectively, indicating that the AOB started to grow in the bioreactor, although their population comprised only ∼1% of the total population. The anammox copy numbers corresponded to an in situ anammox activity of 10 fmol N · cell−1 · day−1. An increase in amoA gene copy numbers, which was congruent with the FISH results, was observed subsequently. At day 353, after more than 200 days of extended enrichment at 12°C, the copy numbers of hzsA and amoA genes were ∼2 × 107 copies · ml−1 and ∼8.6 × 106 copies · ml−1, respectively. At this stage, the in situ anammox and AOB activities were 10.5 and 24 fmol N · cell−1 · day−1, respectively.
The effect of temperature and oxygen on activities of different trophic groups.
In the 100 days after the introduction of O2, there was a decrease in the observed anammox activity from 16.6 to 12 nmol N · mg total protein−1 · min−1 in the nitritation-anammox SBR. This decrease was most probably due to the fact that the AOB were now comprising a larger part of the total biomass and consuming part of the ammonium. AOB activity doubled with increasing O2 and stabilized at around ∼27 nmol N · mg total protein−1 · min−1 at 12°C. NOB activity was undetectable during the whole experimental period, which indicated that all substrates were consumed by anammox and AOB.
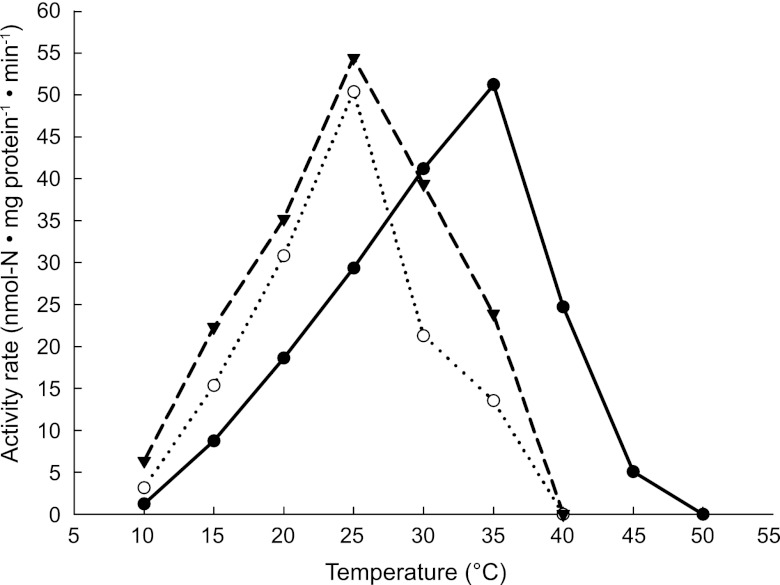
When the nitritation-anammox SBR was operated at 12°C for 120 days, anammox activity increased from 12 to about 18 nmol N · mg total protein−1 · min−1, which indicated a successful adaptation to low temperature (Table 1). To investigate the effect of temperature on anammox activity, biomass from the 12°C nitritation-anammox SBR and the anammox SBR was sampled and incubated at different temperatures. The optimum temperature for the anammox biomass from the nitritation-anammox SBR enriched at 12°C was 25°C, 10°C lower than that for the biomass originating from the anammox SBR operated at 30°C. Interestingly, the maximum specific activities (activity per anammox protein) of anammox bacteria grown at 30°C and 12°C were almost identical (Fig. 2). Moreover, cold-adapted biomass still oxidized ammonium at a rate of 5 nmol N · mg protein−1 · min−1 at 12°C (Fig. 2), which was a high enough rate to remove all nitrogen supplied to the system. The AOB from the cold-adapted reactor had the maximum specific activity (activity per AOB protein) at 25°C and an activity of 10 nmol N · mg protein−1 · min−1 at 12°C.
Table 1.
Activities of anammox, AOB, and NOB at different operation stages
| Day | Temp (°C) | Activity ofa: |
||||
|---|---|---|---|---|---|---|
| Anammox |
AOB |
NOB (nitrite consumption) | ||||
| Ammonium consumption | Nitrite consumption | Ammonium consumption | Nitrite production | |||
| 13 | 25 | 16.6 | 23.4 | ND | ND | ND |
| 113 | 25 | 11.9 | 13.2 | 12.5 | 14 | ND |
| 137 | 12 | 11.4 | 15.4 | 26.6 | 26 | ND |
| 268 | 12 | 18.4 | 24.2 | 28.4 | 31 | ND |
Values are expressed in nmol N · mg protein−1 · min−1. ND, not detectable.
Fig 2.
Effect of long-term enrichment at 30°C (filled circles, solid line) and 12°C (empty circles, dotted line) on the maximum specific activity of the anammox bacteria. Specific activities of cold-adapted (12°C) AOB at different temperatures are also depicted (filled triangles, dashed line). Protein concentrations on the y axis indicate anammox or AOB protein only.
Anammox bacteria oxidize a part of their substrate nitrite to nitrate for reducing equivalents necessary for cell carbon fixation; therefore, when anammox bacteria are growing, nitrate production is observed. With the decreasing temperature, there was also a decrease in nitrate production by anammox bacteria in the nitritation-anammox SBR. In the 68 days before the temperature was lowered, the average ratio of nitrate produced to ammonium consumed by the anammox bacteria was approximately 0.18, which is similar to the stoichiometric value of 0.26. Conversely, in the last 158 days, when the reactor was operated at 12°C, this ratio decreased to 0.04, suggesting that the anammox bacteria had a lower yield and/or higher maintenance activity.
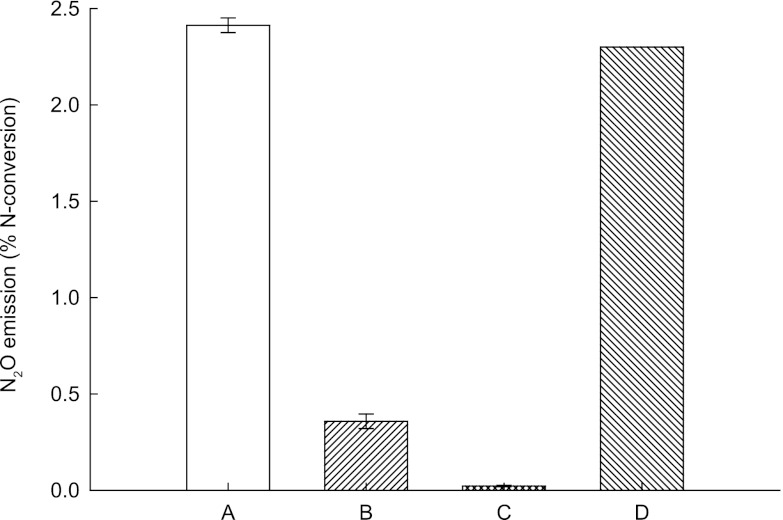
As measured by three grab samples measured in triplicate, N2O emission from the nitritation-anammox SBR at 12°C was ∼2.4% (± 0.1%) of total N removed (Fig. 3).
Fig 3.
N2O emission from the nitritation-anammox SBR (12°C) (A) compared to that from anammox cultures enriched at 30°C (B) and 15°C (C) and a full-scale nitritation-anammox wastewater treatment plant (32 to 33°C) (46) (D).
DISCUSSION
In this study, first the temperature of the bioreactor of an existing anammox culture was lowered from 30 to 25°C within 1 day without adverse effects, indicating that the anammox bacteria had a sufficient overcapacity. Then a coculture of aerobic ammonium-oxidizing bacteria (AOB) and anammox bacteria was enriched in the same reactor by introducing a limiting amount of oxygen to the bioreactor. After establishing a stable coculture consisting of equal amounts of anammox bacteria and AOB (day 127), the temperature of the nitritation-anammox SBR was decreased from 25 to 12°C. It was possible to decrease the temperature of the bioreactor within 10 days without nitrite accumulation, and this decrease did not result in a change in the dominant anammox species in the bioreactor. Conversely, when the temperature of the reactor was lowered to 9°C, there was a gradual (from day 150 to day 152) nitrite accumulation up to ∼6 mg N/liter. Increasing the temperature back to 12°C resulted in the consumption of the accumulated nitrite. This indicates that even though it was not possible to achieve complete nitrite conversion at 9°C, the decrease in activity was reversible. Such a nitrite accumulation may be because AOB have a higher activity than anammox bacteria at 9°C (Fig. 2). Nevertheless, it cannot currently be ruled out that with a slower decrease of temperature or by using a different type of biomass (e.g., granular), complete nitrite conversion can be reached at temperatures lower than 12°C. Considering the long doubling time of anammox bacteria (10 to 25 days) (34), the acclimation period was very short, indicating that sludge from full-scale bioreactors operating at 30°C may be used to seed new bioreactors designed to remove nitrogen from pretreated low-temperature municipal wastewaters without an extended adaptation period.
Growth of the anammox bacteria is always associated with nitrate production because these microorganisms oxidize part of the nitrite to nitrate as the ultimate source for the electrons that are used for cell carbon fixation. The stoichiometric ratio of nitrate production to ammonium consumption for the anammox bacteria is 1:0.26. When the temperature of the nitritation-anammox SBR was decreased from 25°C to 12°C, this ratio decreased from 0.18 (average of the first 68 days) to 0.04 (the average of the last 158 days), indicating that the anammox bacteria had a lower yield and/or much higher cell maintenance activity at a lower temperature. This phenomenon may be the reason why the decrease in temperature resulted in a 100-fold decrease in the copy numbers of the anammox bacteria. Nevertheless, the decrease in temperature did not have an adverse effect on the maximum potential and in situ activity rates of aerobic and anaerobic ammonium-oxidizing bacteria in the nitritation-anammox SBR (Table 1), suggesting that these groups of microorganisms had an overcapacity for the conversion of their substrates. The in situ activities of both groups of microorganisms in the nitritation-anammox SBR, although they were 30% of the maximum (as measured in batch tests), were sufficient to remove more than 90% (average of the last 100 days, 92%) of the supplied ammonium, indicating that the full-scale application of nitritation-anammox reactors at a low temperature and low ammonium concentration may be feasible.
There was a clear optimum temperature shift (35 to 25°C) in the activity of the cold-adapted anammox biomass from the nitritation-anammox SBR, and it was still active at a wide temperature range (10 to 35°C). This indicated that the activity loss caused by low temperature was reversible and that the common seasonal changes in temperature during full-scale applications would not affect the stability of the treatment system.
Currently, nitritation-anammox reactors are operated at temperatures higher than 30°C (10, 11), and previous studies on the physiology of the anammox bacteria reported that the optimum growth temperature for anammox bacteria is around 30 to 35°C (see reference 35 for a review). Moreover, most described AOB have an optimal temperature of around 28°C (36). On the other hand, some studies indicated that nitrite-oxidizing bacteria were capable of growing at lower temperatures ranging from −2°C (37) to 17°C (36) and that at 10°C to 15°C, NOB had a higher activity than AOB (15). If NOB had a competitive advantage over AOB or anammox bacteria below 20 to 25°C, depending on their affinity for nitrite and O2, they could take up the limiting O2 and nitrite before AOB and anammox bacteria, respectively. In turn, this would lead to nitrate production and, eventually, the collapse of the system. Nevertheless, in the natural ecosystems in which aerobic and anaerobic ammonium-oxidizing bacteria thrive (for example, oxygen minimum zones), the temperature is well below 10°C, indicating that both clades of microorganisms are able to compete with (or outcompete) NOB (38, 39). This was also the case in our nitritation-anammox enrichment culture under oxygen limitation: NOB activity was not detected throughout the operation of the reactor (Table 1; see also Fig. S1 in the supplemental material). Even an extended enrichment at 12°C (132 days) did not facilitate the growth of NOB. Our results showed that anammox bacteria directly consumed nitrite produced by AOB, and when the system was both nitrite and O2 limited, NOB had a lower affinity to nitrite than anammox bacteria (40, 41). In our laboratory-scale bioreactor, nitrite oxidation activity could easily be suppressed under oxygen limitation and did not contribute to nitrate formation. Nevertheless, balancing aeration with various ammonium loads may prove difficult to achieve in full-scale applications. Therefore, a thorough study at the pilot scale would be necessary to determine the optimal process conditions and control parameters that would lead to a stable full-scale operation.
Dissolved O2 concentration is also one of the parameters influencing the emission of N2O from nitritation-anammox bioreactors, most of which is attributed to the activity of AOB (42–45). Our results were completely in line with this observation: there was a negligible (0.02%) amount of N2O produced in the anammox SBR (80% enriched) operated at 15°C. On the other hand, in the nitritation-anammox SBR, ∼2.4% of removed nitrogen was detected as N2O (Fig. 3). This value was remarkably similar to the N2O production from full-scale nitritation-anammox bioreactors (∼2.6% of removed nitrogen). Nevertheless, it should be noted that the laboratory-scale nitritation-anammox bioreactor in this study was operated under controlled and stable conditions. On the other hand, in a full-scale application, many parameters, including wastewater quality, efficiency of the previous treatment steps, and temperature, fluctuate and the results obtained here cannot be used to directly estimate N2O emissions from full-scale nitrogen removal systems.
In this study, we present the proof of principle for the application of the nitritation-anammox process for nitrogen removal from pretreated municipal wastewater. It was possible to adapt a nitritation-anammox bioreactor to low temperature and low ammonium load very rapidly. Moreover, the lab-scale nitritation-anammox bioreactor was operated for over 300 days without nitrite accumulation and was able to remove over 90% of the supplied nitrogen at temperatures as low as 12°C. Nevertheless, further studies are necessary to be able to determine the feasibility of the application of such a process at full scale. We believe that these should focus especially on determining process control parameters for the optimal operation of nitritation-anammox bioreactors under variable wastewater conditions and the degree of greenhouse gas emissions (e.g., N2O) from these systems.
ACKNOWLEDGMENTS
This research was supported by a KRW grant (European Union Water Framework Directive, grant number 09035) and STOWA (Foundation for Applied Water Research, The Netherlands). Z.H. and M.S.M.J. were supported by ERC 232937, and B.K. was supported by the Netherlands Organization for Scientific Research (VENI grant 863.11.003).
Footnotes
Published ahead of print 15 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03987-12.
REFERENCES
- 1. Mulder A, van de Graaf AA, Robertson LA, Kuenen JG. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol. Ecol. 16:177–183 [Google Scholar]
- 2. Hamersley MR, Lavik G, Woebken D, Rattray JE, Lam P, Hopmans EC, Damste JSS, Kruger S, Graco M, Gutierrez D, Kuypers MMM. 2007. Anaerobic ammonium oxidation in the Peruvian oxygen minimum zone. Limnol. Oceanogr. 52:923–933 [Google Scholar]
- 3. Kuypers MMM, Lavik G, Woebken D, Schmid M, Fuchs BM, Amann R, Jorgensen BB, Jetten MSM. 2005. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc. Natl. Acad. Sci. U. S. A. 102:6478–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thamdrup B, Dalsgaard T. 2002. Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 68:1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jetten MSM, Horn SJ, van Loosdrecht MCM. 1997. Towards a more sustainable municipal wastewater treatment system. Water Sci. Technol. 35:171–180 [Google Scholar]
- 6. Kartal B, Kuenen JG, van Loosdrecht MCM. 2010. Sewage treatment with anammox. Science 328:702–703 [DOI] [PubMed] [Google Scholar]
- 7. Siegrist H, Salzgeber D, Eugster J, Joss A. 2008. Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci. Technol. 57:383–388 [DOI] [PubMed] [Google Scholar]
- 8. Pynaert K, Smets BF, Wyffels S, Beheydt D, Siciliano SD, Verstraete W. 2003. Characterization of an autotrophic nitrogen-removing biofilm from a highly loaded lab-scale rotating biological contactor. Appl. Environ. Microbiol. 69:3626–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sliekers AO, Derwort N, Gomez JLC, Strous M, Kuenen JG, Jetten MSM. 2002. Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res. 36:2475–2482 [DOI] [PubMed] [Google Scholar]
- 10. Abma WR, Driessen W, Haarhuis R, van Loosdrecht MCM. 2010. Upgrading of sewage treatment plant by sustainable and cost-effective separate treatment of industrial wastewater. Water Sci. Technol. 61:1715–1722 [DOI] [PubMed] [Google Scholar]
- 11. van der Star WRL, Abma WR, Blommers D, Mulder JW, Tokutomi T, Strous M, Picioreanu C, van Loosdrecht MCM. 2007. Startup of reactors for anoxic ammonium oxidation: experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 41:4149–4163 [DOI] [PubMed] [Google Scholar]
- 12. Tchobanoglous G, Burton F, Stensel H. 1991. Waste water engineering, treatment disposal and reuse, 3rd ed McGraw-Hill, Singapore, Singapore [Google Scholar]
- 13. Dosta J, Fernandez I, Vazquez-Padin JR, Mosquera-Corral A, Campos JL, Mata-Alvarez J, Mendez R. 2008. Short- and long-term effects of temperature on the Anammox process. J. Hazard. Mater. 154:688–693 [DOI] [PubMed] [Google Scholar]
- 14. Isaka K, Date Y, Kimura Y, Sumino T, Tsuneda S. 2008. Nitrogen removal performance using anaerobic ammonium oxidation at low temperatures. FEMS Microbiol. Lett. 282:32–38 [DOI] [PubMed] [Google Scholar]
- 15. Yang Q, Peng Y, Liu X, Zeng W, Mino T, Satoh H. 2007. Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities. Environ. Sci. Technol. 41:8159–8164 [DOI] [PubMed] [Google Scholar]
- 16. Hendrickx T, Wang Y, Kampman C, Zeeman G, Temmink H, Buisman C. 2012. Autotrophic nitrogen removal from low strength waste water at low temperature. Water Res. 46:2187–2193 [DOI] [PubMed] [Google Scholar]
- 17. Vazquez-Padin JR, Fernandez I, Morales N, Campos JL, Mosquera-Corral A, Mendez R. 2011. Autotrophic nitrogen removal at low temperature. Water Sci. Technol. 63:1282–1288 [DOI] [PubMed] [Google Scholar]
- 18. Hu B-L, Rush D, van der Biezen E, Zheng P, van Mullekom M, Schouten S, Damste JSS, Smolders AJP, Jetten MSM, Kartal B. 2011. New anaerobic, ammonium-oxidizing community enriched from peat soil. Appl. Environ. Microbiol. 77:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van de Vossenberg J, Rattray JE, Geerts W, Kartal B, van Niftrik L, van Donselaar EG, Damste JSS, Strous M, Jetten MSM. 2008. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production. Environ. Microbiol. 10:3120–3129 [DOI] [PubMed] [Google Scholar]
- 20. Kartal B, Tan NCG, van de Biezen E, Kampschreur MJ, van Loosdrecht MCM, Jetten MSM. 2010. Effect of nitric oxide on anammox bacteria. Appl. Environ. Microbiol. 76:6304–6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Graaf AAV, de Bruijn P, Robertson LA, Jetten MSM, Kuenen JG. 1996. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142:2187–2196 [Google Scholar]
- 22. Kartal B, Koleva M, Arsov R, van der Star W, Jetten MSM, Strous M. 2006. Adaptation of a freshwater anammox population to high salinity wastewater. J. Biotechnol. 126:546–553 [DOI] [PubMed] [Google Scholar]
- 23. Layne E. 1957. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 3:447–454 [Google Scholar]
- 24. Schmid M, Twachtmann U, Klein M, Strous M, Juretschko S, Jetten M, Metzger JW, Schleifer KH, Wagner M. 2000. Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst. Appl. Microbiol. 23:93–106 [DOI] [PubMed] [Google Scholar]
- 25. Schmid M, Schmitz-Esser S, Jetten M, Wagner M. 2001. 16S-23S rDNA intergenic spacer and 23S rDNA of anaerobic ammonium-oxidizing bacteria: implications for phylogeny and in situ detection. Environ. Microbiol. 3:450–459 [DOI] [PubMed] [Google Scholar]
- 26. Wagner M, Rath G, Amann R, Koops HP, Schleifer KH. 1995. In situ identification of ammonia-oxidizing bacteria. Syst. Appl. Microbiol. 18:251–264 [Google Scholar]
- 27. Daims H, Nielsen PH, Nielsen JL, Juretschko S, Wagner M. 2000. Novel Nitrospira-like bacteria as dominant nitrite-oxidizers in biofilms from wastewater treatment plants: diversity and in situ physiology. Water Sci. Technol. 41:85–90 [Google Scholar]
- 28. Juretschko S, Timmermann G, Schmid M, Schleifer KH, Pommerening-Roser A, Koops HP, Wagner M. 1998. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol. 64:3042–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt TM, Delong EF, Pace NR. 1991. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 173:4371–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neef A, Amann R, Schlesner H, Schleifer KH. 1998. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 144:3257–3266 [DOI] [PubMed] [Google Scholar]
- 31. Harhangi HR, Le Roy M, van Alen T, Hu B-L, Groen J, Kartal B, Tringe SG, Quan Z-X, Jetten MSM, Op den Camp HJM. 2012. Hydrazine synthase, a unique phylomarker with which to study the presence and biodiversity of anammox bacteria. Appl. Environ. Microbiol. 78:752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rotthauwe JH, Witzel KP, Liesack W. 1997. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 63:4704–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuenen JG. 2008. Anammox bacteria: from discovery to application. Nat. Rev. Microbiol. 6:320–326 [DOI] [PubMed] [Google Scholar]
- 35. Jetten MSM, Wagner M, Fuerst J, van Loosdrecht M, Kuenen G, Strous M. 2001. Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Curr. Opin. Biotechnol. 12:283–288 [DOI] [PubMed] [Google Scholar]
- 36. Alawi M, Lipski A, Sanders T, Pfeiffer EM, Spieck E. 2007. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J. 1:256–264 [DOI] [PubMed] [Google Scholar]
- 37. Horrigan SG. 1981. Primary production under the Ross Ice Shelf, Antarctica. Limnol. Oceanogr. 26:378–382 [Google Scholar]
- 38. Lam P, Kuypers MMM. 2011. Microbial nitrogen cycling processes in oxygen minimum zones. Ann. Rev. Mar. Sci. 3:317–345 [DOI] [PubMed] [Google Scholar]
- 39. Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D, Dimitri G, Amann R, Jetten MSM, Kuypers MMM. 2009. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc. Natl. Acad. Sci. U. S. A. 106:4752–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blackburne R, Yuan Z, Keller J. 2008. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 19:303–312 [DOI] [PubMed] [Google Scholar]
- 41. Schramm A, De Beer D, Gieseke A, Amann R. 2000. Microenvironments and distribution of nitrifying bacteria in a membrane-bound biofilm. Environ. Microbiol. 2:680–686 [DOI] [PubMed] [Google Scholar]
- 42. Chandran K, Stein LY, Klotz MG, van Loosdrecht MCM. 2011. Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem. Soc. Trans. 39:1832–1837 [DOI] [PubMed] [Google Scholar]
- 43. Kampschreur MJ, Tan NCG, Kleerebezem R, Picioreanu C, Jetten MSM, van Loosdrecht MCM. 2008. Effect of dynamic process conditions on nitrogen oxides emission from a nitrifying culture. Environ. Sci. Technol. 42:429–435 [DOI] [PubMed] [Google Scholar]
- 44. Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, van Loosdrecht MCM. 2009. Nitrous oxide emission during wastewater treatment. Water Res. 43:4093–4103 [DOI] [PubMed] [Google Scholar]
- 45. Tallec G, Garnier J, Billen G, Gousailles M. 2006. Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: effect of oxygenation level. Water Res. 40:2972–2980 [DOI] [PubMed] [Google Scholar]
- 46. Kampschreur MJ, van der Star WRL, Wielders HA, Mulder JW, Jetten MSM, van Loosdrecht MCM. 2008. Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res. 42:812–826 [DOI] [PubMed] [Google Scholar]