Abstract
Sulfadiazine (SDZ)-degrading bacterial cultures were enriched from the topsoil layer of lysimeters that were formerly treated with manure from pigs medicated with 14C-labeled SDZ. The loss of about 35% of the applied radioactivity after an incubation period of 3 years was attributed to CO2 release due to mineralization processes in the lysimeters. Microcosm experiments with moist soil and soil slurries originating from these lysimeters confirmed the presumed mineralization potential, and an SDZ-degrading bacterium was isolated. It was identified as Microbacterium lacus, denoted strain SDZm4. During degradation studies with M. lacus strain SDZm4 using pyrimidine-ring labeled SDZ, SDZ disappeared completely but no 14CO2 was released during 10 days of incubation. The entire applied radioactivity (AR) remained in solution and could be assigned to 2-aminopyrimidine. In contrast, for parallel incubations but with phenyl ring-labeled SDZ, 56% of the AR was released as 14CO2, 16% was linked to biomass, and 21% remained as dissolved, not yet identified 14C. Thus, it was shown that M. lacus extensively mineralized and partly assimilated the phenyl moiety of the SDZ molecule while forming equimolar amounts of 2-aminopyrimidine. This partial degradation might be an important step in the complete mineralization of SDZ by soil microorganisms.
INTRODUCTION
Sulfadiazine (SDZ) belongs to the group of sulfonamide antibiotics, and it is frequently applied in animal husbandry for the prevention of bacterial infections in livestock (1). When 14C-labeled SDZ was administered to pigs, only 4% of the radioactivity remained in the pigs after 10 days and 44% was still detectable in the collected manure as the parent substance, besides 4-hydroxy-sulfadiazine (4-OH-SDZ) and N-acetyl-sulfadiazine (Ac-SDZ) (2). Even during a longer storage of pig manure, SDZ and its principal biotransformation products were shown to be rather stable (3).
SDZ is mainly released to the soil environment either directly by grazing animals or by the use of manure from treated livestock as fertilizer on farmland (4). This poses a potential risk of soil and groundwater contamination (5). Therefore, numerous investigations concerning the mobility (6–8), sorption behavior (9), transformation (10, 11), and mineralization (12), as well as the possible effects on the soil microflora (13, 14) and the spread of antibiotic resistance genes (15), have been performed and published. Once administered to animals and released into the environment, the parent substance may be subject to abiotic and biotic transformation processes (11). The transformation products described to date (10) are generated primarily due to hydroxylation, acetylation, and formylation reactions at either the aniline or the pyrimidine moiety of the SDZ molecule. The disappearance of the parent substance and its metabolites in soil does not necessarily mean that the substances are completely degraded in the sense of mineralization. Förster et al. (16), working with pig slurry containing [14C]SDZ, [14C]4-OH-SDZ, and [14C]Ac-SDZ added to soil, showed that the easily extractable fraction (defined by using a weak extracting agent) decreased exponentially with dissipation half-lives of 16 to 11 days, depending on the soil type. The total radioactivity did not decrease, and after 210 days, about 50% was still extractable with harsh extracting agents and conditions and 50% was nonextractable residues (16). Such an observable dissipation of the parent compound is often called “degradation” in studies of the fate of environmental pollutants (17). Mineralization to carbon dioxide seems to play only a minor role, with less than 2% 14CO2 evolving from14C-labeled SDZ in microcosms in bovine manure, soil, and soil manure slurries (12, 18). However, long-term lysimeter experiments (3 years) with feces from pigs previously treated with 14C-labeled sulfadiazine indicated that presumably substantial mineralization occurred (19). The 14C mass balance revealed a recovery of only about 65% after 3 years of incubation, and this significant loss of radioactivity was attributed to 14CO2 release from the open lysimeters due to the mineralization of SDZ.
The aim of this study was to reassess the SDZ mineralization potential of topsoil taken from the aforementioned lysimeters and, further, to enrich and isolate SDZ-degrading bacteria from these soil samples. We explicitly focused on the isolation of bacteria which not only mediate the primary degradation to the biotransformation products known so far but also are able to extensively degrade or even mineralize SDZ.
MATERIALS AND METHODS
Test items and standards. (i) Species of sulfadiazines.
Nonlabeled SDZ (99.6% purity) was purchased from Riedel-de Haën, Seelze, Germany. The 14C phenyl-ring labeled SDZ ([14C]phenSDZ) had a specific radioactivity of 3.46 MBq mg−1 and was 6-fold labeled in the phenyl ring (Institute of Isotopes Co. Ltd., Budapest, Hungary). The 14C pyrimidine-ring labeled SDZ ([14C]pyrSDZ) had a specific radioactivity of 0.43 MBq mg−1 and was labeled at the C-2 position (BayerHealthCare AG, Wuppertal, Germany).
(ii) Analytical standards.
2-Aminopyrimidine (2-AP; 97% purity) was purchased from Sigma-Aldrich, Taufkirchen, Germany.
Growth conditions, media, and sampling.
All incubations were carried out at room temperature in the dark.
(i) Soil microcosms.
Microcosm experiments were conducted in hermetically closed 250-ml Duran glass bottles with topsoil from the lysimeters in duplicate. SDZ solution ([14C]pyrSDZ and nonlabeled SDZ) was prepared in acetonitrile and added to a calcinated soil aliquot. Acetonitrile was then removed by evaporation, and the spiked aliquot was thoroughly mixed with 100 g of soil to obtain final concentrations of 10 mg (40 μM) SDZ and 3.75 MBq kg−1 soil (dry matter). Afterwards, five subsamples were taken to determine the total radioactivity, and 20 g of dry matter equivalents was apportioned to each of the bottles. Moisture was adjusted to 45% maximum water-holding capacity (WHCmax) for the moist soil and to 200% WHCmax for the slurries. Slurries were shaken on a shaker at 100 rpm. CO2 was trapped in vials filled with 1.5 ml 2 N NaOH and mounted in the headspace of the flasks.
(ii) Enrichment cultures and degradation experiments.
Suspended cultures for enrichment, isolation, and degradation experiments were prepared in 250-ml Erlenmeyer flasks, and the flasks were filled with 100 ml of the respective medium and placed on a rotary shaker at 100 rpm for the incubation period. All incubation mixtures were prepared and sampled under sterile conditions by applying a sterile hood and autoclaved medium and devices. Degradation experiments with liquid growth medium were performed in 250-ml Duran glass bottles as described above for soil microcosms.
(iii) SDZ-mineral medium.
SDZ-degrading cultures were enriched in a medium containing the following per liter: [14C]SDZ or nonlabeled SDZ, 10 mg; NH4Cl, 306 mg; Na2HPO4·12 H2O, 100 mg; MgSO4·7H2O, 50 mg; CaCl2·2H2O, 15 mg; and NaHCO3, 1,130 mg. The mineral medium also contained the following trace elements per liter: MnCl2·4H2O, 99 μg; ZnSO4·7H2O, 6.3 μg; NiSO4·7H2O, 26.3 μg; CoSO4·7H2O, 2.81 μg; and CuSO4·5H2O, 4.99 μg. Finally, Fe-EDTA, 1 ml of the stock solution (EDTA [Titriplex III] at 9.3 g liter−1 and FeSO4 at 6.95 g liter−1), Sørensen buffer (pH 7), and 100 ml of the stock solution (7.268 g liter−1 Na2HPO4 plus 3.522 g liter−1 KH2PO4) were added.
(iv) SDZ medium with complex carbon sources.
Cultivation, enrichment, and isolation steps were carried out in medium spiked with 10 mg liter−1 SDZ. The solid medium used for isolation purposes was Mueller-Hinton agar, and suspended cultures were cultivated in Mueller-Hinton broth (MHB; 1/10 strength; Merck KGaA, Darmstadt, Germany). SDZ was always added to the sterilized medium after chilling to 70°C, and the medium was further agitated on a magnetic stirrer for 2 h to ensure the exhaustive solution of SDZ.
(v) Preparation of samples.
Cell-free samples of bacterial cultures were obtained by centrifugation at 20,000 × g and careful removal of the supernatants. Pellets were washed twice in mineral medium and resuspended before measuring the 14C radioactivity.
Analytical methods. (i) LSC.
Liquid-phase 14C radioactivity was measured by means of liquid scintillation counting (LSC; 2500 TR, Packard Bioscience, Dreieich, Germany). One-milliliter aliquots of the samples were mixed with 10 ml scintillation cocktail (Instant Scint Gel Plus; Canberra Packard, Dreieich, Germany) and 4 ml deionized water. The detection limit was 0.4 Bq, and the limit of quantification was 1.2 Bq.
(ii) Spectrophotometry.
For single-wavelength absorption measurements and absorption scans, a multiplate reader was applied (SoftMax Pro; Molecular Devices Corporation, Sunnyvale, CA). Culture supernatants were screened for dissipation of SDZ by measuring the decrease of UV absorption at 267 nm.
(iii) HPLC.
The high-pressure liquid chromatography (HPLC) system was equipped with an autosampler, binary pump, reversed-phase C18 column (250 by 4.6 mm, 5 μm; Synergi Fusion RP 80; Phenomenex, Aschaffenburg, Germany), diode array detector (UV-D 340S; Dionex, Idstein, Germany), and radio detector (LB 509; Berthold Technologies, Bad Wildbad, Germany). Solvent A was a mixture of water-methanol (49:1) buffered with 1 ml liter−1 phosphoric acid (25%), and solvent B was pure methanol. The elution was performed at a flow rate of 1 ml min−1 and a linear gradient program as follows: 0, 0, 27, 37, 47, 57, 100, and 100% solvent B at 0, 6, 23, 26, 28, 30, 33, and 35 min, respectively. The UV detector was set at 267 nm for SDZ detection and at 298 nm for 2-aminopyrimidine detection.
(iv) HPLC-APCI-MS/MS.
Liquid chromatography (LC)-atmospheric pressure chemical ionization (APCI)-tandem mass spectrometry (MS/MS) was performed with an Agilent 1100 series HPLC (Santa Clara, CA) equipped with an HTC PAL autosampler (CTC Analytics, Zwingen, Switzerland), a binary pump, and a thermostated column oven (40°C) coupled with a Thermo Electron (Waltham, MA) TSQ Quantum triple-quadrupole mass spectrometer. LC separations and MS detection were carried out as described previously (10).
(v) Data analysis.
The measured production of 2-AP was fitted with a logistic function (20) which was generalized. This function accounts for an initial stage of approximately exponential growth of the population, followed by a second stage characterized by limited substrate availability:
| (1) |
where f0 is the initial population, G is the upper limit of the population, and k is the proportionality constant. We assume that the production of 2-AP is directly related to the population of the degrading bacterium. Analogously, the decomposition of SDZ was fitted with a mirrored curve of equation 1, given by
| (2) |
The turning point time (tt), which was characterized by the maximum growth rate and which separated the initial and the consecutive second stage, was defined as
| (3) |
At tt, half of the amount of SDZ is transformed to 2-AP.
(vi) Identification of the isolated strain.
Identification of the isolated strain was conducted by Nadicom GmbH (Karlsruhe, Germany). The primers used were 27f and 1492r, corresponding to the numbering of the Escherichia coli 16S rRNA gene. The amplification products were separated by means of gel electrophoresis in 1% agarose in the presence of a molecular mass standard, followed by ethidium bromide staining. After cleanup and sequencing, the sequence showed 98.96% accordance with the reference sequence of the type strain Microbacterium lacus DSM 18910. Therefore, the isolate was named Microbacterium lacus strain SDZm4, since a cutoff of 98.7% 16S rRNA gene homology should be appropriate for the differentiation of species within a genus (21). The isolate will be made available upon request by the corresponding author.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of M. lacus strain SDZm4 was deposited in the EMBL database under accession number HF571530.
RESULTS
Microcosm experiments with topsoil samples from lysimeters.
Soils from lysimeters expected to show high microbial activity with respect to the mineralization of SDZ were used for degradation experiments in microcosms. In batch experiments with randomly mixed topsoil subsamples, the addition of [14C]pyrSDZ was directly followed by the release of 14CO2 (Fig. 1).
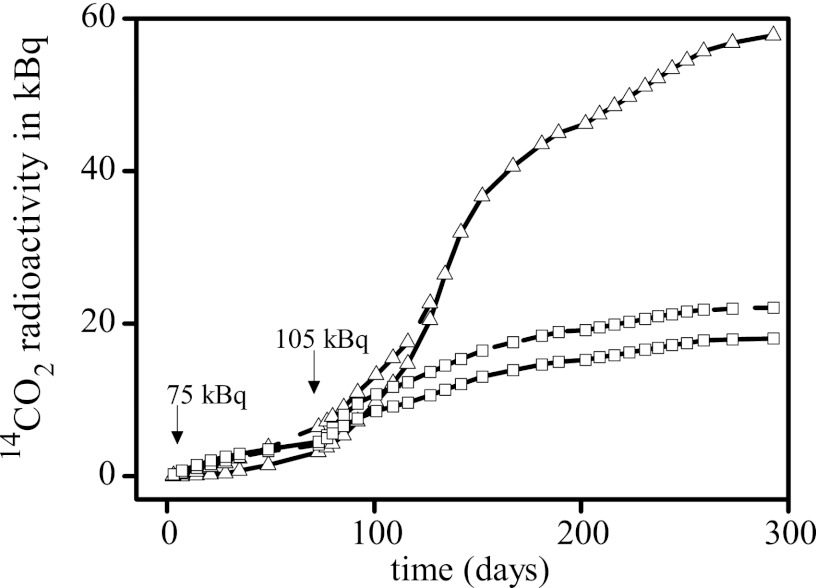
Fig 1.
Cumulative release of 14CO2 radioactivity during incubation of lysimeter topsoil in duplicate samples. Squares, moist soil; triangles, soil slurries. The times of addition of radioactivity and the amounts of radioactivity added are indicated by arrows. The break in one duplicate of the slurry incubation at day 125 is due to the accidental release of alkali from the CO2 trap.
An additional dose of [14C]pyrSDZ after 72 days caused a further increase in 14CO2 release. Duplicates of moist soil and soil slurries were run in parallel, and finally, after 300 days, levels of mineralization were about 12% and up to 32%, respectively. These were far more than the levels reported in the literature for the mineralization of SDZ in field and laboratory studies (12, 18). Furthermore, the soil slurries showed a continuous increase in 14CO2 release, probably indicating an adaptation, an enrichment, and/or the selection of SDZ-degrading bacteria in these microcosms (Fig. 1).
Enrichment and isolation of SDZ-degrading bacteria.
Soil slurries from the microcosm experiments and unlabeled SDZ were applied for the enrichment and selection of SDZ degraders. Because of the numerous cultivation steps and variations, a simple screening method was applied to detect SDZ-degrading cultures. Biotransformation products of SDZ known so far (10) are basically still sulfadiazines which merely differ in their functional groups (N-acetyl-4-hydroxy-sulfadiazine, N-acetyl-sulfadiazine, N-formyl-sulfadiazine, 4-hydroxy-sulfadiazine). For this reason, SDZ and its biotransformation products likewise were easily detectable in culture supernatants due to their UV absorption at about 267 nm. A decrease in UV absorption at 267 nm should therefore indicate degradation of SDZ. This relationship was applied to conduct a fast screening of the culture supernatants on microplates. Several enrichment cultures were positive in that respect, and SDZ degradation accelerated during 3 to 4 consecutive cultivation cycles in unlabeled SDZ-mineral medium but then suddenly ceased. However, some of these cultures regained their degradation potential after amendment with a complex carbon source (MHB). In most cases, however, the addition of even small amounts of complex medium diminished the selective pressure toward SDZ degraders and promoted the growth of any other bacteria. Among 22 pure cultures cultivated in MHB medium containing 40 μM SDZ, only one isolate was able to degrade SDZ. It was identified as Microbacterium lacus and internally designated M. lacus strain SDZm4.
Degradation of SDZ by Microbacterium lacus strain SDZm4.
In order to test whether 40 μM SDZ did not inhibit bacterial growth in our experiments, we doubled the concentration in subsequently conducted batch growth experiments. The specific maximum growth rate (μmax) of M. lacus strain SDZm4 in 1/10 MHB was 0.035 h−1 (25°C), and it was not affected by SDZ up to the highest concentration tested (see Table S1 in the supplemental material).
Further batch experiments were run with M. lacus strain SDZm4 and [14C]pyrSDZ in order to prove the capability of this strain to ultimately degrade SDZ. However, despite the decrease in the absorbance at 267 nm in the culture supernatants, there was no significant release of 14CO2 detected. The entire radioactivity applied remained in solution, although [14C]pyrSDZ completely disappeared (below the detection limit of the HPLC). The decrease in absorption at 267 nm therefore reliably indicated the degradation of SDZ and probably the further degradation of its biotransformation products. However, this was no proof for the mineralization of SDZ. For this reason, instead of absorption measurements at a fixed wavelength (267 nm), UV absorption scans (200 to 400 nm) were accomplished. They clearly indicated the presence of at least one other metabolite with two absorption maxima at 223 nm and 298 nm (Fig. 2).
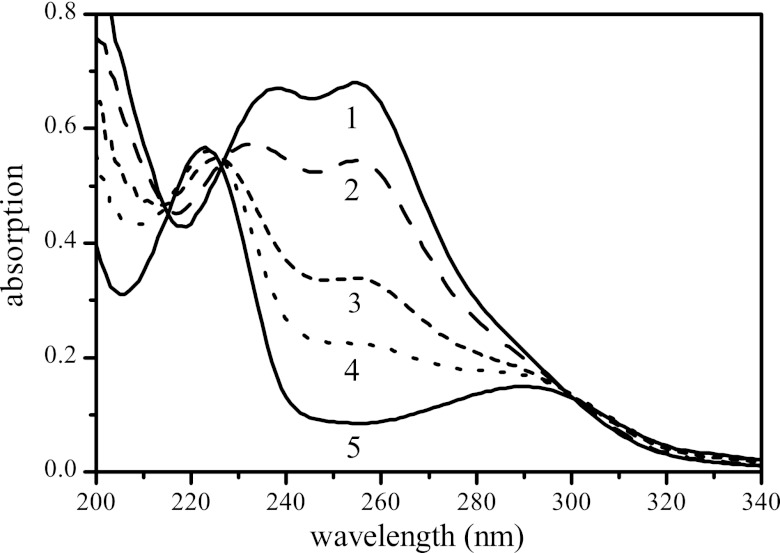
Fig 2.
UV absorption spectra of M. lacus strain SDZm4 culture supernatants in mineral medium. Selected spectra were taken at different subsequent time intervals during the degradation of SDZ (curves 1 to 5). Curve 1, SDZ; curve 5, 2-aminopyrimidine; curves 2 to 4, intermediary-state spectra.
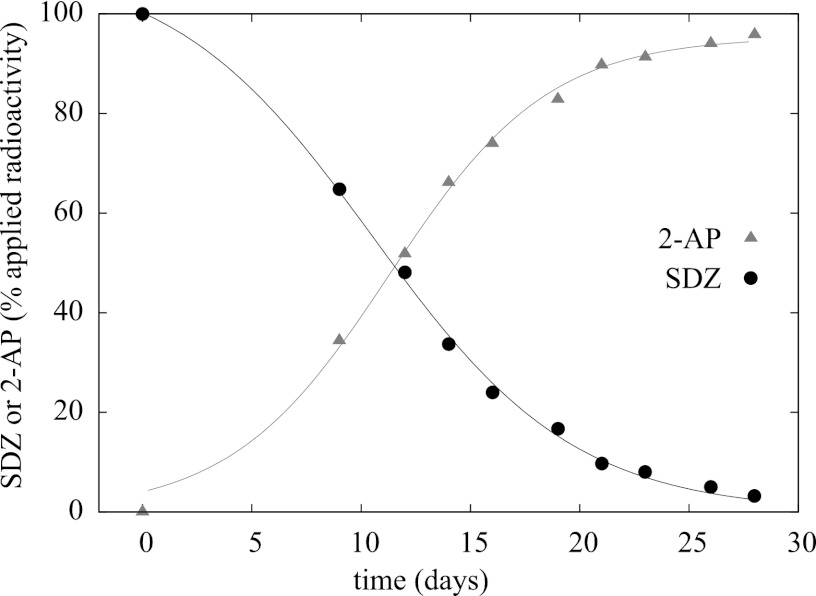
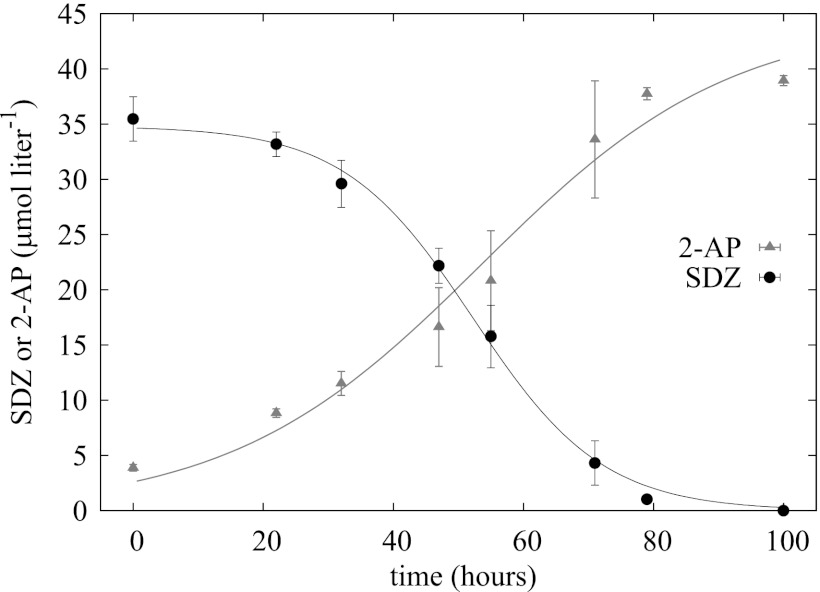
Mass spectrometric analysis of culture supernatants strongly indicated the presence of substantial amounts of 2-aminopyrimidine (2-AP). Additionally, the UV spectra of 2-AP as a reference substance were completely in line with the spectra emerging from M. lacus strain SDZm4 cultures during the dissipation of SDZ. As shown with a radio-HPLC detector, the amount of [14C]2-AP formed during the incubation exactly matched the amount of parent substance ([14C]pyrSDZ) lost (Fig. 3). The incubations were done in triplicate, but the kinetics were considerably different between the parallel assays. However, the qualitative results concerning the dissipation of SDZ and the simultaneous appearance of 2-AP were the same in each incubation. The results of one example of an incubation in mineral medium without an additional carbon and energy source, except for SDZ, are shown in Fig. 3. In contrast, the growth kinetics of M. lacus strain SDZm4 were highly reproducible in medium with additional carbon sources (MHB, 1/10 strength), and the transformation of SDZ to 2-AP was fast (50% was metabolized after about 53 h) and complete (Fig. 4).
Fig 3.
Percentage of radioactivity applied to M. lacus strain SDZm4 culture supernatants in SDZ-amended mineral medium separated into 14C radioactivity of SDZ and 2-AP measured with a radio-HPLC detector. 2-AP and SDZ radioactivities were fitted with equation 1 and equation 2, respectively. tts (see equation 3) were 11.3 days for 2-AP and 10.5 days for SDZ.
Fig 4.
Concentrations of SDZ and 2-AP in M. lacus strain SDZm4 culture supernatants incubated in SDZ-amended Mueller-Hinton broth (1/10 strength plus 40 μmol l−1 SDZ). The SDZ concentration at time zero is reduced due to dilution with a SDZ-free but 2-AP-containing bacterial inoculum (10% of the culture volume); for the same reason, 2-AP was already detectable at time zero. 2-AP and SDZ concentrations were fitted with equation 1 and equation 2, respectively. tts (see equation 3) were 53.5 h for 2-AP and 52.3 h for SDZ.
Due to the equimolar amounts of the parent substance (SDZ) that decreased and of 2-AP that appeared during the incubations, the occurrence of further biotransformation products was rather unlikely. The LC-MS/MS analysis of the supernatants with a focus on the already known biotransformation products ensued, and as before, no evidence for the existence of further biotransformation products was obtained. Concerning the fate of the unlabeled aniline moiety, there was no aniline detectable, but this alone was not proof for the complete degradation of this part of the molecule. However, even though the ability of M. lacus strain SDZm4 to degrade SDZ to 2-AP was clearly demonstrated, the fate of the residual molecule is unclear at this point. All experiments (including the long-term lysimeter studies providing the starting point for this work) were carried out with pyrimidine ring-labeled SDZ ([14C]pyrSDZ).
Degradation of SDZ with different labels by M. lacus strain SDZm4.
Application of an SDZ species with the 14C label in the phenyl moiety ([14C]phenSDZ) instead of pyrimidine-labeled SDZ allowed investigation of the fate of the aniline part of the SDZ molecule (Table 1). For this purpose, culture aliquots from the early stationary phase were transferred to fresh 1/10 MHB–SDZ medium containing either [14C]phenSDZ or [14C]pyrSDZ. The proportions of radioactivity associated with the radiolabeled parent substance, metabolite, trapped 14CO2, and biomass are given in Table 1 for both radiolabeled species of SDZ. In contrast to the approaches involving [14C]pyrSDZ, for the trials with [14C]phenSDZ, more than half of the applied radioactivity was found in the CO2 traps (56%), 16% was associated with biomass, and 21% remained in solution and was not yet identified. Three weeks later, the radioactivity in solution was still 18% of the applied radioactivity, indicating a certain degree of recalcitrance of these unknown substances. For [14C]pyrSDZ, the results clearly confirmed the former findings that the amount of 2-AP that was formed was the same as the amount of parent substance (SDZ) that decreased at any time. Using the mean values of [14C]phenSDZ (Table 1), a carbon balance was calculated by setting the proportion of SDZ carbon equal to 100%. The balance revealed that most of the carbon was metabolized to 2-AP (40%), followed by 34% CO2 C (see Table S2 in the supplemental material).
Table 1.
Distribution of 14C radioactivity in duplicate batch cultures (A and B) of M. lacus strain SDZm4 after complete depletion of SDZa
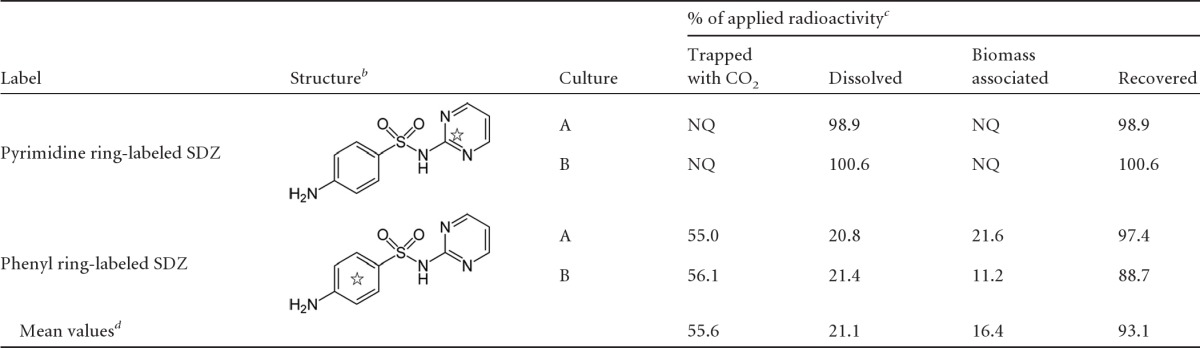
SDZ (40 μM) with two different 14C labels in 1/10 Mueller-Hinton broth.
☆, radiolabel.
NQ, not quantified.
Mean values for phenyl ring-labeled SDZ.
DISCUSSION
Studies of SDZ degradation published so far mainly refer to the dissipation of the parent substance in the respective environments or test systems (22–24) and to the identification of transformation products (23, 25, 26). Mineralization appears to be insignificant, since experiments with 14C-labeled SDZ revealed that only a small fraction (less than 2%) was mineralized to CO2 within moderate time periods (maximum, 218 days) (18). However, soil from lysimeters that had received manure from SDZ-medicated pigs 3 years earlier showed substantial mineralization activity for SDZ in this study (Fig. 1). This indicates that microbial adaptation and selection processes require reasonably long time periods of exposure to take place in pristine soils. Long-term as well as repeated exposure has often been reported to enhance the biodegradation of xenobiotics or other poorly degradable substances like atrazine in soils (27, 28). After long-term and repeated exposure of agricultural soils to veterinary antibiotics, accelerated biodegradation and partial mineralization of sulfamethazine were detectable (29). Untreated control soils showed no degradation of the applied sulfamethazine. Although microbial degradation of sulfonamides (24, 30) and even the utilization of sulfonamides as the only carbon and energy source have been reported (31), detailed knowledge concerning the bacterial strains involved and particularly the extent to which degradation is accomplished is still lacking. Just one study providing profound information about both topics, the microbial sulfonamide degradation and the bacteria involved, was quite recently published by Topp et al. (29). They enriched and isolated a sulfamethazine-degrading Microbacterium sp. from arable soils that had been treated with mixtures of antibiotics (including sulfamethazine) for 10 years.
Microbacteria are widely distributed in many environments (32), namely, marine sediments (33), compost (34), activated sludge (35), wastewater (36), infected human blood (37), cheese (38), arable soils (29; this study), and many more. Accordingly, Microbacterium species are involved in diverse biodegradation reactions and in oxidation and reduction reactions (32, 36). Interestingly, our enrichment and selection efforts also resulted in the isolation of a Microbacterium species. Due to the slow growth of M. lacus strain SDZm4, the enrichment in complex (nonselective) medium probably failed, and separate colonies were accessible only from enrichments in carbon-free mineral medium with SDZ as the only source of nutrients and energy. Most of the fast-growing bacteria were obviously thinned out during consecutive batch cycles in this medium, and analysis for metabolites could be more easily performed because of the very low background level of organic substances. However, SDZ degradation became unstable and unreproducible after several batches in mineral medium, indicating a lack of essential growth factors. Coryneform bacteria are considered to be halotolerant (39), and some of them require vitamins, mostly B vitamins, such as pantothenic acid, as has been shown for M. lacticum (40), or thiamine, biotin, and pantothenic acid, which are necessary for the optimal growth of M. gubbeenese DPC 5286T (38). However, once cultivated in pure culture without competing fast-growing bacterial contaminants, the M. lacus SDZm4 strain isolated in this study was easily culturable in nonselective complex medium. Growth was highly reproducible in Mueller-Hinton broth, and even the highest concentration of SDZ applied (80 μM) did not affect the growth kinetics (see Table S1 in the supplemental material). During SDZ degradation by M. lacus strain SDZm4, none of the many other metabolites described for SDZ in soils could be detected by HPLC or LC-MS/MS. If other metabolites were present at all, only trace amounts were conceivable because 2-AP increased in stoichiometric amounts as the amounts of SDZ decreased. This finding is in accordance with the results of Topp et al. (29) for a sulfamethazine-degrading Microbacterium sp. (strain C448): their isolate likewise mineralized the benzylic portion of the molecule, and the pyrimidine portion was excreted as the end product. The almost concurrent and independent isolation of two bacterial strains belonging to the genus Microbacterium from soils with a history of the presence of sulfonamides (29; this study) strongly indicates that this genus might play an important role within the sulfonamide-degrading soil microbial community.
ACKNOWLEDGMENTS
This study was supported in part by the Deutsche Forschungsgemeinschaft (FOR566, Veterinary Medicines in Soils: Basic Research for Risk Analysis).
We thank Martina Krause and Ulrike Langen for their helpful assistance in the isotope lab.
Footnotes
Published ahead of print 8 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03636-12.
REFERENCES
- 1. Sarmah AK, Meyer MT, Boxall ABA. 2006. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (Vas) in the environment. Chemosphere 65:725–759 [DOI] [PubMed] [Google Scholar]
- 2. Lamshöft M, Sukul P, Zühlke S, Spiteller M. 2007. Metabolism of 14C-labelled and non-labelled sulfadiazine after administration to pigs. Anal. Bioanal. Chem. 388:1733–1745 [DOI] [PubMed] [Google Scholar]
- 3. Lamshöft M, Sukul P, Zühlke S, Spiteller M. 2010. Behaviour of 14C-sulfadiazine and 14C-difloxacin during manure storage. Sci. Total Environ. 408:1563–1568 [DOI] [PubMed] [Google Scholar]
- 4. Jørgensen SE, Halling-Sørensen B. 2000. Drugs in the environment. Chemosphere 40:691–699 [DOI] [PubMed] [Google Scholar]
- 5. Boxall ABA, Fogg LA, Kay P, Blackwell PA, Pemberton EJ, Croxford A. 2003. Prioritisation of veterinary medicines in the UK environment. Toxicol. Lett. 142:207–218 [DOI] [PubMed] [Google Scholar]
- 6. Burkhardt M, Stamm C, Waul C, Singer H, Müller S. 2005. Surface runoff and transport of sulfonamide antibiotics and tracers on manured grassland. J. Environ. Qual. 34:1363–1371 [DOI] [PubMed] [Google Scholar]
- 7. Aust MO, Thiele-Bruhn S, Seeger J, Godlinski F, Meissner R, Leinweber P. 2010. Sulfonamides leach from sandy loam soils under common agricultural practice. Water Air Soil Pollut. 211:143–156 [Google Scholar]
- 8. Stoob K, Singer HP, Mueller SR, Schwarzenbach RP, Stamm CH. 2007. Dissipation and transport of veterinary sulfonamide antibiotics after manure application to grassland in a small catchment. Environ. Sci. Technol. 41:7349–7355 [DOI] [PubMed] [Google Scholar]
- 9. Wehrhan A, Kasteel R, Sǐmùnek J, Groeneweg J, Vereecken H. 2007. Transport of sulfadiazine in soil columns: experiments and modelling approaches. J. Contam. Hydrol. 89:107–135 [DOI] [PubMed] [Google Scholar]
- 10. Sukul P, Lamshöft M, Zühlke S, Spiteller M. 2008. Photolysis of 14C-sulfadiazine in water and manure. Chemosphere 71:717–725 [DOI] [PubMed] [Google Scholar]
- 11. Unold M, Šimùnek J, Kasteel R, Groeneweg J, Vereecken H. 2009. Transport of manure-based applied sulfadiazine and its main transformation products in soil columns, Vadose Zone J. 8:677–689 [Google Scholar]
- 12. Kreuzig R, Höltge S. 2005. Investigations on the fate of sulfadiazine in manured soil: laboratory experiments and test plot studies. Environ. Toxicol. Chem. 24:771–776 [DOI] [PubMed] [Google Scholar]
- 13. Zielezny I, Groeneweg J, Vereecken H, Tappe W. 2006. Impact of sulfadiazine and chlorotetracycline on soil bacterial community structure and respiratory activity. Soil Biol. Biochem. 38:2372–2380 [Google Scholar]
- 14. Hammesfahr U, Kotzerke A, Lamshöft M, Wilke BM, Kandeler E, Thiele-Bruhn S. 2011. Sulfadiazine contaminated fresh and stored manure modifies the function and structure of soil microbial community. Eur. J. Soil Biol. 47:61–68 [Google Scholar]
- 15. Heuer H, Solehati Q, Zimmerling U, Kleineidam K, Schloter M, Müller T, Focks A, Thiele-Bruhn S, Smalla K. 2011. Accumulation of sulfonamide resistance genes in arable soils due to repeated application of manure containing sulfadiazine. Appl. Environ. Microbiol. 77:2527–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Förster M, Laabs V, Lamshöft M, Groeneweg J, Zühlke S, Spiteller M, Krauss M, Kaupenjohann M, Amelung W. 2009. Sequestration of manure applied sulfadiazine residues in soils. Environ. Sci. Technol. 43:1824–1830 [DOI] [PubMed] [Google Scholar]
- 17. Katagi T. 2006. Behavior of pesticides in water-sediment systems. Rev. Environ. Contam. Toxicol. 187:133–251 [DOI] [PubMed] [Google Scholar]
- 18. Schmidt B, Ebert J, Lamshöft M, Thiede B, Schumacher-Buffel R, Ji R, Corvini F-X, Schäffer A. 2008. Fate in soil of 14C-sulfadiazine residues contained in manure of young pigs which were treated with the veterinary antibiotic. J. Environ. Sci. Health Part B 43:8–20 [DOI] [PubMed] [Google Scholar]
- 19. Schulte-Hunsbeck W. 2009. Master's thesis. Ruhr-Universität, Bochum, Germany [Google Scholar]
- 20. Britton NF. 2005. Essential mathematical biology, p 11–12, 3rd printing Springer, London, United Kingdom [Google Scholar]
- 21. Stackebrandt E, Ebers J. 2006. Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today 33:152–155 [Google Scholar]
- 22. Yang JF, Ying GG, Yang LH, Zhao JL, Liu F, Tao R, Yu ZQ, Peng PA. 2009. Degradation behaviour of sulfadiazine in soils under different conditions. J. Environ. Sci. Health Part B 44:241–248 [DOI] [PubMed] [Google Scholar]
- 23. Mohring SAI, Strzysch I, Fernandes MR, Kiffmeyer TK, Tuerk J, Hamscher G. 2009. Degradation and elimination of various sulfonamides during aerobic fermentation: a promising step on the way to sustainable pharmacy? Environ. Sci. Technol. 43:2569–2574 [DOI] [PubMed] [Google Scholar]
- 24. Walker N. 1978. A soil Flavobacterium sp. that degrades sulphanilamide and asulam. J. Appl. Bacteriol. 45:125–129 [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann T, Hofmann D, Klumpp E, Küppers S. 2011. Electrochemistry-mass spectrometry for mechanistic studies and simulations of oxidation processes in the environment. Anal. Bioanal. Chem. 399:1859–1868 [DOI] [PubMed] [Google Scholar]
- 26. Accinelli C, Koskinen WC, Becker JM, Sadowsky MJ. 2007. Environmental fate of two sulfonamide antimicrobial agents in soil. J. Agric. Food Chem. 55:2677–2682 [DOI] [PubMed] [Google Scholar]
- 27. Martinazzo R, Jablonowski ND, Hamacher G, Dick DP, Burauel P. 2010. Accelerated degradation of 14C-atrazine in Brazilian soils from different regions. J. Agric. Food Chem. 58:7864–7870 [DOI] [PubMed] [Google Scholar]
- 28. Zablotowicz RM, Krutz LJ, Reddy KN, Weaver MA, Koger CH, Locke MA. 2007. Rapid development of enhanced atrazine degradation in a Dundee silt loam soil under continuous corn and in rotation with cotton. J. Agric. Food Chem. 55:852–859 [DOI] [PubMed] [Google Scholar]
- 29. Topp E, Chapman R, Devers-Lamrani M, Hartmann A, Marti R, Martin-Laurent F, Sabourin L, Scott A, Sumarah M. 2013. Accelerated biodegradation of veterinary antibiotics in agricultural soil following long-term exposure, and isolation of a sulfamethazine-degrading Microbacterium sp. J. Environ. Qual. 42:173–178 [DOI] [PubMed] [Google Scholar]
- 30. Zhang WW, Wen YY, Niu ZL, Yin K, Xu DX, Chen LX. 2012. Isolation and characterization of sulfonamide degrading bacteria Escherichia sp. HS21 and Acinetobacter sp. HS51. World J. Microbiol. Biotechnol. 28:447–452 [DOI] [PubMed] [Google Scholar]
- 31. Dantas G, Sommer MOA, Oluwasegun RD, Church GM. 2008. Bacteria subsisting on antibiotics. Science 320:100–103 [DOI] [PubMed] [Google Scholar]
- 32. Evtushenko LI, Takeuchi M. 2006. The family Microbacteriaceae, p 1020–1098 In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E. (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed, vol 3 Springer, New York, NY [Google Scholar]
- 33. Kageyama A, Takahashi Y, Matsuo Y, Adachi K, Kasai H, Shizuri Y, Omura S. 2007. Microbacterium flavum sp. nov. and Microbacterium lacus sp. nov., isolated from marine environments. Actinomycetologica 21:53–58 [DOI] [PubMed] [Google Scholar]
- 34. Vaz-Moreira I, Lopes AR, Faria C, Spröer C, Schumann P, Nunes OC, Manaia CM. 2009. Microbacterium invictum sp. nov., isolated from homemade compost. Int. J. Syst. Evol. Microbiol. 59:2036–2041 [DOI] [PubMed] [Google Scholar]
- 35. Chen JA, Li X, Li J, Cao J, Qiu Z, Zhao Q, Xu C, Shu W. 2007. Degradation of environmental endocrine disruptor di-2-ethylhexyl phthalate by a newly discovered bacterium, Microbacterium sp. strain CQ0110Y. Appl. Microbiol. Biotechnol. 75:676–682 [DOI] [PubMed] [Google Scholar]
- 36. Kim DW, Heinze TM, Kim BS, Schnackenberg LK, Woodling KA, Sutherland JB. 2011. Modification of norfloxacin by a Microbacterium sp. strain isolated from a wastewater treatment plant. Appl. Environ. Microbiol. 77:6100–6108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laffineur K, Avesani V, Cornu G, Charlier J, Janssens M, Wauters G, Delmée M. 2003. Bacteremia due to a novel Microbacterium species in a patient with leukemia and description of Microbacterium paraoxydans sp. nov. J. Clin. Microbiol. 41:2242–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mounier J, Rea MC, O'Connor PM, Fitzgerald GF, Cogan TM. 2007. Growth characteristics of Brevibacterium, Corynebacterium, Microbacterium, and Staphylococcus spp. isolated from surface-ripened cheese. Appl. Environ. Microbiol. 73:7732–7739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frings E, Kunte HJ, Galinsky EA. 1993. Compatible solutes in representatives of the genera Brevibacterium and Corynebacterium: occurrence of tetrahydropyrimidines and glutamine. FEMS Microbiol. Lett. 109:25–32 [Google Scholar]
- 40. Dötsch RN, Pelczar MJ. 1949. The growth of Microbacterium lacticum in a chemically defined medium J. Bacteriol. 57:61–62 [DOI] [PubMed] [Google Scholar]