Abstract
Loop-mediated isothermal amplification (LAMP) is an alternative amplification technology which is highly sensitive and less time-consuming than conventional PCR-based methods. Three LAMP assays were developed, two for detection of species of symbiotic blue stain fungi associated with Ips acuminatus, a bark beetle infesting Scots pine (Pinus sylvestris), and an additional assay specific to I. acuminatus itself for use as a control. In common with most bark beetles, I. acuminatus is associated with phytopathogenic blue stain fungi involved in the process of exhausting tree defenses, which is a necessary step for the colonization of the plant by the insect. However, the identity of the main blue stain fungus vectored by I. acuminatus was still uncertain, as well as its frequency of association with I. acuminatus under outbreak and non-outbreak conditions. In this study, we employed LAMP technology to survey six populations of I. acuminatus sampled from the Southern Alps. Ophiostoma clavatum was detected at all sampling sites, while Ophiostoma brunneo-ciliatum, reported in part of the literature as the main blue stain fungus associated with I. acuminatus, was not detected on any of the samples. These results are consistent with the hypothesis that O. clavatum is the main blue stain fungus associated with I. acuminatus in the Southern Alps. The method developed in the course of this work provides a molecular tool by which it will be easy to screen populations and derive important data regarding the ecology of the species involved.
INTRODUCTION
Bark beetles (Coleoptera: Curculionidae, Scolytinae) are among the most economically and ecologically important pests of conifer forests (1, 2). One interesting characteristic of these insects is their widespread mutualistic association with ascomycete fungi, which are transported by the beetles and are important for insect establishment and development in the host plant (3, 4). Some of the fungal species have a directly mutualistic interaction with their vector, serving as nourishment to the larvae in the form of edible hyphae, spores, and exudates (5). Other fungal species are instead thought to be involved in the process of stimulation and exhaustion of plant defenses, which is a necessary step for the insects to overcome host tree resistance and colonize the plant (6). In the latter case, transported fungi are often tree-pathogenic species belonging to the morphologically homogenous group of the ophiostomatoid fungi, also referred to as blue stain fungi (3).
There are several factors that can lead a bark beetle population to rise from a latent non-outbreak phase to a high-density outbreak phase (7, 8); among these factors, host plant resistance is one of the most important (2, 9). However, even though symbiotic blue stain fungi are known to interact with host plant resistance (3, 6), very little is known about their role in insect population dynamics (but see references 2 and 10). In particular, almost no information is available about the possibility that symbiotic blue stain fungi may differ in composition and frequency under bark beetle outbreak and non-outbreak conditions (3). Solheim suggested that aggressive blue stain tree pathogens may play an important role in the initiation and development of bark beetle outbreaks, and they are therefore predicted to occur at a higher frequency in outbreaks than in non-outbreak periods (11, 12). However, this hypothesis has been contradicted by the results of some studies (13, 14) and needs to be investigated further.
The pine engraver beetle Ips acuminatus (Gyll.) is a small bark beetle infesting the thin bark of Scots pine (Pinus sylvestris L.) throughout Europe (15). It is associated with a complex of symbiotic fungi which includes the obligate nutritional fungus Hyalorhinocladiella macrospora (Franke-Grosm.) Harr. (syn. Ambrosiella macrospora Batra) (16–18) and some weakly phytopathogenic blue stain fungi involved in the exhaustion of host plant defenses (3, 6, 19–21). Among these blue stain species, the literature reports a specific fungus which is more consistently associated with the bark beetle vector (17, 22), but the identity of this fungus is still uncertain. Initial records described Ophiostoma clavatum Math.-Käärik as the main associated species (17, 22–25), while subsequently—and without confuting the previous records—many authors reported Ophiostoma brunneo-ciliatum Math. (19–21). This uncertainty could be related to spatial variation in the composition of the symbiotic complex at different sites or to variation in the insect-fungus association according to the epidemic phase of the insect population (11). Alternatively, the physiological and morphological similarities of O. clavatum and O. brunneo-ciliatum (22, 24) may have led to an erroneous species identification. To begin to resolve the uncertainty in this area, efficient methods are required for the accurate identification of blue stain fungi in the insect vector.
Identification of blue stain fungi on the basis of morphological characteristics can be problematic, since species are characterized by simple morphology and many overlapping features (3). The introduction of DNA-based methods has therefore been essential for the correct identification of blue stain fungal species (e.g., see reference 26); however, methods for routine species-specific detection of O. clavatum and O. brunneo-ciliatum have not been reported to date. In recent years, PCR-based methods, for instance multiplex and real-time PCR, have been developed to detect other fungal species directly in their insect vectors (e.g., see references 27 to 29); however, methods based on PCR can be time-consuming and require the extraction of high-quality DNA due to the effects of inhibitors on PCR sensitivity (30, 31). Loop-mediated isothermal amplification (LAMP) is an alternative amplification technology (32) which is highly sensitive, less time-consuming than conventional PCR-based methods, and less prone to inhibition from DNA preparations (33). Amplification by LAMP involves the use of four primers (two internal and two external) and relies on autocycling DNA synthesis by a DNA polymerase with high strand displacement activity. Both the forward and backward inner primers contain two distinct sequences each, corresponding to the sense and the antisense sequences of the target DNA. Amplification products are characterized by the fact that they contain loop regions to which further primers can bind, allowing the amplification to continue isothermally (32). The speed of the reaction is accelerated using additional loop primers that bind to those loops which are of the inverse orientation to the loops to which the internal primers bind (34). LAMP products consist of stem-loop DNAs with numerous inverted repeats of the target in cauliflower-like structures with multiple loops. Like PCR, LAMP reactions can be monitored in real time using intercalating fluorescent dyes (32, 35).
The aims of our study were therefore (i) to develop real-time LAMP assays for the species-specific detection of O. clavatum and O. brunneo-ciliatum directly in the insect vector, and an assay specific to I. acuminatus to be used as an internal control (36); (ii) to use these assays to assess which of these species is the blue stain fungus more consistently associated with I. acuminatus in the Southern Alps; and (iii) to investigate whether outbreak and nonoutbreak populations of I. acuminatus show significant differences in the occurrence of these symbiotic blue stain fungi.
MATERIALS AND METHODS
Sample collection and biological material for assay development.
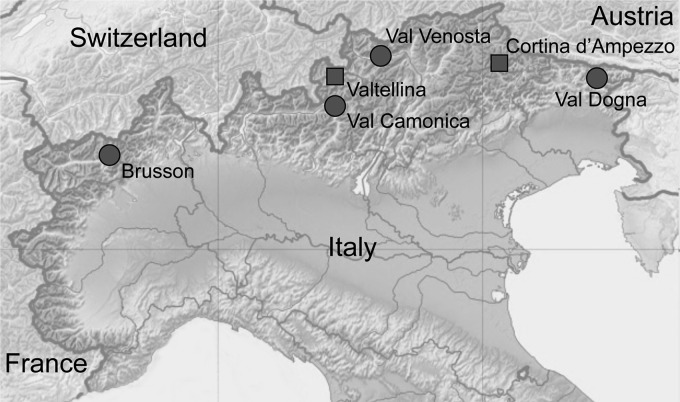
Logs (40 cm long) of Scots pine recently infested by I. acuminatus were collected in winter 2009 from six sites along the Southern Alps, in Italy (Fig. 1). Valtellina and Cortina d'Ampezzo were identified as outbreak population sites on the basis of recently recorded damage on a large scale caused by I. acuminatus (15, 37). The four non-outbreak sites were selected on the basis of reports provided by the Forest Services as locations where there had been only isolated occurrences of small numbers of affected trees. At each site, logs were collected from two different trees, except at Brusson, where only one tree was sampled. After collection, logs were transferred to the University of Padua and kept in individual outdoor rearing cages for insect development. All newly emerged adults were immediately collected, morphologically identified (38), and stored singly at −80°C prior to testing.
Fig 1.
Map of the sampling sites of outbreak populations (squares) and nonoutbreak populations (circles) of Ips acuminatus. The base map is modified from a Wikimedia Commons file originally created by Eric Gaba and NordNordWest and republished under the Creative Commons Attribution-Share Alike 3.0 Unported license.
To develop the O. clavatum and O. brunneo-ciliatum LAMP assays, and test their specificity, 17 isolates of 11 different fungal species were tested (Table 1). Isolates of O. brunneo-ciliatum, Ceratocystis montium (Rumb.) J. Hunt, and H. macrospora were purchased from Centraalbureau voor Schimmelcultures (Utrecht, Netherlands). Isolates of O. clavatum, deposited in the culture collection of the Forestry and Agricultural Biotechnology Institute, University of Pretoria, South Africa, were isolated from I. acuminatus samples collected in Italy and Sweden and identified on the basis of morphological and molecular features. All other fungal isolates belonged to the collection of the Dipartimento di Biotecnologie Agrarie, University of Florence, Italy (39, 40). Species were chosen for their association with I. acuminatus (3) or for their occurrence on Pinus spp. (3, 39, 40).
Table 1.
Characteristics of the fungal isolates used for development of Ophiostoma clavatum and O. brunneo-ciliatum LAMP assays
| Fungal species | Geographic origin or source | Isolate | Collectiona |
|---|---|---|---|
| Ophiostoma clavatum | Lunsen, Uppsala, Sweden | 37983 | CMW |
| Lunsen, Uppsala, Sweden | 37984 | CMW | |
| Val Dogna, Udine, Italy | 37985 | CMW | |
| Val Venosta, Bolzano, Italy | 37986 | CMW | |
| Val Camonica, Brescia, Italy | 37987 | CMW | |
| Ophiostoma brunneo-ciliatum | Atholl, Scotland, Great Britain | 117571 | CBS |
| Kindberg, Styria, Austria | 117591 | CBS | |
| Ceratocystis montium | Weinviertel, Lower Austria, Austria | 117580 | CBS |
| Oregon | 137.36 | CBS | |
| Hyalorhinocladiella macrospora | Sweden | 367.53 | CBS |
| Leptographium serpens | Poggio Valicaia, Florence, Italy | CVLE04 | DiBA |
| Leptographium procerum | Monte Peglia, Siena, Italy | CV05MP01 | DiBA |
| Leptographium lundbergii | Poggio Valicaia, Florence, Italy | CVLE01 | DiBA |
| Leptographium wingfieldii | Alberese, Grosseto, Italy | CVLE03 | DiBA |
| Leptographium guttulatum | Alberese, Grosseto, Italy | CVLE02 | DiBA |
| Sphaeropsis sapinea | S. Rossore, Pisa, Italy | S10 | DiBA |
| Diplodia scrobiculata | Douglas Co., Wisconsin | 215 | DiBA |
CMW, culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa; CBS, Centraalbureau voor Schimmelcultures, Utrecht, Netherlands; DiBA, Dipartimento di Biotecnologie Agrarie, University of Florence, Italy.
Adults of I. acuminatus and other bark beetle species to be used as a comparison during the I. acuminatus LAMP assay development were kindly provided by E. Petrucco Toffolo (DAFNAE, University of Padua, Italy). Tested species and provenances are reported in Table 2.
Table 2.
Description of the insects used for Ips acuminatus LAMP assay development
| Insect species | Geographic origin |
|---|---|
| Ips acuminatus | Lunsen, Uppsala, Sweden |
| Lunsen, Uppsala, Sweden | |
| Cortina d'Ampezzo, Belluno, Italy | |
| Val Dogna, Udine, Italy | |
| Ips sexdentatus | Monfalcone, Gorizia, Italy |
| Ips typographus | Chioggia, Venice, Italy |
| Ips cembrae | Chioggia, Venice, Italy |
| Tomicus piniperda | Verzegnis, Udine, Italy |
| Orthotomicus erosus | Trieste, Italy |
| Crypturgus cinereus | Chioggia, Venice, Italy |
DNA extraction.
DNA was extracted from I. acuminatus samples by following a protocol developed for museum beetle specimens (41), which does not require the homogenization of whole insect tissues. This method was chosen in order to minimize the amount of insect DNA extracted relative to fungal DNA. Total DNA of fungi and insects used for LAMP assay development was extracted following a standard salting-out protocol (42).
Approximate DNA concentrations were determined at 260 nm using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE), and extracts were diluted to 10 ng μl−1 in double-distilled water (Sigma-Aldrich, St. Louis, MO).
Loop-mediated isothermal amplification assay development.
Species-specific LAMP primers for O. clavatum and O. brunneo-ciliatum were designed manually from the β-tubulin gene antisense sequences of O. clavatum (GenBank accession number JX298085) and O. brunneo-ciliatum (GenBank accession numbers HM031559 and JX298086) (43). Species-specific primers for I. acuminatus were designed manually from COX I gene sense sequence (GenBank accession numbers U82585, AF113325, and EF115508) (44–46). BLAST analysis of the listed sequences was performed using the National Center for Biotechnology Information (NCBI) database (47), in order to identify regions with high interspecific variability suitable for primer design. Sequence alignments were performed using ClustalW, in MEGA, version 5, software (48, 49).
Six LAMP primers (external primers F3 and B3, internal primers FIP and BIP, and loop primers F-Loop and B-Loop) were designed for each assay (except the I. acuminatus assay, which lacked the F-Loop primer), according to the strategy described by Notomi et al. (32) and Nagamine et al. (34). Primers were designed to exploit interspecific sequence variability and checked for hairpin, self-dimer, and heterodimer production using Integrated DNA Technologies (Coralville, IA) OligoAnalyzer, version 3.1, software (50). Primers were synthesized by Eurofins MWG Operon (Ebersberg, Germany), and the sequences are reported in Table 3.
Table 3.
Primers used for LAMP assays
| LAMP assay target | Primer | Sequence (5′–3′) | Target region length (bp) |
|---|---|---|---|
| Ophiostoma clavatum | F3 | CCTCGTTGAAGTAGACGCTC | 176 |
| B3 | GATTCCGATCTACGGCTCC | ||
| FIP | GTTGGACGTTGGACGCCGCGAGGCGCTCCAGCTGGAGA | ||
| BIP | GAAGTGGGAGAATACATACACGCCATGGCTGAACTCAACACTGAC | ||
| F-Loop | ACCGCAGCTAACGTAATGTCC | ||
| B-Loop | GCTGTCAAGGCCGTGCTC | ||
| O. brunneo-ciliatum | F3 | GACCGAAAGGACCGGCAC | 217 |
| B3 | GCTCCACCGCGGCATGA | ||
| FIP | CTGTCCCTAGGTACAACGGCACAGATCGACAAGGACGGCAC | ||
| BIP | CCACGACCAACATCAGGGGAGACAGGCAGCAGATTTCCGGC | ||
| F-Loop | CCTCTGGCAACAAGTACGTG | ||
| B-Loop | GCTGTCAAGGCCGTGCTC | ||
| Ips acuminatus | F3 | CATTTCATGGAGCTCAAATTTC | 182 |
| B3 | TCCTGTAAAAAGTGGAAATCATT | ||
| FIP | TCCGGTTAAACCTCCTAGAGTAAATAATCCCTCAAGACTTTGATCT | ||
| BIP | ATGTAGTTGCCCATTTCCATTATGTGGACAATTCCTGCAATAATAG | ||
| B-Loop | ACTTTCAATAGGAGCTGTATTTG |
Real-time LAMP assays were carried out on a Genie II instrument (OptiGene, Horsham, United Kingdom) in 25-μl reaction mixtures containing 15 μl of isothermal master mix at a 1× concentration (OptiGene), 200 nM each external primer, 2 μM each internal primer, and 1 μM each loop primer (35). The isothermal master mix contained a fluorescent double-stranded DNA binding dye to permit the real-time detection of the results. The assays were optimized in terms of reaction time, temperature, and the volume of DNA added per reaction. For the O. clavatum assay, 4 μl of template DNA was added per reaction, and the reaction was held at 66°C for 35 min. For the O. brunneo-ciliatum assay, 2 μl of template DNA was added per reaction, and the reaction was held at 65°C for 30 min. For the I. acuminatus assay, 1 μl of template DNA was added per reaction, and the reaction was held at 63°C for 50 min. After amplification, the nature of the amplification products was confirmed by subjecting the reactions to a slow annealing step (0.05°C per s) from 95°C to 75°C with fluorescence monitoring. An estimate of the average annealing temperature ± the standard deviation (SD) for the amplification product of each assay was calculated on the basis of three repetitions for each target isolate and specimen described in Tables 1 and 2.
To test specificity, assays for O. clavatum and O. brunneo-ciliatum were initially tested using DNA samples from the isolates described in Table 1, and the assay for I. acuminatus was tested on DNA extracted from the species described in Table 2. To evaluate the sensitivity of the three LAMP assays, 10-fold serial dilutions of template DNA were tested. Ophiostoma clavatum and O. brunneo-ciliatum DNA was diluted in DNA extracted from the insect vector, to account for potential inhibitory effects. Serial dilutions of I. acuminatus DNA were prepared in water. For assay optimization and characterization, reactions were carried out in triplicate.
Experimental design.
To survey the occurrence of O. clavatum and O. brunneo-ciliatum on the collected I. acuminatus individuals, 10 randomly chosen insects were tested from each tree, for a total of 110 samples (Table 4). DNA extracted from each sample was tested for the presence of O. clavatum and O. brunneo-ciliatum using real-time LAMP. Reactions were carried out in triplicate, and a sample was considered to be positively associated with each fungus if at least two of the replicates had a positive response. Samples which were negative using both of the fungal assays were tested with the I. acuminatus assay, in order to determine whether DNA extraction had been successful and the sample supported isothermal amplification, as described by Tomlinson et al. (36). In this case, reactions were carried out in one replicate. A negative response with the I. acuminatus assay indicated failure of the DNA extraction procedure for that sample. In total, sufficient samples were tested such that results were successfully obtained for a total of 10 samples from each tree. During sample analysis, each LAMP run included one reaction mixture containing either O. clavatum (isolate CMW 37983), O. brunneo-ciliatum (isolate CBS 117591), or I. acuminatus (collected in Udine, Italy) DNA as a positive control and one reaction mixture containing water as a negative control.
Table 4.
Frequency of association of the blue stain fungi Ophiostoma clavatum and O. brunneo-ciliatum with Ips acuminatus individuals collected in the Southern Alps, Italy (n = 110)
| Site no. | Site locationa (geographic coordinates; altitude above sea level) | Outbreak | Tree | No. of I. acuminatus individuals associated with indicated fungus (no. positives/10 tested samplesb) |
|
|---|---|---|---|---|---|
| O. clavatum | O. brunneo-ciliatum | ||||
| 1 | Brusson, Aosta (45°45′N, 7°45′E; 1,769 m) | No | A | 3 | 0 |
| 2 | Val Camonica, Brescia (46°10′N, 10°22′E; 1,183 m) | No | A | 7 | 0 |
| B | 7 | 0 | |||
| 3 | Valtellina, Sondrio (46°29′N, 10°19′E; 1,644 m) | Yes | A | 3 | 0 |
| B | 4 | 0 | |||
| 4 | Val Venosta, Bolzano (46°38′N, 10°51′E; 1,341 m) | No | A | 5 | 0 |
| B | 6 | 0 | |||
| 5 | Cortina d'Ampezzo, Belluno (46°29′N, 12°10′E; 1,124 m) | Yes | A | 3 | 0 |
| B | 5 | 0 | |||
| 6 | Val Dogna, Udine (46°27′N, 13°22′E; 980 m) | No | A | 8 | 0 |
| B | 8 | 0 | |||
See Fig. 1 for site geographic location.
Ophiostoma clavatum and O. brunneo-ciliatum were tested on the same samples.
Statistical analysis.
Survey data on the occurrence of O. clavatum on the collected I. acuminatus were analyzed with a generalized linear-mixed-effect model (GLMM), using a binomial distribution (51). To account for the nested nature of the experimental design, we used a model with presence or absence of the species on the vector as the response variable, population characteristic (outbreak or non-outbreak) as the fixed effect, and tree within site as the random factor. Data were analyzed in R (52) using the lme4 package (53). Since the presence of O. brunneo-ciliatum was not recorded, statistical analysis on this species was not performed.
RESULTS
Specificity and sensitivity of LAMP assays.
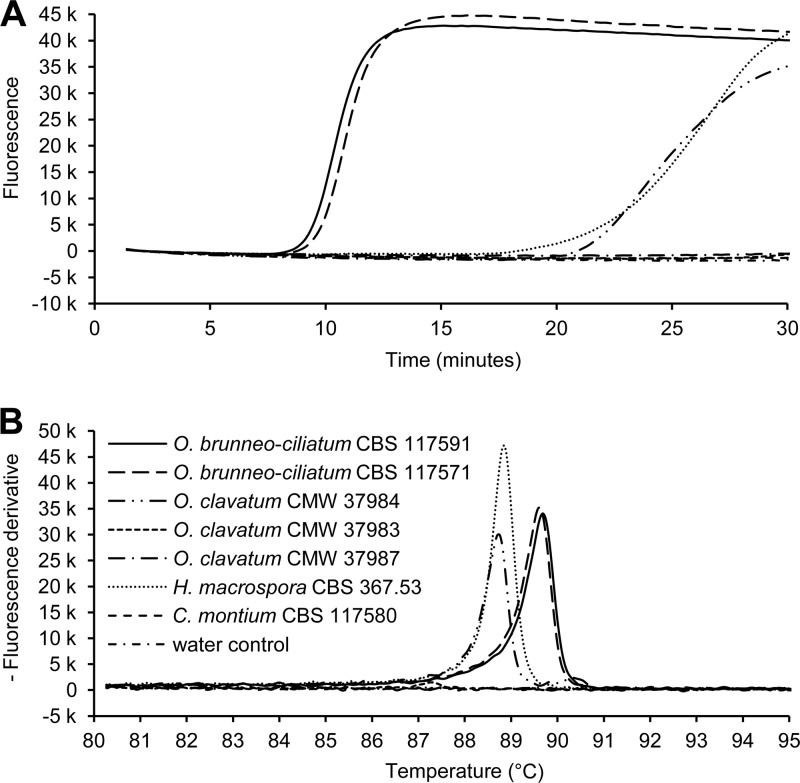
Ophiostoma clavatum and I. acuminatus LAMP assays were specific, producing a positive response only in the presence of the target species. The O. brunneo-ciliatum LAMP assay occasionally also produced nonspecific responses, which were nevertheless always distinguishable from the specific amplification on the basis of annealing temperature (Fig. 2). We hence considered the O. brunneo-ciliatum LAMP assay to be specific provided that the annealing temperature was taken into account for all positive reactions. Annealing temperatures (means ± SDs) for O. clavatum, O. brunneo-ciliatum, and I. acuminatus LAMP assays were 89.33 ± 0.07°C, 89.84 ± 0.07°C, and 80.63 ± 0.25°C, respectively.
Fig 2.
Example of one of the specificity tests for Ophiostoma brunneo-ciliatum real-time LAMP assay. In the amplification plot (A), besides the positive controls for O. brunneo-ciliatum (isolates CBS 117591 and CBS 117571), O. clavatum isolate CMW 37984 and Hyalorhinocladiella macrospora isolate CBS 267.53 produced positive responses. However, in the annealing plot (B), nonspecific amplifications showed an annealing temperature that differed by more than 1°C from the specific ones.
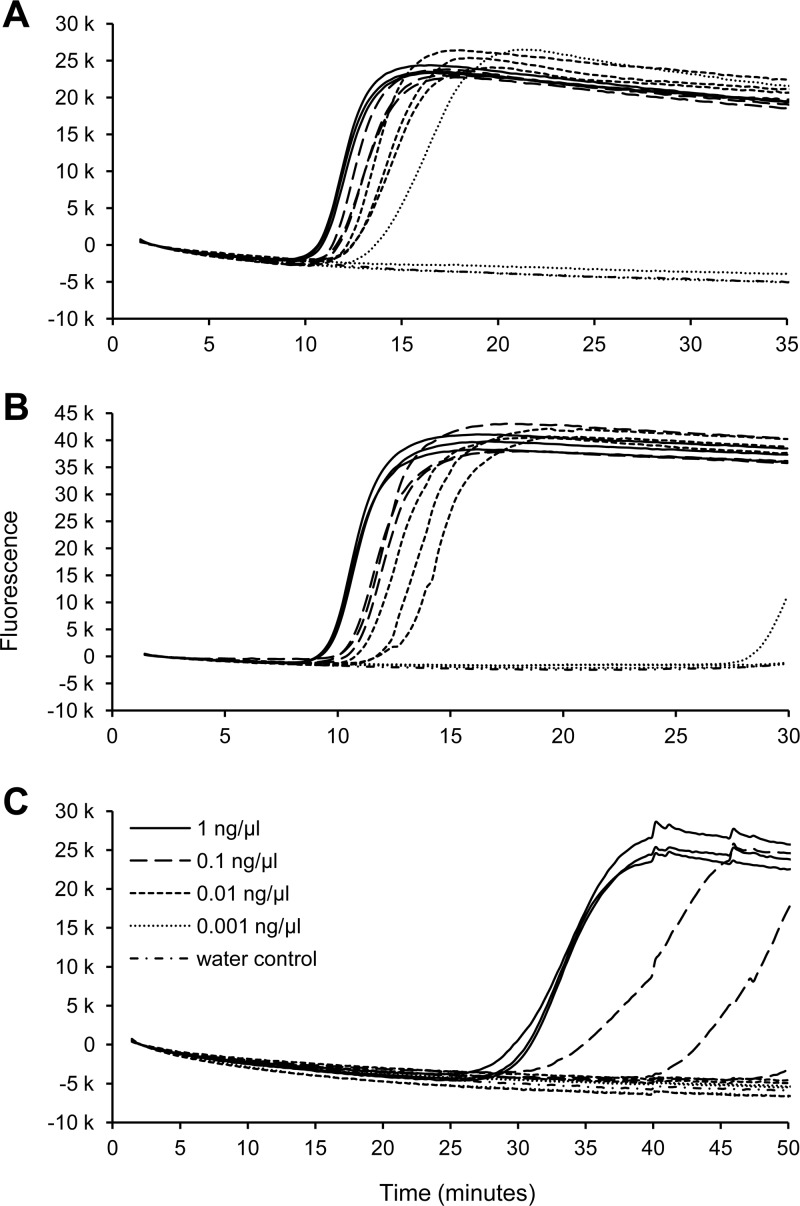
Sensitivity tests for O. clavatum and O. brunneo-ciliatum LAMP assays showed that for both the assays the limit of detection was 0.01 ng μl−1, corresponding to 0.04 ng and 0.02 ng per reaction, respectively. These amounts of DNA produced a positive response in all three replicates for both assays, while at lower concentrations, responses were not consistent between replicates (Fig. 3A and B). The limit of detection of the I. acuminatus assay was 1 ng μl−1, corresponding to 1 ng per reaction; at 0.1 ng μl−1 positive responses were observed, but only in two out of three replicates (Fig. 3C).
Fig 3.
Ophiostoma clavatum (A), O. brunneo-ciliatum (B), and Ips acuminatus (C) real-time LAMP assays. Shown are results of the sensitivity test. Each dilution was run in three replicates. O. clavatum and O. brunneo-ciliatum DNA was diluted in I. acuminatus DNA. I. acuminatus DNA was diluted in water.
Occurrence of O. clavatum and O. brunneo-ciliatum on I. acuminatus.
Of the I. acuminatus samples tested, 19 samples did not react with any of the assays, including the I. acuminatus assay. As this indicated that the testing had been unsuccessful for these samples, they were excluded from further analysis. Of the 110 samples for which results were obtained, 51 samples had a negative response for both fungal LAMP assays but were positive for I. acuminatus DNA, indicating that testing was successful and both fungal species were absent from these samples. Ophiostoma brunneo-ciliatum was not detected in any of the 110 samples for which results were obtained. Ophiostoma clavatum was detected in 59 samples in total, including samples from all trees at all six locations (Table 4).
The generalized linear-mixed-effect model showed that population characteristic (outbreak or non-outbreak) had a significant influence (Wald z statistic = −2.38; P = 0.017) on the frequency of the fungus-vector association, which was 37.5% ± 4.8% (mean value ± standard error [SE]) in the outbreak populations, compared to 62.8% ± 6.8% in the non-outbreak populations.
DISCUSSION
This paper describes the development of three real-time LAMP assays, which were found to be sensitive and specific for the detection of two fungal phytopathogens and their insect vector, and the application of these assays to investigate the frequency of association between these species in populations of I. acuminatus. The two developed fungal assays (specific for O. clavatum and O. brunneo-ciliatum) had a limit of detection of 0.01 ng μl−1, and the total reaction time was less than 40 min. Due to the necessity to exploit the interspecific variability of the COX I gene sequence (44–46), the Ips acuminatus LAMP assay lacked the F-Loop primer. As reported previously (34), this appears to result in this assay being slower than the other assays; however, it was found to be specific, with a limit of detection of 1 ng μl−1.
As shown by numerous studies (33), LAMP is a robust technique, with the potential to bring several advantages in molecular detection and diagnosis. In this study, we applied this method for the first time to the study of bark beetle-associated blue stain fungi, exploiting its high specificity and sensitivity to distinguish two similar fungal species directly in their insect vector. Using LAMP-based detection, we found that O. clavatum, which was detected at all sampling sites and on more than 50% of insects tested, is the main blue stain fungus associated with I. acuminatus in the Southern Alps, while O. brunneo-ciliatum was not detected. The results of this study are in agreement with previous reports by Mathiesen (23, 24), Rennerfelt (25), Mathiesen-Käärik (22), and Francke-Grosmann (17) about the identity of the main blue stain fungus associated with I. acuminatus. Subsequent reports that O. brunneo-ciliatum is consistently associated with I. acuminatus (e.g., see references 19 to 21) are hence probably attributable to an identification mistake due to the high morphological similarity of the two species (22, 24). According to Mathiesen-Käärik (22), O. brunneo-ciliatum is associated with I. sexdentatus (Boern.), a larger species which also may colonize P. sylvestris. The two blue stain fungi can therefore be found in the same tree host, but since they are associated with different vectors which specifically colonize different parts of the stem according to their size and bark thickness, it is unlikely that they can share the same habitat. Taking into account that I. acuminatus has been found to be consistently associated with O. clavatum in Sweden, Germany, and the former Yugoslavia (17), and that we also found the same fungus in the six sites that we sampled, regardless of distance and population characteristics, spatial variation in the complex composition of blue stain fungi associated with I. acuminatus is unlikely, at least concerning the main associated species. However, O. clavatum and O. brunneo-ciliatum are both considered nonaggressive pathogens (19, 24), and in the system bark beetle-P. sylvestris, they probably equally stimulate tree response, assisting bark beetle establishment (6, 21).
In this study, we also found that the occurrence of O. clavatum was significantly lower in outbreak populations than in non-outbreak populations. This result is based on a limited number of samples, but it raises an interesting issue. Since the role of blue stain fungi is to interact with plant defense (6), and outbreaks are believed to occur when some factor reduces the ability of the tree to resist beetle attack (2, 9), we predicted an opposite result, as suggested by Solheim (12). One possible explanation for this phenomenon is that during the outbreak phase, the density of the bark beetle population is so high that the presence of the blue stain fungus, especially if not aggressive, may no longer be critical to overwhelm plant defenses. As explained by Raffa et al. (2), once an eruptive threshold is surpassed, the initial eliciting factors may not be needed to sustain the outbreak. Moreover, the high density of the bark beetle population may elicit an antagonistic pressure on the fungus (54), causing a decrease in the occurrence of O. clavatum. However, the variation in the frequency of blue stain fungus association among sites is regulated by a multitude of factors that are context dependent (54), and our results may therefore have been affected by factors other than population dynamics. A more extensive survey, including a much larger number of replicates, is necessary to investigate these factors in more detail, for instance, by focusing on the incipient phase of an outbreak, when the presence of the associated fungi may play an important role in surpassing the eruptive threshold. Our results may serve as a starting point for these further investigations; furthermore, the LAMP assays described here are potentially valuable tools for the rapid detection of O. clavatum and O. brunneo-ciliatum to expedite future work in this area.
In conclusion, in this study, we used an innovative detection technology to specifically and quickly detect blue stain fungi directly from the vector insects, resolving an uncertainty in the literature about the identity of the main blue stain fungus associated with I. acuminatus. Our results also provided a hint on how the symbiotic fungal complex can vary between outbreak and nonoutbreak populations, although the discussion about the role of blue stain fungi and their importance in the population dynamics of bark beetles is still open. Rapid and accurate methods for species-specific detection of blue stain fungi directly in their insect vectors will be valuable in future investigations in this area. LAMP technology has the potential to bring a considerable improvement not only in diagnostics but also in ecological and biological studies that require quick and reliable identification of symbionts.
ACKNOWLEDGMENTS
This research was supported by EU FP 7 project Q-Detect No. 245047.
We thank staff from Forest Services of Valle d'Aosta, Veneto, Friuli Venezia Giulia and Trentino-Alto Adige Regions for field support, and staff from Comunità Montana Tirano (Sondrio), Parco Adamello (Brescia), Regole Magnifica Comunità Cortina d'Ampezzo (Belluno), and University of Milan, Edolo campus (Brescia), for facilities and logistic support. We also thank E. Petrucco Toffolo for providing insect samples, F. Colombari for help in collecting Ips acuminatus, A. Basso for help in DNA extraction, and M. Simonato and L. Marini for comments and suggestions during the analysis of molecular and statistical data, respectively. We are grateful to P. Gonthier for reviewing an early version of the manuscript and providing valuable comments.
Footnotes
Published ahead of print 8 February 2013
REFERENCES
- 1. Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF. (ed). 2004. Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht, Netherlands [Google Scholar]
- 2. Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH. 2008. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517 [Google Scholar]
- 3. Kirisits T. 2004. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi, p 181–236 In Lieutier F, Day KR, Battisti A, Gŕegoire J-C, Evans HF. (ed), Bark and wood boring insects in living trees in Europe, a synthesis. Springer, Dordrecht, Netherlands [Google Scholar]
- 4. Paine TD, Raffa KF, Harrington TC. 1997. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 42:179–206 [DOI] [PubMed] [Google Scholar]
- 5. Six D. 2012. Ecological and evolutionary determinants of bark beetle-fungus symbioses. Insects 3:339–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lieutier F, Yart A, Salle A. 2009. Stimulation of tree defenses by Ophiostomatoid fungi can explain attack success of bark beetles on conifers. Ann. For. Sci. doi:10.1051/forest/2009066 [Google Scholar]
- 7. Coulson RN. 1979. Population dynamics of bark beetles. Annu. Rev. Entomol. 24:417–447 [Google Scholar]
- 8. Wallner WE. 1987. Factors affecting insect population dynamics: differences between outbreak and non-outbreak species. Annu. Rev. Entomol. 32:317–340 [Google Scholar]
- 9. Raffa KF, Berryman AA. 1983. The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: Scolytidae). Ecol. Monogr. 53:27–49 [Google Scholar]
- 10. Hofstetter RW, Cronin JT, Klepzig KD, Moser JC, Ayres MP. 2006. Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 147:679–691 [DOI] [PubMed] [Google Scholar]
- 11. Solheim H. 1993. Ecological aspects of fungi associated with Ips typographus in Norway, p 235–242 In Wingfield MJ, Seifert KA, Webber JF. (ed), Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. APS Press, St. Paul, MN [Google Scholar]
- 12. Solheim H. 1993. Fungi associated with the spruce bark beetle Ips typographus in an endemic area in Norway. Scand. J. For. Res. 8:118–122 [Google Scholar]
- 13. Harding S. 1989. The influence of mutualistic blue-stain fungi on bark beetle population dynamics. Ph.D. thesis Royal Veterinary and Agricultural University, Copenhagen, Denmark [Google Scholar]
- 14. Kirisits T. 2001. Studies on the association of ophiostomatoid fungi with bark beetles in Austria with special emphasis on Ips typographus and Ips cembrae and their associated fungi Ceratocystis polonica and Ceratocystis laricicola. Ph.D. thesis Universität für Bodenkultur, Vienna, Austria [Google Scholar]
- 15. Colombari F, Schroeder L, Battisti A, Faccoli M. 2013. Spatio-temporal dynamics of an Ips acuminatus outbreak and implications for management. Agric. For. Entomol. 15:34–42 [Google Scholar]
- 16. Batra LR. 1967. Ambrosia fungi: a taxonomic revision, and nutritional studies of some species. Mycologia 59:976–1017 [Google Scholar]
- 17. Francke-Grosmann H. 1963. Die Übertragung der Pilzflora bei dem Borkenkäfer Ips acuminatus Gyll.: ein Eintrag zur Kenntnis der Ipiden-Symbiosen. J. Appl. Entomol. 52:355–361 [Google Scholar]
- 18. Harrington TC, Aghayeva DN, Fraedrich SW. 2010. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111:337–361 [Google Scholar]
- 19. Guérard N, Dreyer E, Lieutier F. 2000. Interactions between Scots pine, Ips acuminatus (Gyll.) and Ophiostoma brunneo-ciliatum (Math.): estimation of the critical thresholds of attack and inoculation densities and effects on hydraulic properties in the stem. Ann. For. Sci. 57:681–690 [Google Scholar]
- 20. Lieutier F, Garcia J, Yart A, Vouland G, Pettinetti M, Morelet M. 1991. Ophiostomatales (Ascomycetes) associated with Ips acuminatus Gyll (Coleoptera, Scolytidae) in Scots pine (Pinus sylvestris L) in South-Eastern France, and comparison with Ips sexdentatus Boern. Agronomie 11:807–817 [Google Scholar]
- 21. Villari C, Battisti A, Chakraborty S, Michelozzi M, Bonello P, Faccoli M. 2012. Nutritional and pathogenic fungi associated with the pine engraver beetle trigger comparable defenses in Scots pine. Tree Physiol. 37:867–879 [DOI] [PubMed] [Google Scholar]
- 22. Mathiesen-Käärik A. 1953. Eine Übersicht uber die gewöhnlichsten mit Borkenkäfern assoziierten Bläuepilze in Schweden und einige für Sweden neue Bläuepilze. Meddn. St. SkogsforskInst. 43:1–74 [Google Scholar]
- 23. Mathiesen A. 1950. Über einige mit Borkenkäfern assoziierte Bläuepilze in Schweden. Oikos 2:275–308 [Google Scholar]
- 24. Mathiesen A. 1951. Einige neue Ophiostoma-Arten in Schweden. Sven. Bot. Tids. 45:203–232 [Google Scholar]
- 25. Rennerfelt E. 1950. Über den Zusammenhang zwischen dem Verblauen des Holzes und den Insekten. Oikos 2:120–137 [Google Scholar]
- 26. Zipfel RD, de Beer ZW, Jacobs K, Wingfield BD, Wingfield MJ. 2006. Multi-gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Stud. Mycol. 55:75–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luchi N, Mancini V, Feducci M, Santini A, Capretti P. 2011. Leptoglossus occidentalis and Diplodia pinea: a new insect-fungus association in Mediterranean forests. For. Pathol. 42:246–251 [Google Scholar]
- 28. Roets F, Wingfield MJ, Dreyer LL, Crous PW, Bellstedt DU. 2006. A PCR-based method to detect species of Gondwanamyces and Ophiostoma on surfaces of insects colonizing Protea flowers. Can. J. Bot. 84:989–994 [Google Scholar]
- 29. Schweigkofler W, Otrosina WJ, Smith SL, Cluck DR, Maeda K, Peay KG, Garbelotto M. 2005. Detection and quantification of Leptographium wageneri, the cause of black-stain root disease, from bark beetles (Coleoptera: Scolytidae) in Northern California using regular and real-time PCR. Can. J. For. Res. 35:1798–1808 [Google Scholar]
- 30. Haugland RA, Brinkman N, Vesper SJ. 2002. Evaluation of rapid DNA extraction methods for the quantitative detection of fungi using real-time PCR analysis. J. Microbiol. Methods 50:319–323 [DOI] [PubMed] [Google Scholar]
- 31. Ma Z, Michailides TJ. 2007. Approaches for eliminating PCR inhibitors and designing PCR primers for the detection of phytopathogenic fungi. Crop Prot. 26:145–161 [Google Scholar]
- 32. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:E63 doi:10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parida M, Sannarangaiah S, Dash PK, Rao PVL, Morita K. 2008. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 18:407–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagamine K, Hase T, Notomi T. 2002. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 16:223–229 [DOI] [PubMed] [Google Scholar]
- 35. Tomlinson JA, Ostoja-Starzewska S, Adams IP, Miano DW, Abidrabo P, Kinyua Z, Alicai T, Dickinson MJ, Peters D, Boonham N, Smith J. 2012. Loop-mediated isothermal amplification for rapid detection of the causal agents of cassava brown streak disease. J. Virol. Methods doi:10.1016/j.jviromet.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 36. Tomlinson JA, Dickinson MJ, Boonham N. 2010. Rapid detection of Phytophthora ramorum and P. kernoviae by two-minute DNA extraction followed by isothermal amplification and amplicon detection by generic lateral flow device. Phytopathology 100:143–149 [DOI] [PubMed] [Google Scholar]
- 37. Lozzia GC, Rigamonti IE. 2002. Notes on Ips acuminatus Gyll. damaging Pinus sylvestris in Valtellina (Northern Italy) [Lombardy]. Monti Boschi 53:20–22 [Google Scholar]
- 38. Faccoli M. 2004. A morphological illustrated key to European species of the genus Ips DeGeer (Coleoptera: Scolytidae). Coleopterist 13:103–119 [Google Scholar]
- 39. Luchi N, Capretti P, Surico G, Orlando C, Pazzagli M, Pinzani P. 2005. A real-time quantitative PCR assay for the detection of Sphaeropsis sapinea from inoculated Pinus nigra shoots. J. Phytopathol. 153:37–42 [Google Scholar]
- 40. Sabbatini Peverieri G, Capretti P, Tiberi R. 2006. Associations between Tomicus destruens and Leptographium spp. in Pinus pinea and P. pinaster stands in Tuscany, central Italy. For. Pathol. 36:14–20 [Google Scholar]
- 41. Gilbert MTP, Moore W, Melchior L, Worobey M. 2007. DNA extraction from dry museum beetles without conferring external morphological damage. PLoS One 2:e272 doi:10.1371/journal.pone.0000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patwary MU, Kenchington EL, Bird CJ, Zouros E. 1994. The use of random amplified polymorphic DNA markers in genetic studies of the sea scallop Placopecten magellanicus (Gmelin, 1791). J. Shellfish Res. 13:547–553 [Google Scholar]
- 43. Linnakoski R, de Beer ZW, Ahtiainen J, Sidorov E, Niemela P, Pappinen A, Wingfield MJ. 2010. Ophiostoma spp. associated with pine- and spruce-infesting bark beetles in Finland and Russia. Persoonia 25:72–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cognato AI, Sperling FAH. 2000. Phylogeny of Ips DeGeer species (Coleoptera: Scolytidae) inferred from mitochondrial cytochrome oxidase I DNA sequence. Mol. Phylogenet. Evol. 14:445–460 [DOI] [PubMed] [Google Scholar]
- 45. Cognato AI, Sun JH. 2007. DNA based cladograms augment the discovery of a new Ips species from China (Coleoptera: Curculionidae: Scolytinae). Cladistics 23:539–551 [DOI] [PubMed] [Google Scholar]
- 46. Stauffer C, Lakatos F, Hewitt GM. 1997. The phylogenetic relationships of seven European Ips (Scolytidae, Ipinae) species. Insect Mol. Biol. 6:233–240 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203–214 [DOI] [PubMed] [Google Scholar]
- 48. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Owczarzy R, Tataurov AV, Wu Y, Manthey JA, McQuisten KA, Almabrazi HG, Pedersen KF, Lin Y, Garretson J, McEntaggart NO, Sailor CA, Dawson RB, Peek AS. 2008. IDT SciTools: a suite for analysis and design of nucleic acid oligomers. Nucleic Acids Res. 36:W163–W169 doi:10.1093/nar/gkn198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Springer, New York, NY [Google Scholar]
- 52. R Development Core Team 2011, posting date R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- 53. Bates D, Maechler M, Bolker B. October 2011, posting date lme4: linear mixed-effects models using S4 classes. R package version 0.999375-42. http://CRAN.R-project.org/package=lme4
- 54. Klepzig KD, Six DL. 2004. Bark beetle-fungal symbiosis: context dependency in complex associations. Symbiosis 37:189–205 [Google Scholar]