Abstract
Members of the genus Listeria are fastidious bacteria with respect to their nutritional requirements, and several minimal media described in the literature fail to support growth of all Listeria spp. Furthermore, strict limitation by a single nutrient, e.g., the carbon source, has not been demonstrated for any of the published minimal media. This is an important prerequisite for defined studies of growth and physiology, including “omics.” Based on a theoretical analysis of previously published mineral media for Listeria, an improved, well-balanced growth medium was designed. It supports the growth, not only of all tested Listeria monocytogenes strains, but of all other Listeria species, with the exception of L. ivanovii. The growth performance of L. monocytogenes strain Scott A was tested in the newly designed medium; glucose served as the only carbon and energy source for growth, whereas neither the supplied amino acids nor the buffering and complexing components (MOPS [morpholinepropanesulfonic acid] and EDTA) supported growth. Omission of amino acids, trace elements, or vitamins, alone or in combination, resulted in considerably reduced biomass yields. Furthermore, we monitored the specific growth rates of various Listeria strains cultivated in the designed mineral medium and compared them to growth in complex medium (brain heart infusion broth [BHI]). The novel mineral medium was optimized for the commonly used strain L. monocytogenes Scott A to achieve optimum cell yields and maximum specific growth rates. This mineral medium is the first published synthetic medium for Listeria that has been shown to be strictly carbon (glucose) limited.
INTRODUCTION
In general, nutritionally rich media, such as brain heart infusion broth (BHI), satisfactorily support the growth of most known strains of Listeria. However, for controlled scientific investigations, including growth studies, transcriptome and proteome analyses (1), or metabolomics (2), the use of an undefined complex medium is not optimal. For such media, knowledge of the elemental composition and protein- or carbohydrate-derived ingredients is mostly poor, and furthermore, the medium composition can change from batch to batch (3, 4). Several defined mineral media for Listeria reported to support more or less good growth of various strains of Listeria have been described previously (4–10). Nonetheless, Premaratne and coworkers and others complained that most chemically defined media fail to support growth of the frequently used strains of Listeria monocytogenes, including Scott A and ATCC 19115 (9, 10). Despite the fact that several mineral media for cultivating Listeria have been published, only limited and incomplete data on growth in these media are available (4, 11). Most importantly, none of the media using glucose as the principal carbon and energy source have been reported to be strictly glucose limited, which is a prerequisite to study bacterial growth physiology under defined growth conditions.
In the present study, mineral media previously published for the cultivation of Listeria spp. were analyzed for their elemental compositions, considering that the principal carbon source (in all cases glucose) was the limiting carbon/energy source, as described by Pirt (12) and Egli and Fiechter (13). Based on this analysis, an improved mineral medium was designed with the goal of obtaining a medium that supports clearly carbon-limited growth of different Listeria strains. Subsequently, data were collected for the growth of a selection of Listeria strains, including maximum specific growth rates (μmax). This allowed us to compare growth characteristics for a range of Listeria spp. Moreover, the influence of trace elements (TE), amino acids, and buffers on bacterial growth was examined. To our knowledge, this is the first published defined mineral medium for Listeria shown to be strictly carbon-limited.
MATERIALS AND METHODS
Bacterial strains.
All bacterial strains used in this study were stored in cryoculture at −80°C before use. Before each experiment, the required cryoculture was streaked onto BHI agar plates and incubated for 24 h at 37°C. Subsequently, the different strains used were precultivated in liquid batch culture using media indicated in the individual experiments. Precultures were prepared freshly for each experiment.
The medium was optimized for the growth of L. monocytogenes Scott A (serotype 4b; clinical isolate), which is frequently used as a reference strain in Listeria research; recently, its genome has been sequenced (14). Additionally, the following strains were used: L. monocytogenes WSLC 1042 (serotype 4b), L. monocytogenes EGDe (serotype 1/2a), L. monocytogenes ATCC 19115 (serotype 1/2b), L. monocytogenes ATCC 19112 (serotype 1/2c), Listeria ivanovii subsp. ivanovii DSM 20751, Listeria innocua DSM 20649, Listeria seeligeri DSM 20751, Listeria grayi DSM 20601, and Listeria welshimeri DSM 20650. All strains were obtained from the culture collection of the Institute of Food, Nutrition and Health at the ETH Zurich, Switzerland.
Medium preparation.
To prevent precipitation or denaturation of components, the medium was prepared as follows (according to the recipe given in Table 1). Stock solutions of autoclaved KH2PO4 (100-fold concentrated), Na2HPO4·H2O (100×), MgSO4·7H2O (100×), and (NH4)2SO4 (50×) were mixed in 500 ml MilliQ water according to their stock concentrations. Subsequently, autoclaved EDTA, as a chelating agent (15), and 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, as a buffering agent (9), were added. Glucose, prepared as a stock solution (0.555 M) and autoclaved separately, was added afterward to the mixture. Also, the trace element stock solution (100×) (prepared according to the recipe in Table S1 in the supplemental material [16]) was added to the medium mixture. Finally, the vitamins and amino acids were added. Vitamins were made as a 1,000× stock solution (17), filter sterilized, and kept refrigerated. The amino acid stock solutions (100×) were prepared fresh prior to each experiment; l-cysteine and l-glutamine were prepared separately from the other amino acids. After mixing all components, the volume was brought up to 1,000 ml and the medium was sterilized by filtering through sterile 0.22-μm membrane filters.
Table 1.
Composition of glucose-limited mineral medium E for the cultivation of Listeria
| Compound | Mass concn | Molar concn |
|---|---|---|
| EDTA | 1.4612 g liter−1 | 5.00 mmol liter−1 |
| KH2PO4 | 0.656 g liter−1 | 4.82 mmol liter−1 |
| Na2HPO4·H2O | 2.047 g liter−1 | 12.80 mmol liter−1 |
| MgSO4·7H2O | 1.55 g liter−1 | 6.29 mmol liter−1 |
| (NH4)2SO4 | 7.07 g liter−1 | 53.50 mmol liter−1 |
| Glucose | 2.5 g liter−1 | 13.88 mmol liter−1 |
| MOPS | 20.93 g liter−1 | 100.01 mmol liter−1 |
| Trace elementsa | ||
| CaCO3 | 80 mg liter−1 | 0.40 mmol liter−1 |
| FeCl3·6H2O | 38.7 mg liter−1 | 143.18 μmol liter−1 |
| MnCl2·4H2O | 5.75 mg liter−1 | 29.05 μmol liter−1 |
| CuSO4·5H2O | 0.73 mg liter−1 | 2.92 μmol liter−1 |
| CoCl2·6H2O | 0.65 mg liter−1 | 2.73 μmol liter−1 |
| ZnO | 2 mg liter−1 | 24.57 μmol liter−1 |
| H3BO3 | 0.62 mg liter−1 | 10.03 μmol liter−1 |
| EDTA·Na4·2H2O | 396 mg liter−1 | 0.94 mmol liter−1 |
| MgCl2·6H2O | 67.1 mg liter−1 | 0.33 mmol liter−1 |
| Na2MoO4·2H2O | 5.2 mg liter−1 | 21.49 μmol liter−1 |
| Vitaminsb | ||
| Biotin | 20 μg liter−1 | 81.86 pmol liter−1 |
| Folic acid | 20 μg liter−1 | 45.31 pmol liter−1 |
| Pyridoxine | 100 μg liter−1 | 591.09 pmol liter−1 |
| Thiamine | 50 μg liter−1 | 166.22 pmol liter−1 |
| Riboflavin | 50 μg liter−1 | 132.85 pmol liter−1 |
| Niacin | 50 μg liter−1 | 406.14 pmol liter−1 |
| Cobalamin | 50 μg liter−1 | 36.89 pmol liter−1 |
| Pantothenic acid | 50 μg liter−1 | 228.07 pmol liter−1 |
| 4-Aminobenzoic acid | 50 μg liter−1 | 364.60 pmol liter−1 |
| Lipoic acid | 50 μg liter−1 | 242.34 pmol liter−1 |
| Nicotinamide | 50 μg liter−1 | 409.42 pmol liter−1 |
| Amino acidsa | ||
| Cysteine | 100 mg liter−1 | 825.37 μmol liter−1 |
| Glutamine | 600 mg liter−1 | 4105.53 μmol liter−1 |
| Methionine | 100 mg liter−1 | 670.19 μmol liter−1 |
| Histidine | 100 mg liter−1 | 644.52 μmol liter−1 |
| Tryptophan | 100 mg liter−1 | 489.66 μmol liter−1 |
| Leucine | 100 mg liter−1 | 762.35 μmol liter−1 |
| Isoleucine | 100 mg liter−1 | 762.35 μmol liter−1 |
| Valine | 100 mg liter−1 | 853.63 μmol liter−1 |
| Arginine·HCl | 120.9 mg liter−1 | 573.91 μmol liter−1 |
Added from 100× stock solution.
Added from 1,000× stock solution.
For growth on solid nutrient agar, the mineral medium (MM) was prepared as a 1.1×-concentrated medium in only 900 ml instead of 1,000 ml MilliQ water. Subsequently, this mineral medium was filter sterilized and mixed with 100 ml of autoclaved agar (10× concentrated [150 g liter−1; Sigma-Aldrich Chemie GmbH, Germany]), both prewarmed to 60°C. The resulting 1×-concentrated MM agar was poured into culture dishes.
Calculation of elemental excess factors and nutrient concentrations.
Mineral media for Listeria were analyzed based on average growth yield factors (YX/E = g [dry weight] cells per g element) deduced from the composition of bacterial and yeast dry biomass from published data (12, 13, 15). Using these growth yields for the published media, theoretical excess factors (Fc), with respect to carbon as the limiting nutrient, were calculated for the different individual elements based on the following equation (13): FC = (YX/E × cE)/(YX/C × cC), where FC is the theoretical excess factor with respect to carbon, YX/E is the average growth yield for element E [g cell dry weight (g element)−1], YX/C is the average growth yield for carbon [g cell dry weight (g carbon)−1], cE is the concentration of element E (g element liter−1), and cC is the concentration of carbon (g carbon liter−1).
Based on this analysis, the new glucose-limited mineral medium was designed according to the methods of Egli and Fiechter (13) and Egli (15).
Bacterial cultivation in MM.
Listeria spp. were cultivated in batch culture at 37°C either in Erlenmeyer flasks (medium-to-flask-volume, ≤20%) under constant magnetic stirring or in 24-well plates (Multiwell 24 well; Becton, Dickinson and Co., Franklin Lakes, NJ; 1 ml MM per well) for cultivation in an automated microplate reader (Synergy Mx; Bio Tek Instruments) under moderate shaking. For each experiment, MM was prepared fresh, sterilized by filtration, and prewarmed to 37°C before inoculation with a defined number of Listeria cells (as described for individual experiments). All Listeria strains were precultivated in MM to adapt the cells to the medium conditions prior to each experiment.
Optical density measurement.
Cell growth was determined by measuring the optical density (OD). All OD measurements of Listeria in batch cultures were performed at a wavelength of 600 nm, either in the automated microplate reader (Synergy Mx; Bio Tek Instruments) or using a Uvikon 860 spectrophotometer (Kontron Instruments, Switzerland). Optical density at 600 nm (OD600) values from the microplate reader corresponded to OD600 values from the Uvikon spectrometer divided by a factor of 3.
Flow cytometric analysis.
Cell numbers in Listeria cultures were determined flow cytometrically using a CyFlow Space instrument (Partec, Münster, Germany). Cells were counted by staining 1 ml of the culture with 10 μl SYBR green I (Molecular Probes, Basel, Switzerland) diluted 100× in dimethyl sulfoxide (Fluka Chemie AG, Buchs, Switzerland). The stained cells were measured using a 200-mW solid-state laser emitting at a wavelength of 488 nm. Cell signals were detected at the combined 520-nm/630-nm dot plot with the trigger set on the 520-nm channel (for more information, see Hammes et al. [18]). If the cell concentration exceeded the machine's quantification limit of 1,000 cell counts s−1 (standard deviation, less than 5%), the samples were diluted appropriately using 0.22-μm-filtered Evian mineral water.
Determination of the biomass growth yield.
For calculation of the biomass growth yield, 20 ml of sample from a culture was harvested and filtered through a preweighed membrane filter with a pore size of 0.22 μm (Durapore polyvinylidene difluoride [PVDF] hydrophilic filters; Merck Millipore). Subsequently, the filters with the bacterial pellets were washed with 20 ml demineralized water to remove medium residues. After drying the filters at 105°C for 24 h and cooling them in a desiccator, the dry weight of the filtered cells was measured. Yields were determined in triplicate.
Residual glucose determination.
Glucose concentrations were determined using an enzymatic assay based on glucose oxidase (from Aspergillus niger; Sigma-Aldrich, Buchs, Switzerland) and horseradish peroxidase (Sigma-Aldrich, Buchs, Switzerland) utilizing 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich, Buchs, Switzerland) as a chromogenic substrate (19, 20). In short, 20 μl of sample was mixed with 80 μl sodium phosphate buffer (0.1 mol liter−1, pH 7), and subsequently, 40 μl glucose oxidase solution (100 U ml buffer−1) was added. After adding 840 μl ABTS solution (1 mmol liter−1) and 40 μl peroxidase (20 U ml buffer−1), the assay was incubated for 20 min at 37°C. Extinction was measured at 415 nm, and glucose concentrations in samples were calculated from a standard curve.
RESULTS
Theoretical excess factors of different media for Listeria.
The media described by Welshimer (7) (WB; medium A), Premaratne et al. (10) (MWB; medium B), Phan-Thanh and Gormon (4) (IMM; medium C), and Tsai and Hodgson (9) (HTM; medium D) were analyzed with respect to their theoretical excess factors, assuming that carbon was the limiting element. The FC values obtained are listed in Table 2. Values higher than 1 indicate a theoretical excess of the particular element, whereas FC values below 1 suggest a theoretical lack of an element. Most clearly, three of them (with the exception of Phan-Thanh and Gormon's medium [medium C]) are theoretically limited by nitrogen. Furthermore, excess factors for sulfur and magnesium in the media are close to 1 and, therefore, may easily become limiting factors. Interestingly, the FC values of iron are below optimal levels in all tested recipes: medium A contains no iron, while the makers of media B and C used the same concentration of ferric citrate. In medium C, the makers also tested higher concentrations of ferric citrate and hemin, but they concluded that higher concentrations of hemin have a toxic effect on Listeria (9). However, in medium E, the concentration of ferric chloride was increased to get an FC value of 2.0 without any adverse effect observable for Listeria. Since no trace elements were added in media A, B, and C, the FC values for these mostly essential nutrients for microorganisms (12, 15), namely, Ca, Mn, Zn, Cu, and Co, were zero. Only Tsai and Hodgson (medium D) tested the addition of some trace elements, but they omitted them after they found, surprisingly, no improvement in growth (9).
Table 2.
FC analysis for essential nutritional elements with respect to carbon in four published mineral media for Listeria (A to D) and FC values for the improved mineral medium designed in this study (E)
| Element |
FC (excess factor with respect to carbon) for mediuma |
||||
|---|---|---|---|---|---|
| Ab | B | C | D | E | |
| C | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| N | 0.5 | 0.4 | 2.1 | 0.3 | 8.5 |
| S | 2.4 | 2.3 | 1.7 | 6.2 | 120.3 |
| P | 17.2 | 37.1 | 29.0 | 4.1 | 11.0 |
| K | 19.4 | 41.8 | 32.6 | 4.7 | 11.5 |
| Mg | 1.7 | 1.8 | 1.4 | 2.0 | 20.7 |
| Fe | − | 0.9 | 0.7 | # | 2.0 |
| Ca | − | − | − | − | 2.0 |
| Mn | − | − | − | − | 19.5 |
| Zn | − | − | − | − | 19.7 |
| Cu | − | − | − | − | 22.7 |
| Co | − | − | − | − | 19.7 |
| μmax (h−1) | 0.26/0.08c | 0.1/0.21c | 0.47d | 0.26–0.50e | 0.52g |
| Max TCC (cells ml−1) | − | ∼1.1 × 107d | ∼1.5 × 109d | NAf | 3.9 × 109d |
FC values below 1 indicate a theoretical lack of an element, while elements with values higher than 1 should be available in excess. Included in the table are some selected growth parameters published for various L. monocytogenes strains. Medium A, Welshimer (7); medium B, Premaratne et al. (10); medium C, Phan-Thanh and Gormon (4); medium D, Tsai and Hodgson (9); medium E, medium designed in this study with 2.5 g glucose liter−1. −, not added; #, different Fe concentrations tested.
No growth of strain L. monocytogenes Scott A was supported in medium A trough sequential subcultures, as reported by Premaratne et al. (10).
Published by Jones et al. (11) for L. monocytogenes strains NCTC 7973 (left) and NCTC 4885 (right) at 30°C.
Published by Phan-Thanh and Gormon (4) for strain EGDe at 37°C. TCC values were estimated from published growth curves.
Different values were published for different Fe concentrations and amino acids by Tsai and Hodgson (9) for L. monocytogenes strain 10403. No growth of L. monocytogenes Scott A was supported.
NA, only optical density data are available; OD600 = 0.134 to 1.217.
Design of an improved carbon-limited balanced growth medium for Listeria.
Based on the existing mineral media, theoretical assumed excess factors, and the FC values computed from Listeria media, we designed an improved medium (medium E), considering that all nutrients, trace elements, and vitamins essential for growth should be available in excess with respect to carbon, allowing unrestricted, controllable, and well-balanced growth that is strictly carbon limited.
The assumed excess factors used for this calculation were suggested by Egli (15); they are based on the elemental composition of microbial dry mass (12, 13). First, EDTA (1.46 g liter−1) was added to the medium to avoid the formation of insoluble precipitates due to mixing of mineral salts and trace metals (15). Trace elements were added to the medium to supply Listeria with nutrients known to be mostly essential for bacteria (12). Furthermore, a mixture of vitamins, already used in other studies (21), was added to the medium. Even though only four of these vitamins, namely, biotin, lipoic acid, riboflavin, and thiamine, have been reported to be essential for Listeria in some studies (7, 9, 10, 22), while a slight or no adverse effect of the additional components was observed by others (4). The nine amino acids most often listed in earlier reports on Listeria media (Cys, Gln, Met, His, Trp, Leu, Ile, Val, and Arg) were integrated into the new recipe (7, 10, 22). Although it is known that only some of these amino acids are essential for some strains (9, 22), all nine amino acids were chosen, which can potentially support a broad range of different Listeria species. The elemental composition and the FC of our improved medium are listed in Table 2.
Carbon limitation.
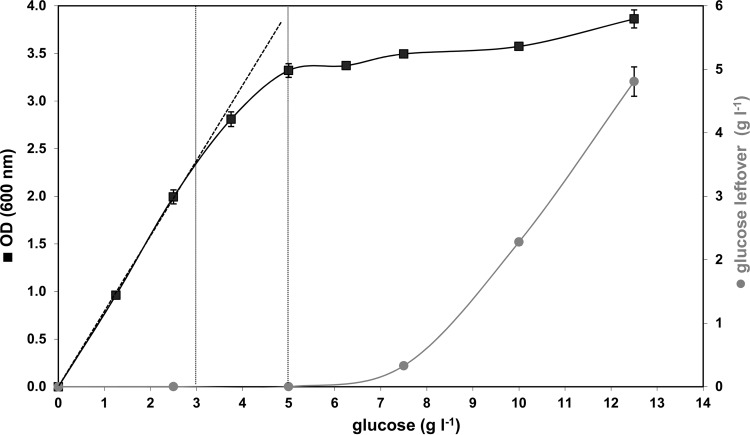
The medium presented in Table 1, designed to support glucose-limited growth of Listeria up to 10 g liter−1, was tested in batch culture experiments amended with increasing glucose concentrations from 0 to 12.5 g liter−1 by determining the final biomass in stationary phase. Since, the medium was intended to be strictly glucose limited, in a plot of the maximum biomass reached (xm) or final OD600, a linear correlation between biomass and initial glucose concentration (S0) should exist, with a slope that gives the growth yield Y (12). From Fig. 1, it is evident that the linear correlation between OD600 and S0 is given only for glucose concentrations in the range from 0 to 2.5 g liter−1 in this mineral medium (corresponding to 0 to 1 g carbon liter−1 from glucose). At higher glucose concentrations, the linear relationship between biomass and glucose breaks down. This is nicely demonstrated in Fig. 1 in the range of glucose concentrations between 3 and 5 g liter−1 (vertical gray dashed lines), where the slope of the growth yield starts to decrease slowly, and glucose is obviously not the only limiting factor any longer. Thus, in this range, the growth of Listeria must be influenced by factors other than the pure carbon source availability. Consequently, the synthetic medium presented here is strictly limited for glucose in the range from 0 to 2.5 g liter−1 and, therefore, should not be used with higher levels of glucose, unless all the other medium component concentrations are enhanced proportionally to the glucose concentration.
Fig 1.
Plot showing the final OD600s of L. monocytogenes batch cultured in carbon concentrations from 0 to 12.5 g glucose liter−1. A linear correlation between the final OD600 and the available carbon concentration in the medium is only given between 0 and 2.5 g glucose liter−1 (linear correlation is shown by the dashed lines). This indicates that the mineral medium is only glucose limited for concentrations up to 2.5 g liter−1. The gray curve (right axis) represents the residual carbon after the bacteria reached stationary phase. The error bars indicate the standard deviations of triplicate samples.
Do C-containing compounds other than glucose support growth?
Potentially, components other than glucose contained in our medium, such as amino acids, MOPS buffer, or chelators, like EDTA (23), can serve as potential sources of carbon supporting bacterial growth. Therefore, we tested whether these carbonaceous components can be used as sole C sources for growth by Listeria spp. by omitting glucose from the growth medium. The results shown in Table 3 clearly demonstrate that neither the added amino acids nor EDTA or MOPS was used by L. monocytogenes as an alternative C source for growth. Therefore, the medium presented here is strictly glucose limited, and only carbon from glucose is used for growth. When the MOPS buffer was abolished from the synthetic medium, only minor differences in the early growth phase of L. monocytogenes were observed. However, in the later exponential phase, cultures with MOPS grew better and reached cell numbers 22% higher than those without buffer (Table 3), which is most probably an effect of a pH change in the medium due to missing buffer capacity (9). In all buffered versions of this medium, the pH remained throughout the whole batch growth cycle in the range of 7.2 (start) to 6.8 (end).
Table 3.
L. monocytogenes Scott A in batch culture at 37°C grown in mineral medium omitting selected componentsa
| Medium | Final cell concn (cells ml−1)b | Growth potential (cells ml−1)c | Max specific growth rate μmax (h−1)d |
|---|---|---|---|
| MM completee | 3.86E9 | 3.86E9 | 0.509 |
| MM −MOPSf | 3.02E9 | 3.02E9 | 0.397 |
| MM with Cys, Metg | 1.01E6 | 9.55E5 | 0.121 |
| MM complete −Cys, Meth | 1.00E6 | 9.52E5 | 0.108 |
| MM −amino acidsi | 7.98E5 | 7.48E5 | 0.144 |
| MM −Gluc +amino acidsj | 5.66E4 | 0 | 0 |
| MM −Gluc +Cys, Metk | 5.00E4 | 0 | 0 |
The data represent the measurements of an independent series of experiments in which all media were tested once in the same run (therefore, no standard deviations are indicated). However, all media were tested at least three times in other series, and the results obtained for corresponding media were within ±5%.
Final cell concentration reached.
Growth potential, final cell concentration of L. monocytogenes measured minus the initial number of cells inoculated.
Maximum specific growth rate measured in the exponential phase.
Medium according to the recipe in Table 1.
Medium without MOPS buffer.
Medium without amino acids l-Gln, l-His, l-Trp, l-Leu, l-Ile, l-Val, and l-Arg.
Medium without the two amino acids l-Cys and l-Met.
Medium without amino acids.
Medium with amino acids l-Cys, l-Met, l-Gln, l-His, l-Trp, l-Leu, l-Ile, l-Val, l-Arg, without glucose.
Medium with amino acids l-Cys and l-Met, without glucose.
Influence of trace elements on the μmax and yield of L. monocytogenes.
According to the stoichiometric excess factor analysis (Table 2), the relatively low FC values for Fe and Ca in our medium might constrain the growth of Listeria. Since both elements are incorporated in the TE solution that was added to the medium, we tested the effect of increased TE concentration on the yield and growth performance of L. monocytogenes Scott A. The following concentrations of TE contained in the mineral medium were tested (assuming the concentration of TE in the recipe [Table 1] was 1×): no addition of TE (0× TE), 1× TE (normal recipe), 2× TE, and 4× TE. As presented in Table 4, the final cell yields (OD600) were marginally increased with increasing trace element concentrations. In contrast, when looking at μmax as a kinetic parameter, an influence of elevated TE concentrations can be observed. The highest maximum specific growth rate was reached with single-concentrated trace elements (1× TE), and this value decreased by 38.4% with the 4× TE concentration. No growth was observed when the trace elements were omitted. Since the μmax was reached with 1× TE and the cell yield (OD600) increased only negligibly, the standard TE concentration in our medium was set as shown in Table 1 (corresponding to 1× TE).
Table 4.
Influence of TE on the growth performance of L. monocytogenes Scott Aa
| TE concn | μmax (h−1) | Final OD600 (plate reader) |
|---|---|---|
| 0× | ND | ND |
| 1× | 0.516 ± 0.05 | 0.51 ± 0.005 |
| 2× | 0.438 ± 0.02 | 0.53 ± 0.006 |
| 4× | 0.318 ± 0.04 | 0.55 ± 0.003 |
Shown are the maximum specific growth rate and the final optical density when grown in batch culture with mineral medium using concentrations of trace elements from 0× to 4×. The values are mean values of triplicate experiments ± standard deviations. ND, not detectable.
Influence of amino acids on the growth performance of Listeria strains.
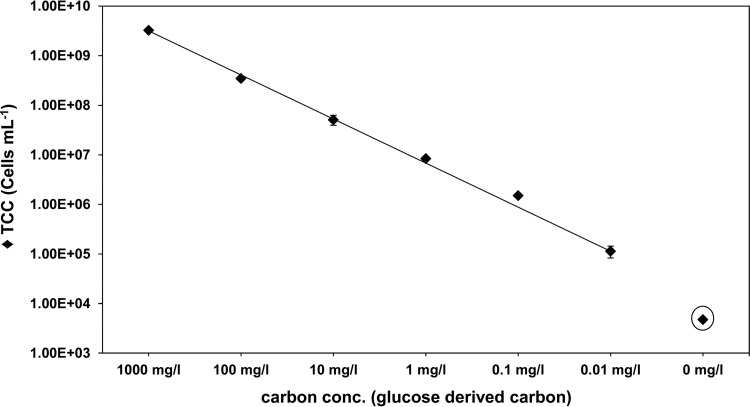
Tsai and Hodgson (9) found only the two amino acids cysteine (Cys) and methionine (Met) to be essential for growth of L. monocytogenes strain 10403. Accordingly, the effects of amino acids on the growth of our strain, Scott A, were tested. As depicted in Table 3, L. monocytogenes Scott A grew only poorly on mineral medium with Cys and Met as the only amino acids added. The yield in this medium was reduced by more than a factor of 4,000 compared to the yield in the complete medium. Also, the μmax was markedly reduced in the medium with Cys and Met only. Similar findings were made when all seven other amino acids were added while omitting Cys and Met; in this medium, the numerical cell yield was reduced by over 3 orders of magnitude, with a low μmax of 0.11 h−1. When all amino acids were omitted from the mineral medium, slight growth was still possible. When all amino acids but no glucose were added to the mineral medium, no growth of Listeria was observed (see Fig. 3).
Fig 3.
L. monocytogenes Scott A growth in mineral medium with serially diluted glucose concentrations at 30°C. Batch cultures were inoculated with an initial cell concentration (conc.) of 5,000 cells ml−1 (0 mg liter carbon−1, circled). The final cell number (TCC) was measured by flow cytometry (FCM) and recorded after Listeria reached stationary phase. The error bars indicate standard deviations of triplicate batch cultures.
The absence of potentially essential amino acids could be an explanation for the poor growth of some Listeria strains. Therefore, we added 19 amino acids to the mineral medium and compared the growth performance of Listeria in this mineral medium. The missing 20th amino acid, asparagine (Asn), was not found to be essential for any Listeria strain in previous studies (5, 22) and therefore was concluded not to be relevant in this experiment. As presented in Table 5, the addition of the amino acids did not have the same unequivocal effect on the μmax for all the Listeria strains tested. Whereas the additional amino acids had no influence on L. monocytogenes strain Scott A, the extra amino acids had a stimulating effect on the L. monocytogenes strains WSLC 1042 and ATCC 19112 with respect to their maximum specific growth rates. The strains L. innocua DSM 20649 and L. welshimeri DSM showed decreases in their μmax values when extra amino acids were added. The two strains L. seeligeri DSM 20751 and L. grayi DSM 20601 showed equal (for L. seeligeri) or better growth performance when extra amino acids were added. Most clearly, the L. ivanovii strain (DSM 20750) did not start to grow even after adding the extra amino acids, which indicated that the strain is strictly dependent on substances from nutritional groups other than amino acids that are not present in our medium. Growth of L. ivanovii could not be stimulated by adding traces of BHI to the MM (final BHI concentration, 1:1,000). Thus, it can be assumed that essential nutrients other than trace elements or vitamins (normally needed only in small amounts) are lacking in the MM to stimulate growth of L. ivanovii. However, the differences in the growth performance between the different strains were becoming much clearer under the mineral medium conditions than in BHI medium (0.5× concentrated), where most strains reached similar μmax values.
Table 5.
μmax values of Listeria strains in mineral medium with 2.5 g glucose liter−1 batch cultured at 37°C
| Strain | μmax (h−1) |
||
|---|---|---|---|
| MM (9 amino acids)a | MM (19 amino acids)b | 0.5× BHIc | |
| L. monocytogenes Scott A | 0.52 ± 0.01 | 0.52 ± 0.02 | 1.03 ± 0.03 |
| L. monocytogenes WSLC 1042 | 0.44 ± 0.02 | 0.62 ± 0.01 | 1.13 ± 0.03 |
| L. monocytogenes ATCC 19112 | 0.22 ± 0.00 | 0.47 ± 0.01 | 0.84 ± 0.02 |
| L. monocytogenes EGDe | 0.47 ± 0.00 | 0.50 ± 0.00 | 0.77 ± 0.02 |
| L. ivanovii DSM 20750 | No growth | No growth | 0.97 ± 0.02 |
| L. innocua DSM 20649 | 0.46 ± 0.00 | 0.35 ± 0.03 | 1.14 ± 0.02 |
| L. seeligeri DSM 20751 | 0.15 ± 0.00 | 0.17 ± 0.03 | 0.96 ± 0.02 |
| L. grayi DSM 20601 | 0.32 ± 0.01 | 0.50 ± 0.02 | 0.74 ± 0.01 |
| L. welshimeri DSM 20650 | 0.34 ± 0.05 | 0.17 ± 0.03 | 0.91 ± 0.03 |
Listeria grown in MM as given in Table 1 (9 amino acids added: l-Cys, l-Gln, l-Met, l-His, l-Trp, l-Leu, l-Ile, l-Val, and l-Arg). The means ± standard deviations of triplicates are shown.
Listeria grown in MM with additional amino acids (19 amino acids: l-Ala, l-Arg, l-Asp, l-Cys, l-Gln, l-Glu, Gly, l-His, l-Ile, l-Leu, l-Lys, l-Met, l-Phe, l-Pro, l-Ser, l-Thr, l-Trp, l-Tyr, and l-Val). The means ± standard deviations of triplicates are shown.
Listeria cultivated in half-concentrated BHI medium (0.5× BHI). The means ± standard deviations of triplicates are shown.
Yield of L. monocytogenes in mineral medium E.
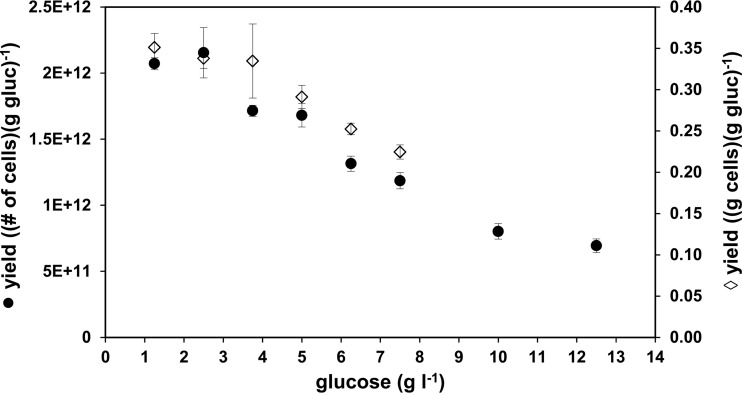
L. monocytogenes Scott A was batch cultured in mineral medium with glucose concentrations increasing from 2.5 to 7.5 g glucose liter−1. After the cells entered stationary phase, the growth yield of each batch at the distinct levels of glucose was determined and calculated either as the yield of biomass (g cells per g glucose) or as the yield of cell numbers (number of cells per g glucose) (Fig. 2). The highest yields were reached at a glucose concentration of 1.25 g liter−1 and at 2.5 g glucose liter−1, whereas the yields for both cell number and dry biomass were decreased at glucose concentrations of 3.75, 5.0, 6.25, and 7.5 g liter−1.
Fig 2.
Growth yields of L. monocytogenes Scott A cultivated in batch culture at 37°C in mineral medium E as a function of the initial glucose concentrations. Left axis (●), numerical cell yield of L. monocytogenes Scott A grown in mineral medium with increasing glucose (gluc) concentrations from 1.25 g liter−1 up to 12.5 g liter−1 in triplicate samples; right axis (♢), biomass yield of cells (L. monocytogenes Scott A) grown in mineral medium with different concentrations of glucose (1.25, 2.5, 3.75, 5.0, 6.25, and 7.5 g liter−1). The error bars indicate the standard deviations of triplicate samples.
Growth of L. monocytogenes in MM at low concentrations of substrate.
A good correlation between the cell yield and the substrate concentration in MM E for L. monocytogenes was demonstrated for high glucose concentrations up to approximately 2.5 g liter−1 (Fig. 1). However, for investigations concerning growth of L. monocytogenes under low-nutrient concentrations, resembling environmental conditions, it is more relevant to know about the growth performance of Listeria cultivated at low concentrations of glucose or similar substrates. In Fig. 3, the final cell concentration of L. monocytogenes Scott A batch cultured in MM at different glucose concentrations from 25 μg liter−1 (corresponding to 0.01 mg carbon liter−1) to 2.5 g liter−1 (1,000 mg carbon liter−1) is shown. These data demonstrate that the new MM is well suited to studying bacterial growth at low substrate concentrations without any loss of cell yield or other restrictions. The linearity between glucose-derived carbon and the final cell number (TCC) of Listeria reached is also valid at very low concentrations of 0.01 mg carbon liter−1. Additionally, it was shown that no growth of L. monocytogenes is possible in MM E without addition of glucose (no other carbohydrates were tested to determine whether they can replace glucose). This demonstrates that L. monocytogenes is also able to grow under oligotrophic concentrations with respect to the only accessible growth substrate (glucose).
Growth of various strains of Listeria in mineral medium.
Since none of the previously published media were tested to determine whether they supported the growth of all Listeria strains (9), the medium presented here was tested with a number of different strains of Listeria. In Table 5, the μmax values that were reached with the particular strains in medium E are listed. The species L. ivanovii (strain DSM 20750) did not grow in our medium, and L. seeligeri showed a very poor specific growth rate. Also, an additional tested L. ivanovii strain (SLCC 4769) showed no growth in the MM.
Growth of Listeria spp. on solid mineral medium.
According to Tsai and Hodgson (9), growing Listeria in liquid medium is a poor criterion for the definition of a minimal medium, since some mineral media, such as MWB (10), support growth of Listeria in a liquid but not in a solid state (9). Hence, the mineral medium presented here was solidified and used as mineral medium agar. A colony of each of the Listeria strains listed in Table 5 (in addition to L. monocytogenes strain ATCC 19115) was streaked out onto a prepared mineral medium agar plate and subsequently incubated at 37°C for 24 h to 48 h. In agreement with the findings of the culture in liquid mineral medium, all the strains grew and formed easily visible colonies on the mineral medium agar, with the exception of L. seeligeri strain DSM 20751, which grew only very slowly, and of L. ivanovii strain DSM 20750, where no formation of colonies was visible at all, even after 48 h of incubation (see Figure S2 in the supplemental material). All strains showing growth on the agar formed colonies after a second consecutive subculture on mineral medium agar.
DISCUSSION
In this article, we present a balanced synthetic mineral medium for L. monocytogenes that is strictly glucose limited for concentrations up to 2.5 g liter−1 and in which good values for the maximum specific growth rate and high yields can be achieved. The importance of having a mineral medium for Listeria (i) the composition of which is entirely known and (ii) where the limiting nutrient for growth is defined has been highlighted in previous publications, such as that by Stoll and coworkers (1), where they pointed out the immense influence of the type of growth medium selected on the outcome of a research question. Also, Slaghuis and colleagues (3) underlined in their article the importance of knowing the nutritional conditions and their influence on the physiology of Listeria. As Pirt stated in his comprehensive book about microbe and cell cultivation (12), “probably the most common first cause of failure to maintain constant exponential growth is a change in the environment.” Such changes can include physical factors, such as pH, temperature, and availability of oxygen or nutrients, such as glucose. If these important prerequisites are ignored, the organism may grow under conditions that are not controllable, and there occur processes the complexity of which exceeds the investigators' intention (24). Several mineral medium recipes have been published for Listeria (4–10), but none of them has been shown to be strictly growth limiting for one specific nutrient, usually the carbon source. Typically, media for heterotrophic microbes are designed to be carbon limited, as carbon is the constituent consumed in the largest amounts (25). Under the nutritional conditions presented in this report, with glucose as the only growth-limiting substrate, the growth of L. monocytogenes can be examined under well-balanced, highly controllable, and predictable growth conditions.
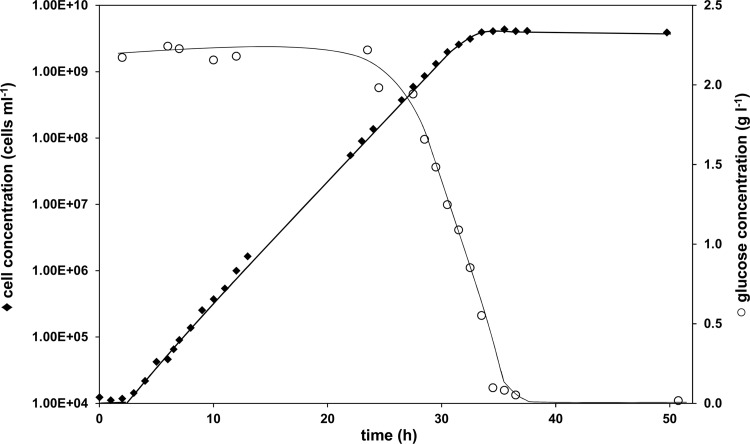
In the theoretical excess factor analysis (Table 2) of four previously published mineral media, we demonstrated that they differ substantially with respect to their elemental compositions. In particular, the role of trace elements in most of the previously reported media has been neglected. Our medium composition (Table 1) deviates most from older media in the addition of trace elements and having adapted the glucose concentrations to a value where true glucose-limiting conditions during Listeria growth were achieved (Fig. 1). Since the glucose concentrations of older mineral media (e.g., media A to D) are usually set to 10 g liter−1, i.e., four times higher than in our medium, a correspondingly larger cell crop in older media should be expected. However, none of the available data on either optical densities or cell numbers reached in the older media exceed our findings. Therefore, we conclude that glucose concentrations in older mineral media are generally too high, and one must assume that glucose is not the limiting growth factor in these media. Striking evidence for the fact that the older media are not well balanced is provided in Phan-Thanh and Gormon's publication (4), where they show growth curves of L. monocytogenes EGD in medium B (10) and their own improved medium C (4). Compared to the growth curve of L. monocytogenes Scott A reported in the present study (Fig. 4), the growth patterns in media B (10) and C (4) in Phan-Thanh and Gormon's report (4) exhibited no extended exponential growth phases but rather long and undefined transitions from exponential to stationary phase. In the medium presented here, the three typical phases of bacterial growth (lag phase, exponential phase, and stationary phase) are clearly distinguishable from each other, and furthermore, the residual glucose levels measured during growth indicate that bacterial growth is limited due to glucose levels being depleted (Fig. 4). Remarkably, the doubling time reported in medium C is not much different at 88 min (4) (corresponding to a μmax of 0.47 h−1) than our reported μmax (medium E) of 0.51 (±0.03) h−1, and the final cell numbers for the two media are comparable. This example demonstrates clearly that for the choice of an optimal medium, it is important to compare not only specific growth rates and yields, but growth patterns/curves, as well.
Fig 4.
Representative growth curve of L. monocytogenes Scott A grown in mineral medium at 37°C in batch culture (diamonds). The residual glucose concentration (right axis; circles) was measured during the experiment. Using cell number as a growth parameter allows the demonstration of exponential growth over more than 5 orders of magnitude.
In order to improve the present medium, we tried to adjust potential compositional bottlenecks in the recipe according to our elemental analysis of those that have a high chance of being limiting (Table 2). For example, higher concentrations of TE were found to be unfavorable for the maximum specific growth rates of Listeria, while the cell yield only marginally increased. However, we demonstrated that the trace elements are an indispensable part of the minimal media and that their concentration has an essential influence on growth.
Furthermore, the concentrations of the vitamins biotin, thiamine, and riboflavin were distinctly reduced in our medium compared to earlier reports, except for the lipoic acid concentration, which was increased. In earlier reports, observations concerning the benefits of some vitamins are contradictory and obviously not applicable to all strains of Listeria. Therefore, we did not attempt to reduce the list of vitamins added, especially since none of them seemed to influence bacterial growth negatively. Various studies have shown a massive influence of sugars, alternative carbon sources, and other factors on the gene expression and physiology of Listeria in liquid culture (26–28). These data demonstrate that it is extremely important to know the exact composition of a given medium with respect to carbon sources that Listeria is able to use alternatively. This study demonstrates that L. monocytogenes Scott A is not able to grow on carbon from amino acids, as already stated by Premaratne and coworkers (10), in the absence of a primary carbon and energy source, such as glucose.
The data on the maximum specific growth rates of different Listeria strains (Table 5) demonstrate the heterogeneity of the genus Listeria and, particularly, of different strains within one species, as shown in this study for representatives of the species L. monocytogenes. The mineral medium, even when complemented with 19 amino acids, shows inconsistent results and once again emphasizes the heterogeneity of the genus. Since not all strains showed good growth, most clearly L. ivanovii DSM 20750, components other than amino acids are essential for their growth. As demonstrated by supplementing the MM with BHI traces, it can also be assumed that substances other than TE or vitamins, e.g., principal carbohydrates other than glucose or increased concentrations of amino acids, are essential to stimulate the growth of L. ivanovii.
The data presented demonstrate that Listeria spp. are fastidious bacteria with respect to their nutrient requirements, and furthermore, the data for maximum specific growth rates and yields differ significantly among the different strains. This demonstrates that selection of the appropriate strain to study Listeria under defined conditions is a very crucial point and should be considered carefully. However, due to different growth physiologies influenced by different requirements for nutrients, the replacement of pathogenic L. monocytogenes by apathogenic Listeria species, such as L. innocua (29), as a model organism could lead to data that are neither comparable nor representative.
In conclusion, our minimal medium supports good growth of L. monocytogenes, particularly the strain Scott A, and other strains of the genus Listeria. It can be recommended as a viable alternative to complex media, such as BHI or lysogeny broth (LB), where the exact nutritional composition is essentially unknown, and therefore, a change in the growth environment due to effects such as limitation of essential nutrients cannot be avoided (30).
ACKNOWLEDGMENTS
This study, as a part of the project BactFlow, was funded by the Competence Center Environment and Sustainability (CCES) directed by the Swiss Federal Institute of Technology Zurich ETHZ.
Footnotes
Published ahead of print 1 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03538-12.
REFERENCES
- 1. Stoll R, Mertins S, Joseph B, Müller-Altrock S, Goebel W. 2008. Modulation of PrfA activity in Listeria monocytogenes upon growth in different culture media. Microbiology 154:3856–3876 [DOI] [PubMed] [Google Scholar]
- 2. Friedman ME, Alm WL. 1962. Effect of glucose concentration in the growth medium on some metabolic activities of Listeria monocytogenes. J. Bacteriol. 84:375–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slaghuis J, Joseph B, Goebel W. 2007. Metabolism and physiology of Listeria monocytogenes, p 63–80 In Goldfine H, Shen H. (ed), Listeria monocytogenes: pathogenesis and host response. Springer, New York, NY [Google Scholar]
- 4. Phan-Thanh L, Gormon T. 1997. A chemically defined minimal medium for the optimal culture of Listeria. Int. J. Food Microbiol. 35:91–95 [DOI] [PubMed] [Google Scholar]
- 5. Siddiqi R, Khan MA. 1989. Amino acid requirement of six strains of Listeria monocytogenes. Zentralbl. Bakteriol. 271:146–152 [DOI] [PubMed] [Google Scholar]
- 6. Trivett TL, Meyer EA. 1971. Citrate cycle and related metabolism of Listeria monocytogenes. J. Bacteriol. 107:770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welshimer HJ. 1963. Vitamin requirements of Listeria monocytogenes. J. Bacteriol. 85:1156–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedman ME, Roessler WG. 1961. Growth of Listeria monocytogenes in defined media. J. Bacteriol. 82:528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsai H-N, Hodgson DA. 2003. Development of a synthetic minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 69:6943–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Premaratne RJ, Lin WJ, Johnson EA. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones CE, Shama G, Andrew PW, Roberts IS, Dorothy J. 1995. Comparative study of the growth of Listeria monocytogenes in defined media and demonstration of growth in continuous culture. J. Appl. Microbiol. 78:66–70 [DOI] [PubMed] [Google Scholar]
- 12. Pirt SJ. 1975. Principles of microbe and cell cultivation. Blackwell Scientific Publications, Oxford, United Kingdom [Google Scholar]
- 13. Egli T, Fiechter A. 1981. Theoretical analysis of media used in the growth of yeasts on methanol. J. Gen. Microbiol. 123:365–369 [Google Scholar]
- 14. Briers Y, Klumpp J, Schuppler M, Loessner MJ. 2011. Genome sequence of Listeria monocytogenes Scott A, a clinical isolate from a food-borne listeriosis outbreak. J. Bacteriol. 193:4284–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egli T. 2009. Nutrition, microbial, p 308–324 In Schaechter M. (ed), Encyclopedia of microbiology, 2nd ed Academic Press, San Diego, CA [Google Scholar]
- 16. Kötzsch S, Egli T, Bucheli-Witschel M. 2010. Beurteilung von Kunststoffen in Kontakt mit Trinkwasser: BioMig: Ein verbessertes Methodenpaket zur Bestimmung des Verkeimungspotenzials. Fachz. Gas Wasser Abwasser 90:797–810 [Google Scholar]
- 17. Egli T, Weilenmann HU, El-Banna T, Auling G. 1988. Gram-negative, aerobic, nitrilotriacetate-utilizing bacteria from wastewater and soil. Syst. Appl. Microbiol. 10:297–305 [Google Scholar]
- 18. Hammes F, Berney M, Wang Y, Vital M, Koster O, Egli T. 2008. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 42:269–277 [DOI] [PubMed] [Google Scholar]
- 19. Bruss ML, Black AL. 1978. Enzymatic microdetermination of glycogen. Anal. Biochem. 84:309–312 [DOI] [PubMed] [Google Scholar]
- 20. Bateman RC, Jr, Evans JA. 1995. Using the glucose oxidase/peroxidase system in enzyme kinetics. J. Chem. Educ. 72:240–241 [Google Scholar]
- 21. Füchslin HP, Schneider C, Egli T. 2012. In glucose-limited continuous culture the minimum substrate concentration for growth, smin, is crucial in the competition between the enterobacterium Escherichia coli and Chelatobacter heintzii, an environmentally abundant bacterium. ISME J. 6:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ralovich B, Shahamat M, Woodbine M. 1977. Further data on the characters of Listeria strains. Med. Microbiol. Immunol. 163:125–139 [DOI] [PubMed] [Google Scholar]
- 23. Witschel M, Nagel S, Egli T. 1997. Identification and characterization of the two-enzyme system catalyzing oxidation of EDTA in the EDTA-degrading bacterial strain DSM 9103. J. Bacteriol. 179:6937–6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovárová-Kovar K, Egli T. 1998. Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiol. Mol. Biol. Rev. 62:646–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egli T, Lendenmann U, Snozzi M. 1993. Kinetics of microbial growth with mixtures of carbon sources. Antonie Van Leeuwenhoek 63:289–298 [DOI] [PubMed] [Google Scholar]
- 26. Gilbreth SE, Benson AK, Hutkins RW. 2004. Catabolite repression and virulence gene expression in Listeria monocytogenes. Curr. Microbiol. 49:95–98 [DOI] [PubMed] [Google Scholar]
- 27. Larsen MH, Kallipolitis BH, Christiansen JK, Olsen JE, Ingmer H. 2006. The response regulator ResD modulates virulence gene expression in response to carbohydrates in Listeria monocytogenes. Mol. Microbiol. 61:1622–1635 [DOI] [PubMed] [Google Scholar]
- 28. Becker LA, Cetin MS, Hutkins RW, Benson AK. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houtsma PC, Kusters BJM, de Wit JC, Rombouts FM, Zwietering MH. 1994. Modelling growth rates of Listeria innocua as a function of lactate concentration. Int. J. Food Microbiol. 24:113–123 [DOI] [PubMed] [Google Scholar]
- 30. Baev M, Baev D, Radek A, Campbell J. 2006. Growth of Escherichia coli MG1655 on LB medium: determining metabolic strategy with transcriptional microarrays. Appl. Microbiol. Biotechnol. 71:323–328 [DOI] [PubMed] [Google Scholar]