Abstract
A specific real-time PCR quantification method combined with a propidium monoazide sample treatment step was developed to determine quantitatively the viable population of the Photobacterium phosphoreum species group in raw modified-atmosphere-packed salmon. Primers were designed to amplify a 350-bp fragment of the gyrase subunit B gene (gyrB) of P. phosphoreum. The specificity of the two primers was demonstrated by using purified DNA from 81 strains of 52 different bacterial species. When these primers were used for real-time PCR in pure culture, a good correlation (R2 of 0.99) was obtained between this method and conventional enumeration on marine agar (MA). Quantification was linear over 5 log units as confirmed by using inoculated salmon samples. On naturally contaminated fresh salmon, the new real-time PCR method performed successfully with a quantification limit of 3 log CFU/g. A correlation coefficient (R2) of 0.963 was obtained between the PCR method and classic enumeration on MA, followed by identification of colonies (290 isolates identified by real-time PCR or by 16S rRNA gene sequencing). A good correlation with an R2 of 0.940 was found between the new PCR method and an available specific conductance method for P. phosphoreum. This study presents a rapid tool for producing reliable quantitative data on viable P. phosphoreum bacteria in fresh salmon in 6 h. This new culture-independent method will be valuable for future fish inspection, the assessment of raw material quality in fish processing plants, and studies on the ecology of this important specific spoilage microorganism.
INTRODUCTION
Modified-atmosphere-packed (MAP) fresh fish is increasingly popular in Europe and widely sold in supermarkets as a chilled product. Compared to aerobically stored fresh fish, this kind of packaging, with typical headspace CO2 concentrations of 20 to 60%, modifies the dominating microbiota mainly by reducing growth of aerobic Gram-negative bacteria like Pseudomonas (1, 2). This results in an extended shelf life which facilitates the chilled distribution and marketing of fresh MAP fish. However, this packaging allows the growth of CO2-resistant bacteria, including Gram-positive lactic acid bacteria and the Gram-negative bacterium Photobacterium phosphoreum (2–4). The latter has been identified as the specific spoilage organism (SSO) responsible for trimethylamine production and sensory spoilage of MAP cod (5, 6) and as the main spoilage bacterial species of several chilled marine fish, including cod, garfish, halibut, saithe, salmon, and shrimp (7–15).
Multilocus analysis, based on 16S rRNA and gyrB and luxABFE genes, divided strains originally isolated and identified as P. phosphoreum into three distinct clades of P. phosphoreum, Photobacterium iliopiscarium, and Photobacterium kishitanii (16, 17). The members of the P. phosphoreum species group have been reported as important for spoilage of raw MAP fish, and they include both luminous and nonluminous variants (5, 18). It is therefore relevant to detect and enumerate this species group in fish products.
As no specific medium is available for enumeration of either the P. phosphoreum species group or the individual species in seafood, the control of these important spoilage bacteria is problematic. There is a specific conductance method for quantifying the P. phosphoreum species group in fish samples (19) which is quantitative, sensitive, and selective. However, it is not very rapid, with analyses taking 12 to 50 h depending on the bacterial concentration in the sample. Instruments required to run this conductance method are available, e.g., from SY-LAB in Austria, but they are far less widespread than real-time PCR thermocyclers. Therefore, the development of a rapid and specific real-time PCR quantification method for viable cells of the P. phosphoreum species group in fresh fish is clearly of interest. One of the limitations of PCR and real-time PCR methods for the quantification of bacteria in food samples is that both live and dead cells are detected (20). To overcome this problem, propidium monoazide (PMA) can be used before DNA extraction. PMA is an intercalating DNA agent which enters dead cells and binds to DNA, inhibiting subsequent PCR amplification and thereby ensuring quantification of viable bacteria (21). PMA has been used in combination with several real-time PCR methods for quantification of viable bacteria in food, e.g., Campylobacter (22), Listeria monocytogenes (23), Brochothrix thermosphacta (24, 25), Vibrio parahaemolyticus (26, 27), and Escherichia coli O157:H7 (28).
In the present study, we describe a new rapid assay to quantify and identify the P. phosphoreum species group in raw salmon using real-time PCR. This new method has been validated for artificially inoculated and naturally contaminated fresh MAP salmon and compared to both the classic enumeration method on culture media followed by colony identification and the conductance method specifically developed for these bacteria. The term P. phosphoreum will be used below to refer collectively to the closely related members of the P. phosphoreum species group.
MATERIALS AND METHODS
Raw salmon samples.
To establish a standard curve on artificially contaminated fish, 24 samples of fresh salmon were inoculated with different concentrations of P. phosphoreum and analyzed. In order to evaluate the real-time PCR quantification method using naturally contaminated samples, it was compared with viable plate counting with 13 samples of MAP salmon steaks. These were obtained from a local French processing plant or purchased from a local supermarket and were packed with a headspace gas composition of approximately 50% CO2 and 50% N2. With the aim of comparing the real-time PCR method and a conductance method for quantification of P. phosphoreum, 26 naturally contaminated MAP salmon steaks were assessed. These samples were obtained from a Danish processing plant and were packed with a headspace gas composition of approximately 20% CO2 and 80% N2.
Bacterial strains and DNA extraction.
Bacterial strains used in this study are listed in Table 1. P. phosphoreum strains were grown in brain heart infusion broth (BHI; Biokar Diagnostics, Beauvais, France) supplemented with 2.0% NaCl and incubated at 15°C for 24 h.
Table 1.
Bacterial isolates used to develop and evaluate the specificity of a real-time PCR assay for detection of the P. phosphoreum species group
| Bacterial species | Strain | Source | No. of strains | Real-time PCR resulta |
|---|---|---|---|---|
| Photobacterium phosphoreum group | ||||
| P. phosphoreum | CIP 102511T | Fish | 1 | + |
| P. phosphoreum | DSM 2167 | Hake (Merluccius capensis) | 1 | + |
| P. iliopiscarium | DSM 9896 | Pyloric ceca of herring | 1 | + |
| P. kishitanii | LMG 23890 | Light organ of Physiculus japonicus | 1 | + |
| P. phosphoreum | Oniris/Ifremer Collection | Spoiled salmon steak or fillets under MAP | 23 | + |
| Other Photobacterium species | ||||
| P. leiognathi | CIP 66.5T | Light organ of teleostean fish (Leiognathus equula) | 1 | − |
| P. angustum | CIP 75.10T | Seawater at depth of 750 m | 1 | − |
| P. damselae subsp. piscicida | CIP 103910 | White perch | 1 | − |
| P. damselae subsp. damselae | DSM 7482 | Fish ulcer | 1 | − |
| P. indicum | LMG 22857 | Marine mud | 1 | − |
| P. lypolyticum | LMG 23071 | Light organ of teleostean fish (family Leiognathidae) | 1 | − |
| P. rosenbergii | LMG 22223 | Tissue extract of bleached coral | 1 | − |
| Non-Photobacterium strains | ||||
| Aeromonas hydrophila | Oniris/Ifremer Collection | Spoiled shrimp under MAP | 1 | − |
| Aeromonas salmonicida subsp. salmonicida | Oniris/Ifremer Collection | Spoiled shrimp under MAP | 1 | − |
| Buttiauxela gaviniae | Oniris/Ifremer Collection | Spoiled shrimp under MAP | 1 | − |
| Brochothrix campestris | DSM 4712 | Soil | 1 | − |
| Brochothrix thermosphacta | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Carnobacterium maltaromaticum | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| C. maltaromaticum | NCDO 2762 | Kidney of adult cutthroat trout | 1 | − |
| Carnobacterium divergens | NCDO 2763 | Vacuum-packed minced beef | 1 | − |
| C. divergens | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Escherichia coli | ATCC 25922 CIP 76.24 | Clinical isolate | 1 | − |
| Enterococcus faecalis | CIP 105042 | Poultry cecum | 1 | − |
| Enterococcus faecium | CIP 106742 | Dairy products | 1 | − |
| Hafnia alvei | Oniris/Ifremer Collection | Spoiled salmon fillet under MAP | 1 | − |
| H. alvei | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Listeria monocytogenes | CIP 78.35 | Spinal fluid | 1 | − |
| Lactococcus lactis | CNRZ 1075 | Origin not available | 1 | − |
| Lactococcus piscium | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Lactobacillus raffinolactis | Oniris/Ifremer Collection | Spoiled salmon fillet under MAP | 1 | − |
| Lactobacillus curvatus | Oniris/Ifremer Collection | Cold-smoked salmon | 1 | − |
| Lactobacillus fuchuensis | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Lactobacillus sakei | NBIMCC 3453 | NBIMCC | 1 | − |
| Leuconostoc gasicomitatum | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Listeria innocua | CIP 80.11T | Bovine brain | 1 | − |
| Moellerella wisconsensis | Oniris/Ifremer Collection | Spoiled salmon fillet under MAP | 1 | − |
| Morganella morganii subsp. morganii | CIP A231 | A case of summer diarrhea | 1 | − |
| Morganella psychrotolerans | Oniris/Ifremer Collection | Spoiled salmon fillet under MAP | 1 | − |
| M. psychrotolerans | CIP 109403 | Cold-smoked tuna | 1 | − |
| Proteus mirabilis | CIP A235 | Origin not available | 1 | − |
| Pseudomonas fluorescens | CIP 69.13T | Prefilter tanks | 1 | − |
| Pseudoalteromonas sp. | Oniris/Ifremer Collection | Spoiled shrimp under MAP | 1 | − |
| Psychrobacter sp. | Oniris/Ifremer Collection | Spoiled shrimp under MAP | 1 | − |
| Salmonella enterica | CIP 81.3 | Feces | 1 | − |
| Serratia proteamaculans | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Serratia quinivorans | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Serratia sp. | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
| Shewanella putrefaciens | ATCC 8071 | Surface of tainted butter | 1 | − |
| Shewanella baltica | Oniris/Ifremer Collection | Spoiled salmon fillet under MAP | 1 | − |
| S. baltica | Oniris/Ifremer Collection | Spoiled shrimp under MAP | 1 | − |
| Staphylococcus aureus | ATCC 25923 CIP 76.25 | Clinical isolate | 1 | − |
| Vibrio anguillarum | CIP 63.36T | Ulcerous lesion of cod (Gadus morhua) | 1 | − |
| Vibrio fischeri | DSM 2168 | Fish | 1 | − |
| Vibrio orientalis | CIP102891T | Seawater | 1 | − |
| Vibrio parahaemolyticus | CIP 75.2T | Shirasu food poisoning | 1 | − |
| Vibrio sp. | Oniris/Ifremer Collection | Spoiled shrimp under MAP | 1 | − |
| Vagococcus fluvialis | CIP 102976T | Chicken feces | 1 | − |
| Vagococcus fluvialis/carniphilus | Oniris/Ifremer Collection | Trout | 1 | − |
| Yersinia intermedia | Oniris/Ifremer Collection | Spoiled salmon steak under MAP | 1 | − |
+, amplification product with a Tm of 84°C obtained in the method's range of use; −, no PCR product obtained in the method's range of use.
Photobacterium isolates from culture collections were grown in BHI (Biokar) with 2.0% NaCl between 15°C and 25°C depending on the strain and for 24 to 48 h. Other strains were grown in BHI (Biokar) at 20°C for 24 to 48 h except Escherichia coli ATCC 25922/CIP 76.24, Enterococcus faecalis CIP 105042, Enterococcus faecium CIP 106742, Staphylococcus aureus ATCC 25923/CIP 76.25, and Salmonella enterica CIP 81.3, which were grown at 37°C, and Listeria innocua CIP 80.11T, Listeria monocytogenes CIP 78.35, and Pseudomonas fluorescens CIP 69.13T, which were grown at 30°C. Vibrio isolates from culture collections were grown in marine broth (Difco Laboratories, Detroit, MI) at 20°C for 24 to 48 h except for Vibrio parahaemolyticus, which was grown at 37°C. To validate primer specificity, a total of 27 P. phosphoreum isolates were tested as targets for inclusivity tests while 54 non-P. phosphoreum isolates belonging to 49 different species were used for exclusivity testing (Table 1). These 81 strains were tested in vitro using genomic DNA extracted from 1.5 ml of bacterial cultures at a concentration of at least 7 log CFU/ml (data not shown). The chromosomal DNA of all bacterial isolates was extracted using the Qiagen DNeasy blood and tissue kit (Qiagen, S.A., Courtaboeuf, France).
Extraction of bacterial DNA from salmon flesh.
For each salmon sample, a 30-g portion was aseptically weighed in a sterile stomacher plastic bag and 120 ml sterile peptone water (0.85% NaCl, 0.1% peptone) was added to obtain a 5-fold dilution which was then homogenized for 2 min using a stomacher 400 (Seward Medical, London, United Kingdom). In order to separate the eukaryotic cells and DNA of salmon from the bacterial cells, 9 ml of homogenized suspension (previously treated with PMA for naturally contaminated samples) was filtered on a NucleoSpin Plant L column (Macherey-Nagel, Hoerdt, France) by centrifugation at 11,700 × g for 10 min at 4°C. The supernatant was carefully removed, and the bacterial cell pellet was resuspended in 400 μl of enzymatic lysis solution (20 mM Tris-HCl [pH 8.0], 2 mM sodium EDTA, 1.2% Triton X-100, 20 mg/ml lysozyme, 11.6 U mutanolysin) in a 2-ml microtube and then incubated at 37°C for 1 h. Reagents used in this step were purchased from Sigma-Aldrich. A mechanical lysis step was then performed by adding about 0.2 g of glass beads (150 to 200 μm diameter) and shaking twice for 2 min in a bead beater MM200 (30 Hz) (BioSpec Products, Bartlesville, OK). Proteins were digested by proteinase K (20 mg/ml) during incubation at 56°C for 30 min and with 200 μl of AL buffer (DNeasy blood and tissue kit; Qiagen). To pellet the glass beads, centrifugation at 9,500 × g for 3 min was carried out and the supernatant was transferred into a 2-ml microtube to perform nucleic acid precipitation by addition of 200 μl of ice-cold absolute ethanol. DNA purification was carried out using a DNeasy blood and tissue kit as described in the Qiagen instruction manual.
PMA treatment of samples.
Prior to DNA extraction, a preliminary treatment with PMA (Biotium, Hayward, CA) was carried out in order to reduce amplification of DNA from dead cells. PMA was dissolved in 20% dimethyl sulfoxide to provide a stock solution of 2.5 mM. To obtain a final PMA concentration of 50 μM in samples, 20 μl of the PMA stock solution was added to 980 μl of P. phosphoreum pure culture or 180 μl of the PMA stock solution was added to 8,820 μl of homogenized and naturally contaminated salmon. The tubes were then shaken and placed in the dark at room temperature for 5 min to allow the PMA to penetrate the dead cells and bind to DNA. Finally, to photoactivate the PMA, the tubes were placed in crushed ice with their lids removed and exposed for 5 min to light from a 500-W halogen lamp positioned at a distance of 20 cm. They were shaken occasionally to ensure complete cross-linking of the available DNA (24).
Artificially contaminated pieces of salmon flesh.
Salmon flesh was prepared and ionized as previously described by Macé et al. (29). A 60-g portion of ionized raw salmon cubes was aseptically weighed into a sterile plastic bag, and 240 ml of sterile physiological saline solution (0.85% NaCl, 0.1% peptone) was added to obtain a 5-fold dilution. The suspension was homogenized for 2 min in a stomacher 400. Eight sterile plastic tubes were filled with 25 ml of the sterile salmon homogenate. Seven of them were inoculated with 1 ml of 10-fold serially diluted suspensions of P. phosphoreum CIP 102511, previously treated with PMA, and one noninoculated tube was used as a control. The concentrations of P. phosphoreum in the salmon samples were prepared in order to range from approximately 7.7 to 1.7 log CFU/g. DNA was extracted from each homogenate as described above. Concentrations of bacteria in the homogenates were determined by real-time PCR and viable counting in duplicate using marine agar (MA; Difco Laboratories, Detroit, MI) incubated at 15°C 7 days. These conditions were used for P. phosphoreum growth in a previous study (30). The viable counts were expressed as the arithmetic mean of the two plate counts in log CFU/g. This experiment was repeated three times independently.
Primer design and real-time PCR assay.
Alignment of the gyrase subunit B (gyrB) gene DNA sequence from P. phosphoreum and closely related species was performed using the CLC DNA Workbench 6.5 (CLC bio, Aarhus, Denmark) and BioEdit sequence alignment software (31) (Fig. 1). Primers specific to the P. phosphoreum species group were designed in a conserved region so that they excluded other Photobacterium spp. and the closely related Vibrio fischeri (Fig. 1). The forward primer MO627 (5′-TACTGTTGAAGTGGCGAT-3′) matched positions 461 to 478, and the reverse primer MO628 (5′-TCTGCTGGGCTTTCTAAT-3′) matched positions 794 to 811 of the P. phosphoreum type strain gyrB gene (ATCC 11040T/CIP 102111T, accession number AY455875.1). The primers amplified a 350-bp specific fragment. Primer specificity was tested in silico using the Basic Local Alignment Search Tool (BLAST) program (Fig. 1) and Primer BLAST (32). The hybridization temperature of the primers was optimized using the temperature gradient test on a Chromo4 real-time PCR detection system (Bio-Rad Laboratories, Marnes-La-Coquette, France). Real-time PCR amplification was performed using the IQ SYBR green Supermix (Bio-Rad Laboratories, Marnes-La-Coquette, France) in 15-μl reaction volumes containing 7.5 μl of 2× IQ SYBR green Supermix; 2.5 μM each primer (Fisher Bioblock Scientific, Illkirch, France) and 2 μl of template DNA or 1 ng for the specificity test on the pure strains, which are listed in Table 1. The amplification reaction was conducted using the Chromo4 real-time PCR detection system (Bio-Rad Laboratories). The optimum cycling parameters were as follows: 94°C hold temperature for 3 min for initial denaturation, 35 cycles of amplification (94°C for 30 s, 58°C for 1 min, and 72°C for 1 min), followed by a final extension at 72°C for 5 min. A melting curve between 70°C and 90°C was determined after the last amplification cycle and at a temperature transition rate of 0.5°C/s. The background limit was set at 0.02 when the cycle threshold (CT) values were determined. All amplification reactions were run in triplicate in three independent assays. The P. phosphoreum-specific PCR product had a melting temperature (Tm) of 84°C, and a response was considered positive only when this was observed. Real-time PCR assays were interpreted as positive for P. phosphoreum if the determined CT values were ≤27.5 for DNA from pure cultures or ≤31 for DNA from inoculated fish samples. Higher CT values were considered out of the range for this assay. These cutoff values were determined as the lowest observed CT for the non-P. phosphoreum isolates when evaluating specificity (Table 1).
Fig 1.

Sequence alignment of the gyrase subunit B gene (gyrB) from Photobacterium species and Vibrio fischeri. The accession numbers of the sequences used for the alignment are shown. Sequences used for primer design are boxed.
Standard curves and amplification efficiency.
Two different calibration curves were constructed to determine the sensitivity and efficiency of the real-time PCR method. A standard curve was obtained by using purified genomic DNA from P. phosphoreum CIP 102511T, extracted from serial dilutions of a bacterial suspension. The culture was diluted in sterile peptone water (0.85% NaCl, 0.1% peptone) to obtain concentrations ranging from 1 to 8 log CFU/ml.
The other standard curve was obtained from bacterial DNA extracted from independent triplicate pieces of salmon flesh previously inoculated with 10-fold serially diluted concentrations of the P. phosphoreum type strain. The different bacterial concentrations (log CFU/g) were plotted against the corresponding CT values. The slope of the linear relation of this curve was used to determine the amplification efficiency (E) by applying the following equation: E = 10−1/slope − 1 (33).
Comparison of real-time PCR and plate counting for quantification of P. phosphoreum in naturally contaminated salmon.
A total of 13 samples of MAP salmon steaks with a headspace gas composition of approximately 50% CO2 and 50% N2was obtained from a local French processing plant or purchased from a local supermarket. The samples were stored at 4°C for 3 days and then at 8°C for 7 days after a break of 2 h at 20°C, according to a French shelf life protocol (34). At different times during the storage period, bacterial DNA was extracted from salmon samples and P. phosphoreum was quantified using the real-time PCR method described above. The CT values obtained by real-time PCR were used to calculate the concentration (log CFU/g) present in each sample, based on the standard curve established with artificially contaminated pieces of salmon flesh. All the real-time PCRs were carried out in triplicate, and the mean values were used. Results were compared to viable counts on MA (Difco), followed by determination of the ratio of P. phosphoreum bacteria in the samples. This was done by testing about 20 colonies per countable plate using real-time PCR (see below). The concentration of P. phosphoreum was then calculated by multiplying the viable counts on MA and the proportion of P. phosphoreum isolates for each tested sample. The correlation between the concentrations obtained by plate counts and real-time PCR quantification of P. phosphoreum was determined by linear regression.
Identification of bacterial isolates from MA plates.
MA is a nonselective medium used for total viable counting so identification was required to determine the proportion of P. phosphoreum colonies on this medium for each naturally contaminated sample. From each of the 13 MAP salmon samples, about 20 colonies were randomly selected from MA plates of the highest dilution showing growth. The 290 isolates collected were purified twice on BHI agar (Biokar) supplemented with 2.0% NaCl. In order to determine whether or not they belonged to the P. phosphoreum species group, all 290 isolates were analyzed by the real-time PCR assay described above and following extraction of chromosomal DNA using the Qiagen DNeasy blood and tissue kit (Qiagen). They were thus classified as P. phosphoreum or non-P. phosphoreum isolates. To confirm these results, from the 290 isolates, 50 from 4 different salmon samples were identified by partial 16S rRNA gene sequencing as described in a previous study (11). In brief, the partial nucleotide sequence (about 600 bp) of the amplified 16S rRNA gene was determined with an automated sequencer (Beckman Coulter Genomics, Takeley, United Kingdom) using the primer SP1 (35) for the 50 strains. The resulting sequences were then submitted to BLAST, available at the National Center for Biotechnology Information (NCBI; Bethesda, MD) (http://www.ncbi.nlm.nih.gov/), for representation of sequence and similarity searches in the GenBank database.
Comparison of real-time PCR with a conductance method for quantification of P. phosphoreum in naturally contaminated salmon.
A total of 26 MAP salmon steaks with a headspace gas composition of approximately 20% CO2 and 80% N2 was obtained from a Danish processing plant. The samples were divided into two batches which were stored at 2°C or 4°C for 9 days. Each day, the concentration of P. phosphoreum was determined by using both an available specific conductance method (19) and the new real-time PCR method described above. An Mx3000P thermocycler (Stratagene, AH Diagnostics, Aarhus, Denmark) was applied, and a standard curve was developed for the real-time PCR method when used with this instrument. The correlation between the results obtained by the two methods was determined by linear regression.
Linear regression and statistical analysis.
Linear regression was performed using Fig.P software (version 2.98; Biosoft, Cambridge, United Kingdom). Analysis of variance (ANOVA), the Fisher least significant difference (LSD) test (95.0%), the Kruskal-Wallis test, and the Mood test on medians were all performed on the nonpairwise P. phosphoreum concentration data obtained from the real-time PCR and viable count methods on naturally contaminated samples. ANOVA was used to compare variances within and between results obtained from the two methods for 13 samples. The Fisher LSD test (95.0%) was used to measure variability in samples by displaying the standard deviation of means, the Kruskal-Wallis test was used to compare medians, and the Mood test was used to analyze the distribution of data around medians. These tests were carried out using STATGRAPHICS Centurion XVI version 16.1.11 software (Statistical Graphics Corp.).
RESULTS
Specificity of the real-time PCR assay.
Using Primer BLAST (32), the selected primers, MO627 and MO628, targeted only P. phosphoreum-related species, and by using the BLASTn program, they showed 100% maximum identity with P. phosphoreum-related species. The primers developed were specific to P. phosphoreum-related species, and all 27 culture collection strains studied were correctly identified (Table 1). The threshold of the assay was set at a fluorescent background value of 0.02, resulting in CT values ranging from 9 to 14 for the 27 target isolates, with a CT mean value of 10.3 ± 2.3. The melting temperature calculated at the end of each real-time PCR assay was 84°C. CT values above 27.5 were obtained for all 54 non-P. phosphoreum isolates, and these values were considered out of the specificity range. The CT threshold obtained was 27.5 for DNA from pure cultures and 31.0 for bacterial DNA extracted from salmon flesh.
The specificity of these primers, when tested against a large collection of nontarget bacteria (Table 1) including other species of Photobacterium and other bacterial species frequently encountered in seafood products, shows that they can be used for specific detection of P. phosphoreum.
Sensitivity and quantification limits of the assay.
The detection and quantification limits of the PCR assay when used for pure cultures were determined by analyzing the genomic DNA of 10-fold serial dilutions of a P. phosphoreum CIP 102511T overnight culture at 15°C. The linear relation determined over a 5-log CFU/ml range, from 2.2 to 7.2 log CFU/ml, was CT = −3.15 · viable count (log CFU/ml) + 34.6 (R2 = 0.999), which indicates an efficiency of 108% (Fig. 2). The quantification was considered specific up to a CT value of 27.5 (specificity limit described above), corresponding to 2.3 log CFU/ml.
Fig 2.
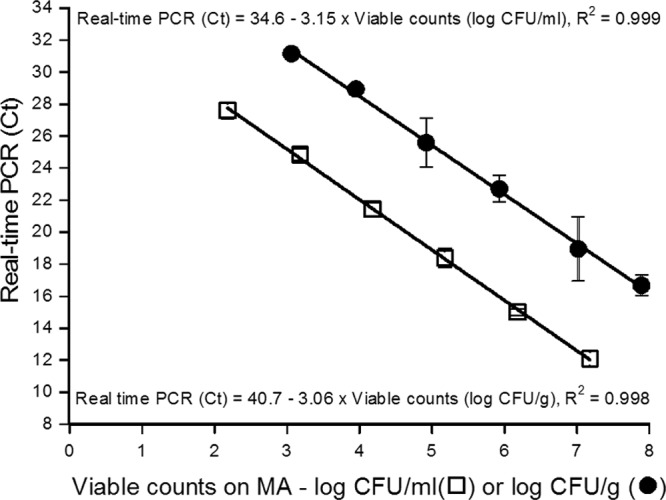
Standard curves for real-time PCR quantification of P. phosphoreum diluted in physiological saline solution (□) or in artificially contaminated salmon (●). Viable counts were determined using MA. The average efficiencies of the real-time amplification were 108% with physiological saline and 112% with artificially contaminated salmon.
Sensitivity was then investigated by using sterile salmon flesh artificially contaminated with decreasing amounts of P. phosphoreum CIP 102511T. The linear quantification (R2 of 0.998) was determined from 3 to 8 log CFU/g, achieving about 5 logs of magnitude. The equation was CT = −3.06 · viable count (log CFU/g) + 40.73, corresponding to an efficiency of about 112% (Fig. 2).
On this salmon model, the quantification was specific with a limiting CT value of 31 enabling P. phosphoreum to be enumerated in concentrations between 3 and 8 log CFU/g.
Bacterial counts in noninoculated ionized salmon flesh were below the enumeration threshold (0.7 log CFU/g) (data not shown). Thus, ionized flesh was validated as not contaminated.
Enumeration of P. phosphoreum in naturally contaminated MAP salmon steaks.
A total of 13 MAP salmon steaks was analyzed to confirm the accuracy of real-time PCR for P. phosphoreum quantification. P. phosphoreum was detected in the range of 3.3 to 7.7 log CFU/g by real-time PCR (Table 2). Using MA plate counting and colony identification, P. phosphoreum viable counts varied from 2.9 to 7.6 log CFU/g for the 13 samples analyzed (Table 2). A good correlation was obtained (R2 = 0.963) between real-time PCR and enumeration of P. phosphoreum by viable counting on MA followed by identification of colonies (Fig. 3). The root mean square error (RMSE) was 0.34 log CFU/g. Compared to the accuracy of plate counting on MA, the relative accuracy of P. phosphoreum detection by the real-time PCR method varied from 87.7 to 115.3% (Table 2). The ANOVA, Fisher test, Kruskal-Wallis test, and Mood test statistical analyses revealed that the differences between plating and real-time PCR results were not significant (P > 0.05). The suitability of the real-time PCR assay for identifying P. phosphoreum isolates was also confirmed by partial sequencing of the 16S rRNA gene for 50 isolates from 4 of the 13 salmon samples. The identification results were exactly the same for the two methods, confirming the specificity of real-time PCR for P. phosphoreum detection (Table 3).
Table 2.
Viable counts on MA and real-time PCR enumeration of P. phosphoreum in naturally contaminated MAP salmon steaks (log CFU/g) and their relative accuracy
| Sample | Real-time PCR (log CFU/g) | CT valuesa | Viable count (log CFU/g)b | Relative accuracy (%)c |
|---|---|---|---|---|
| 1 | 3.32 | 30.57 ± 0.56 | 2.88 | 115.3 |
| 2 | 5.37 | 24.29 ± 0.77 | 5.49 | 97.8 |
| 3 | 7.13 | 18.89 ± 0.14 | 7.03 | 101.4 |
| 4 | 7.34 | 18.27 ± 0.17 | 7.38 | 99.5 |
| 5 | 3.47 | 30.11 ± 0.10 | 3.57 | 97.2 |
| 6 | 3.85 | 28.93 ± 0.47 | 4.42 | 87.1 |
| 7 | 7.06 | 19.11 ± 0.30 | 7.06 | 100 |
| 8 | 7.66 | 17.28 ± 0.20 | 7.61 | 100.7 |
| 9 | 7.47 | 17.85 ± 0.21 | 7.31 | 102.2 |
| 10 | 3.84 | 28.96 ± 0.19 | 4.54 | 84.7 |
| 11 | 5.36 | 24.32 ± 0.16 | 5.60 | 95.6 |
| 12 | 7.40 | 18.06 ± 0.17 | 7.20 | 102.8 |
| 13 | 6.76 | 20.02 ± 0.32 | 7.29 | 92.8 |
Mean and standard deviation of three CT values.
Viable counts were determined using MA and identification of colonies.
Degree of correspondence between the results obtained with a conventional plating technique and with a real-time PCR assay. The relative accuracy is expressed as the percentage of numbers of log (CFU/g) calculated by the real-time PCR assay versus the conventional method (45).
Fig 3.
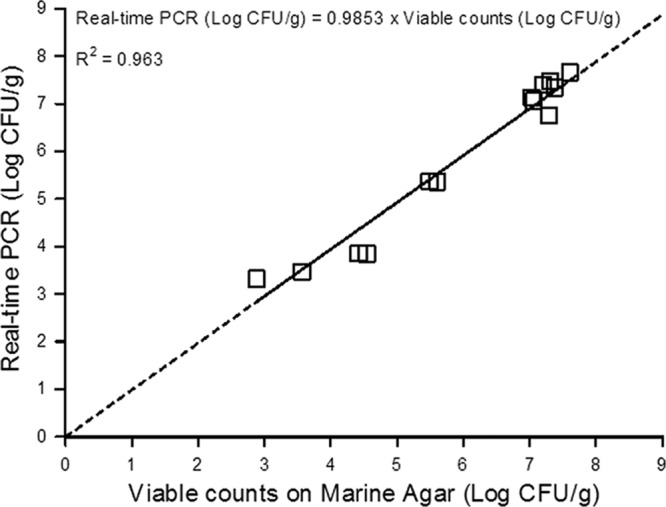
Correlation between concentrations of P. phosphoreum determined by real-time PCR and viable counting in 13 samples of naturally contaminated fresh MAP salmon steaks.
Table 3.
Identification of 50 isolates from MA plates using partial 16S rRNA gene sequencing and P. phosphoreum identification by real-time PCR method
| Sample | Isolate | Species or genus identification by partial 16S rRNA gene sequencing | P. phosphoreum identification using real-time PCR methoda |
|---|---|---|---|
| 1 | 1 | Serratia fonticola | − |
| 2 | Pseudomonas sp. | − | |
| 3 | Pseudomonas sp. | − | |
| 4 | Pseudomonas sp. | − | |
| 5 | Pseudomonas sp. | − | |
| 6 | Acinetobacter sp. | − | |
| 7 | Pseudomonas sp. | − | |
| 8 | Pseudomonas sp. | − | |
| 9 | Pseudomonas sp. | − | |
| 10 | Pseudomonas sp. | − | |
| 11 | Shewanella sp. | − | |
| 12 | Pseudomonas sp. | − | |
| 13 | Myroides odoratimimus | − | |
| 14 | Acinetobacter sp. | − | |
| 15 | Nonworkable sequence | − | |
| 16 | Shewanella sp. | − | |
| 17 | P. phosphoreum | + | |
| 18 | Nonworkable sequence | − | |
| 19 | Pseudomonas sp. | − | |
| 20 | Pseudomonas sp. | − | |
| 2 | 1 | P. phosphoreum | + |
| 2 | P. phosphoreum | + | |
| 3 | P. phosphoreum | + | |
| 4 | P. phosphoreum | + | |
| 5 | P. phosphoreum | + | |
| 6 | P. phosphoreum | + | |
| 7 | P. phosphoreum | + | |
| 8 | P. phosphoreum | + | |
| 9 | P. phosphoreum | + | |
| 10 | Shewanella sp. | − | |
| 3 | 1 | P. phosphoreum | + |
| 2 | P. phosphoreum | + | |
| 3 | Nonworkable sequence | + | |
| 4 | P. phosphoreum | + | |
| 5 | P. phosphoreum | + | |
| 6 | Shewanella sp. | − | |
| 7 | P. phosphoreum | + | |
| 8 | Shewanella sp. | − | |
| 9 | P. phosphoreum | + | |
| 10 | P. phosphoreum | + | |
| 4 | 1 | Shewanella sp. | − |
| 2 | Nonworkable sequence | + | |
| 3 | Nonworkable sequence | + | |
| 4 | P. phosphoreum | + | |
| 5 | P. phosphoreum | + | |
| 6 | P. phosphoreum | + | |
| 7 | P. phosphoreum | + | |
| 8 | P. phosphoreum | + | |
| 9 | P. phosphoreum | + | |
| 10 | Photobacterium sp. | + |
+, amplification product with a Tm of 84°C obtained in the method's range of use; −, no PCR product obtained in the method's range of use.
Comparison of real-time PCR and a conductance method for quantification of P. phosphoreum in fresh MAP salmon.
P. phosphoreum was detected in the range of 2.5 to 8.5 log CFU/g by real-time PCR and of 1 to 8 log CFU/g by the conductance method. In one of the 26 samples tested, P. phosphoreum was not detected by either of these two methods. Three samples presenting P. phosphoreum concentrations from 1 to 2 log CFU/g were quantified by the conductance method only (data not shown). One sample was quantified by the real-time PCR method, but the level of bacteria was out of range, with a concentration of about 2.5 log CFU/g. However, this count was well correlated with that of the conductance method (2.7 log CFU/g), so this result was used for the correlation curve. Based on data for the remaining 22 samples, a high correlation with an R2 of 0.940 and an RMSE of 0.49 log CFU/g was obtained (Fig. 4).
Fig 4.
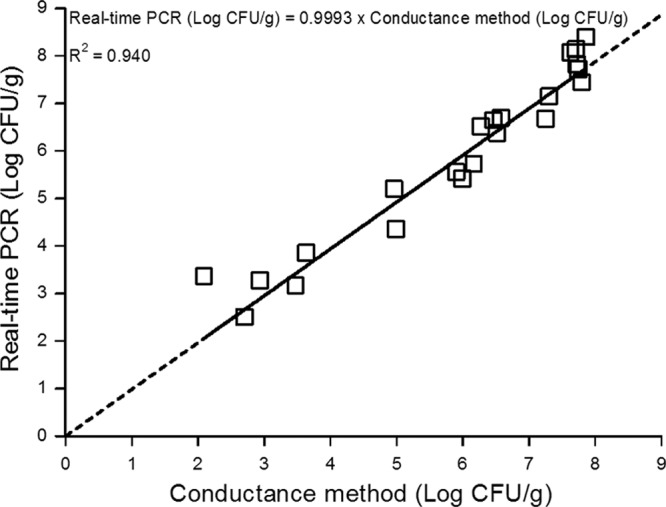
Correlation curve between P. phosphoreum enumeration by the specific conductance method and real-time PCR on 22 samples of naturally contaminated MAP salmon steaks.
DISCUSSION
Microbiological quality control of fresh seafood products remains a challenge due to their high sensitivity to microbial contamination and growth. By improving good hygiene practices in processing plants and by using MAP, the shelf life of fresh fish can be increased by several days (2, 4). The detection and quantification of SSO can support and document efforts with good hygiene practices and thereby contribute to increasing quality and reducing product losses. In recent years, culture-independent molecular methods like real-time PCR have been increasingly applied to detect and quantify target microorganisms in food (36, 37). Several real-time PCR methods have been developed to enumerate or detect pathogenic bacteria in seafood products, including Vibrio parahaemolyticus (26, 38) and mesophilic Gram-negative histamine-producing bacteria (39, 40). Spoilage bacteria like Pseudomonas (41) and Brochothrix thermosphacta (24) have also been enumerated by these methods. In this work, real-time PCR was chosen for our objective of quantifying the principal SSO of MAP fish, P. phosphoreum, since the specific enumeration of this bacterium is difficult using classic methods. To our knowledge, this is the first report to present a rapid molecular quantification tool for P. phosphoreum using real-time PCR. Moreover, the use of PMA allowed exclusive quantification of viable cells (21, 42) and, consequently, only the P. phosphoreum cells active in spoilage.
When real-time PCR was used previously for the enumeration of bacteria in a meat or fish matrix, linear quantifications were reported over a range of at least 5 logs, and quantification limits without enrichment varied from about 2 to 3 log CFU/g (22, 24, 25, 37, 43, 44), as obtained in the present study.
When enumeration by viable counting and that by real-time PCR are compared, a relative accuracy of 100% indicates total agreement between the two methods. The relative accuracy values obtained in the present study for the naturally contaminated salmon samples (88 to 115%) are better than those reported in several earlier studies for other bacteria (24, 25) and similar to those obtained by Rodríguez-Lázaro et al. (45) for the quantification of Listeria monocytogenes (89 to 116%). Martin et al. (43) presented better relative accuracy and higher R2 values for Lactobacillus sakei with less variability (91 to 98%), but these results were based on only 7 tested samples, compared to 13 in the present study. The correlation curve obtained here on 13 samples gave an R2 value of 0.963, which is higher than the correlation coefficients reported for several real-time PCR methods applied to more than 10 samples of meat or seafood products (R2 values ranging from 0.798 to 0.911) (22, 24, 25, 41). This confirms the reliability of our new real-time PCR method dedicated to the quantification of P. phosphoreum.
The accuracy of this method was validated by comparison with the available specific conductance method. In fact, the correlation curve obtained by plotting the quantification results of the 22 tested MAP salmon samples showed a high R2 of 0.940. The RMSE values obtained by comparing real-time PCR with the viable count (0.34 log CFU/g) or conductance method (0.49 log CFU/g) are lower than the currently accepted error value of 0.5 to 0.8 log for plate counting on solid matrices (46). With a quantification limit of 3 log CFU/g, the new real-time PCR method is slightly less sensitive than the available conductance method, which detects less than 1 log CFU/g (19). However, among the naturally contaminated samples, only a few (3 out of the 26 samples tested) were out of range of the real-time PCR method and could be quantified only by the conductance method. The major advantage of the real-time PCR method is its rapidity; it provides results in less than 6 h (including DNA extraction and real-time PCR analysis), which is much faster than the available conductance method, requiring 12 to 50 h (19), or the viable counting method, requiring more than a week, as colonies must be identified.
Reducing the detection level is not required for P. phosphoreum as much as for pathogenic bacteria, since this organism induces spoilage rejection of products at a high population density, e.g., above 6.5 to 7 log CFU/g (5–7, 29). This species is also known as a histamine producer and is involved in histamine food poisoning (47, 48) due to the consumption of cold-smoked tuna, dried sardines, and fresh tuna. Nevertheless, histamine poisoning has never been reported following salmon consumption, as the histidine content of salmon flesh is too low for toxic concentrations of histamine to be formed (8, 49). The real-time PCR method could thus be adapted for P. phosphoreum quantification in other fish, including transformed products such as cooked tuna, with an appropriate DNA extraction method. Additionally, detection from 3 log CFU/g is sufficient because histamine is produced when P. phosphoreum concentrations are above about 6 log CFU/g (8, 48, 49). Consequently, this real-time PCR method should provide a powerful tool to evaluate the contamination of seafood products by P. phosphoreum before spoilage appears or histamine is formed.
However, in the case of its application in combination with predictive microbiology models, a lower detection level could be useful, for example, by employing the Seafood Spoilage and Safety Predictor (http://sssp.dtuaqua.dk), which includes models for growth of P. phosphoreum in fresh MAP cod, plaice, and salmon (50). To improve the sensitivity of the new real-time PCR method, an enrichment step in broth, prior to the PCR assay, could be used, e.g., in the same way as applied for the available conductance method for sensitive enumeration of P. phosphoreum (19). Enrichment steps in the broth or food samples have already been used to enhance the sensitivity of real-time PCR methods, for both qualitative and quantitative detection of the target bacteria (27, 44, 51). In fact, we tested this approach by using quantitative enrichment in Photobacterium phosphoreum differential medium (PPDM) at 15°C (19). Unfortunately, this enrichment medium seemed to inhibit the real-time PCR. Thus, on slightly contaminated samples with less than 3 log CFU/g of P. phosphoreum, this real-time PCR method is currently suitable only for qualitative purposes.
In conclusion, this method is now applicable to enumerating P. phosphoreum in salmon and, according to preliminary tests in progress on cod, it could also be used for other fish.
Thanks to the PMA treatment step, it could be used by industry to detect and quantify viable P. phosphoreum contamination in production plants and thus target processing steps which need to be controlled. This method could also be used to test the shelf life of newly packaged products, to quantify the growth of this very important fish spoiler, and to determine shelf life, as discussed previously for the available conductance method (4, 13).
Moreover, the development of new methods to quantify food spoilage organisms, like P. phosphoreum or other bacterial species (24, 25, 41, 51), could assist in the establishment of more accurate microbial hygienic criteria than those usually applied (e.g., total flora count). This would help to reduce food losses and to improve sustainable development in food production. Photobacterium spp. have recently been reported as part of beef and pork spoilage microbiota (52, 53). Thus, this new quantitative method could clearly be used by the scientific community as a complementary tool to study P. phosphoreum distribution and abundance in bacterial populations.
ACKNOWLEDGMENTS
The Ph.D. project of Sabrina Macé was supported financially by the Bretagne and Pays de la Loire regions and by Oniris (Nantes).
We thank Nadereh Samieian from DTU Food, Lyngby, Denmark, for technical assistance in the study of the conductance method. We are grateful to Monique Zagorec (UMR INRA Sécalim, Nantes, France) for her valuable comments on the manuscript.
Footnotes
Published ahead of print 8 February 2013
REFERENCES
- 1. Gram L, Huss HH. 1996. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 33:121–137 [DOI] [PubMed] [Google Scholar]
- 2. Sivertsvik M, Jeksrud J, Rosnes JT. 2002. A review of modified atmosphere packaging of fish and fishery products—significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 37:107–127 [Google Scholar]
- 3. Gram L, Ravn L, Rasch M, Bruhn JB, Christensen AB, Givskov M. 2002. Food spoilage—interactions between food spoilage bacteria. Int. J. Food Microbiol. 78:79–97 [DOI] [PubMed] [Google Scholar]
- 4. Dalgaard P. 2000. Fresh and lightly preserved seafood, p 110–139 In Man CD, Jones AA. (ed), Shelf life evaluation of foods, 2nd ed Aspen Publishing, Inc., Frederick, MD [Google Scholar]
- 5. Dalgaard P. 1995. Qualitative and quantitative characterization of spoilage bacteria from packed fish. Int. J. Food Microbiol. 26:319–333 [DOI] [PubMed] [Google Scholar]
- 6. Dalgaard P, Gram L, Huss HH. 1993. Spoilage and shelf-life of cod fillets packed in vacuum or modified atmospheres. Int. J. Food Microbiol. 19:283–294 [DOI] [PubMed] [Google Scholar]
- 7. Dalgaard P, Mejholm O, Christiansen TJ, Huss HH. 1997. Importance of Photobacterium phosphoreum in relation to spoilage of modified atmosphere-packed fish products. Lett. Appl. Microbiol. 24:373–378 [Google Scholar]
- 8. Emborg J, Laursen BG, Rathjen T, Dalgaard P. 2002. Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere-packed salmon (Salmo salar) at 2 degrees C. J. Appl. Microbiol. 92:790–799 [DOI] [PubMed] [Google Scholar]
- 9. Hansen AA, Morkore T, Rudi K, Rodbotten M, Bjerke F, Eie T. 2009. Quality changes of prerigor filleted Atlantic salmon (Salmo salar L.) packaged in modified atmosphere using CO2 emitter, traditional MAP, and vacuum. J. Food Sci. 74:M242–M249 [DOI] [PubMed] [Google Scholar]
- 10. Hovda MB, Sivertsvik M, Lunestad BT, Lorentzen G, Rosnes JT. 2007. Characterisation of the dominant bacterial population in modified atmosphere packaged farmed halibut (Hippoglossus hippoglossus) based on 16S rDNA-DGGE. Food Microbiol. 24:362–371 [DOI] [PubMed] [Google Scholar]
- 11. Macé S, Cornet J, Chevalier F, Cardinal M, Pilet Dousset M-FX, Joffraud J-J. 2012. Characterisation of the spoilage microbiota in raw salmon (Salmo salar) steaks stored under vacuum or modified atmosphere packaging combining conventional methods and PCR-TTGE. Food Microbiol. 30:164–172 [DOI] [PubMed] [Google Scholar]
- 12. Reynisson E, Lauzon HL, Magnusson H, Jonsdottir R, Olafsdottir G, Marteinsson V, Hreggvidsson GO. 2009. Bacterial composition and succession during storage of North-Atlantic cod (Gadus morhua) at superchilled temperatures. BMC Microbiol. 9:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalgaard P. 2006. Microbiology of marine muscle foods, p 53-51-53–20 In Hui YH. (ed), Handbook of food science, technology and engineering, vol 1 Taylor & Francis, CRC Press, Boca Raton, FL [Google Scholar]
- 14. Van Spreekens K. 1974. The suitability of Long and Hammer's medium for the enumeration of more fastidious bacteria from fresh fishery products. Arch. Lebensmittelhyg. 25:213–219 [Google Scholar]
- 15. López-Caballero ME, Goncalves A, Nunes ML. 2002. Effect of CO2/O2-containing modified atmosphere on packed deepwater shrimp (Parapenaeus longirostris). Eur. Food Res. Technol. 214:192–197 [Google Scholar]
- 16. Ast JC, Dunlap PV. 2005. Phylogenetic resolution and habitat specificity of members of the Photobacterium phosphoreum species group. Environ. Microbiol. 7:1641–1654 [DOI] [PubMed] [Google Scholar]
- 17. Urbanczyk H, Ast JC, Dunlap PV. 2011. Phylogeny, genomics, and symbiosis of Photobacterium. FEMS Microbiol. Rev. 35:324–342 [DOI] [PubMed] [Google Scholar]
- 18. Dalgaard P, Madsen HL, Samieian N, Emborg J. 2006. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone)—effect of modified atmosphere packaging and previous frozen storage. J. Appl. Microbiol. 101:80–95 [DOI] [PubMed] [Google Scholar]
- 19. Dalgaard P, Mejlholm O, Huss HH. 1996. Conductance method for quantitative determination of Photobacterium phosphoreum in fish products. J. Appl. Microbiol. 81:57–64 [Google Scholar]
- 20. Wolffs P, Norling B, Radström P. 2005. Risk assessment of false-positive quantitative real-time PCR results in food, due to detection of DNA originating from dead cells. J. Microbiol. Methods 60:315–323 [DOI] [PubMed] [Google Scholar]
- 21. Nocker A, Cheung Camper C-YAK. 2006. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 67:310–320 [DOI] [PubMed] [Google Scholar]
- 22. Josefsen MH, Löfström C, Hansen TB, Christensen LS, Olsen JE, Hoorfar J. 2010. Rapid quantification of viable Campylobacter bacteria on chicken carcasses, using real-time PCR and propidium monoazide treatment, as a tool for quantitative risk assessment. Appl. Environ. Microbiol. 76:5097–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan Y, Breidt F. 2007. Enumeration of viable Listeria monocytogenes cells by real-time PCR with propidium monoazide and ethidium monoazide in the presence of dead cells. Appl. Environ. Microbiol. 73:8028–8031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mamlouk K, Macé S, Guilbaud M, Jaffrès E, Ferchichi M, Prévost H, Pilet Dousset M-FX. 2012. Quantification of viable Brochothrix thermosphacta in cooked shrimp and salmon by real-time PCR. Food Microbiol. 30:173–179 [DOI] [PubMed] [Google Scholar]
- 25. Pennacchia C, Ercolini D, Villani F. 2009. Development of a real-time PCR assay for the specific detection of Brochothrix thermosphacta in fresh and spoiled raw meat. Int. J. Food Microbiol. 134:230–236 [DOI] [PubMed] [Google Scholar]
- 26. Zhu R-G, Li T-P, Jia Y-F, Song L-F. 2012. Quantitative study of viable Vibrio parahaemolyticus cells in raw seafood using propidium monoazide in combination with quantitative PCR. J. Microbiol. Methods 90:262–266 [DOI] [PubMed] [Google Scholar]
- 27. Robert-Pillot A, Copin S, Gay M, Malle P, Quilici ML. 2010. Total and pathogenic Vibrio parahaemolyticus in shrimp: fast and reliable quantification by real-time PCR. Int. J. Food Microbiol. 143:190–197 [DOI] [PubMed] [Google Scholar]
- 28. Elizaquível P, Sánchez G, Selma MV, Aznar R. 2012. Application of propidium monoazide-qPCR to evaluate the ultrasonic inactivation of Escherichia coli O157:H7 in fresh-cut vegetable wash water. Food Microbiol. 30:316–320 [DOI] [PubMed] [Google Scholar]
- 29. Macé S, Joffraud Cardinal J-JM, Malcheva M, Cornet J, Lalanne V, Chevalier F, Sérot T, Pilet Dousset M-FX. 2013. Evaluation of the spoilage potential of bacteria isolated from spoiled raw salmon (Salmo salar) fillets stored under modified atmosphere packaging. Int. J. Food Microbiol. 160:227–238 [DOI] [PubMed] [Google Scholar]
- 30. Dalgaard P, Manfio GP, Goodfellow M. 1997. Classification of Photobacteria associated with spoilage of fish products by numerical taxonomy and pyrolysis mass spectrometry. Zentralbl. Bakteriol. 285:157–168 [DOI] [PubMed] [Google Scholar]
- 31. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 32. Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T. 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klein D, Janda P, Steinborn R, Müller M, Salmons B, Günzburg WH. 1999. Proviral load determination of different feline immunodeficiency virus isolates using real-time polymerase chain reaction: influence of mismatches on quantification. Electrophoresis 20:291–299 [DOI] [PubMed] [Google Scholar]
- 34. Union du mareyage Français (UMF) 2010. Guide des bonnes pratiques et application de l'HACCP (J. officiel référence no. 5941/2010). Fonds européens pour la pêche et FranceAgrimer, Paris, France [Google Scholar]
- 35. Matamoros S, André S, Hue I, Prévost H, Pilet MF. 2010. Identification of lactic acid bacteria involved in the spoilage of pasteurized “foie gras” products. Meat Sci. 85:467–471 [DOI] [PubMed] [Google Scholar]
- 36. Juste A, Thomma B, Lievens B. 2008. Recent advances in molecular techniques to study microbial communities in food-associated matrices and processes. Food Microbiol. 25:745–761 [DOI] [PubMed] [Google Scholar]
- 37. Postollec F, Falentin H, Pavan S, Combrisson J, Sohier D. 2011. Recent advances in quantitative PCR (qPCR) applications in food microbiology. Food Microbiol. 28:848–861 [DOI] [PubMed] [Google Scholar]
- 38. Nordstrom JL, Vickery MCL, Blackstone GM, Murray SL, DePaola A. 2007. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 73:5840–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bjornsdottir-Butler K, Jones JL, Benner R, Burkhardt W., III 2011. Development of a real-time PCR assay with an internal amplification control for detection of Gram-negative histamine-producing bacteria in fish. Food Microbiol. 28:356–363 [DOI] [PubMed] [Google Scholar]
- 40. Bjornsdottir-Butler K, Jones JL, Benner RA, Jr, Burkhardt W., III 2011. Quantification of total and specific gram-negative histamine-producing bacteria species in fish using an MPN real-time PCR method. Food Microbiol. 28:1284–1292 [DOI] [PubMed] [Google Scholar]
- 41. Reynisson E, Lauzon HL, Magnusson H, Óli Hreggvidsson G, Marteinsson VT. 2008. Rapid quantitative monitoring method for the fish spoilage bacteria Pseudomonas. J. Environ. Monit. 10:1357–1362 [DOI] [PubMed] [Google Scholar]
- 42. Nocker A, Sossa-Fernandez P, Burr MD, Camper AK. 2007. Use of propidium monoazide for live/dead distinction in microbial ecology. Appl. Environ. Microbiol. 73:5111–5117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin B, Jofré A, Garriga M, Pla M, Aymerich T. 2006. Rapid quantitative detection of Lactobacillus sakei in meat and fermented sausages by real-time PCR. Appl. Environ. Microbiol. 72:6040–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takahashi H, Kimura B, Tanaka Y, Shinozaki J, Suda T, Fujii T. 2009. Real-time PCR and enrichment culture for sensitive detection and enumeration of Escherichia coli. J. Microbiol. Methods 79:124–127 [DOI] [PubMed] [Google Scholar]
- 45. Rodríguez-Lázaro D, Jofré A, Aymerich T, Hugas M, Pla M. 2004. Rapid quantitative detection of Listeria monocytogenes in meat products by real-time PCR. Appl. Environ. Microbiol. 70:6299–6301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Afssa 2008. Avis de l'Agence française de sécurité sanitaire des aliments concernant les références applicables aux denrées alimentaires en tant que critères indicateurs d'hygiène des procédés. Saisine liée no. 2006-SA-0215, Saisine no. 2007-SA-0174 Afssa, Maison-Alfort, France [Google Scholar]
- 47. Emborg J, Laursen BG, Dalgaard P. 2005. Significant histamine formation in tuna (Thunnus albacares) at 2°C—effect of vacuum- and modified atmosphere-packaging on psychrotolerant bacteria. Int. J. Food Microbiol. 101:263–279 [DOI] [PubMed] [Google Scholar]
- 48. Kanki M, Yoda T, Ishibashi M, Tsukamoto T. 2004. Photobacterium phosphoreum caused a histamine fish poisoning incident. Int. J. Food Microbiol. 92:79–87 [DOI] [PubMed] [Google Scholar]
- 49. Jorgensen LV, Huss HH, Dalgaard P. 2000. The effect of biogenic amine production by single bacterial cultures and metabiosis on cold-smoked salmon. J. Appl. Microbiol. 89:920–934 [DOI] [PubMed] [Google Scholar]
- 50. Dalgaard P, Buch P, Silberg S. 2002. Seafood Spoilage Predictor—development and distribution of a product specific application software. Int. J. Food Microbiol. 73:343–349 [DOI] [PubMed] [Google Scholar]
- 51. Ranieri ML, Ivy RA, Mitchell WR, Call E, Masiello SN, Wiedmann M, Boor KJ. 2012. Real-time PCR detection of Paenibacillus spp. in raw milk to predict shelf life performance of pasteurized fluid milk products. Appl. Environ. Microbiol. 78:5855–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pennacchia C, Ercolini D, Villani F. 2011. Spoilage-related microbiota associated with chilled beef stored in air or vacuum pack. Food Microbiol. 28:84–93 [DOI] [PubMed] [Google Scholar]
- 53. Nieminen T, Bjorkroth J, Dalgaard P. 2012. Volatile compounds and microbiota associated with the spoilage of high-oxygen modified atmosphere-packaged raw pork meat, poster 273. Abstr. FOODMICRO 2012-23rd International ICFMH Symposium, Istanbul, Turkey, 3 to 7 September 2012 [Google Scholar]


