Abstract
Here we show that a bacterial endosymbiont, Regiella insecticola, protects pea aphids (Acyrthosiphon pisum) from the aphid-specific fungal entomopathogen Zoophthora occidentalis but not from the generalist insect fungal pathogen Beauveria bassiana. This finding highlights the complex influence of fungi on the dynamics of this economically important agricultural pest.
TEXT
Symbiotic relationships between invertebrates and vertically transmitted microbes are widespread. One feature of this mutualistic relationship is that symbionts depend on host resources for their own survival and reproduction (1), and theory therefore predicts that in the absence of manipulation of host reproduction, beneficial symbionts must provide a fitness advantage to spread through a host population (2). Studying ecologically relevant traits conferred to hosts by symbionts is critical for understanding host-microbe dynamics, and researchers have therefore searched for fitness advantages of harboring symbionts in a number of systems. Several recent studies have shown that one advantage conferred by some symbionts is protection against pathogens and parasites (3–7). For example, pea aphids (Acyrthosiphon pisum), which are a model system for the study of host-symbiont dynamics, are protected against the fungal entomopathogen Pandora neoaphidis (Zygomycota: Entomophthorales) by several facultative, vertically transmitted bacteria, including the gammaproteobacteria Regiella insecticola (8, 9). Fungi are important natural pathogens of aphids (10) and are used in biocontrol (11, 12), and symbiont-mediated protection to fungi is likely an important factor influencing the population dynamics of aphids and their symbionts. However, aphids encounter several diverse species of fungal pathogens in the wild (13). It is not known if Regiella-conferred protection is specific to Pandora or if it extends to other species of fungus as well, which would suggest that multiple fungal species are influencing aphid-Regiella dynamics. We therefore exposed pea aphids, with and without symbionts, to two additional species of fungal pathogens: Zoophthora occidentalis (Zygomycota: Entomophthorales), a highly aphid-specific entomopathogen, and Beauveria bassiana (Ascomycota: Hypocreales), a generalist that has been found in a variety of hosts, including species of Coleoptera, Hemiptera, and Diptera (13–15). These fungal species are highly divergent (with some estimates as high as 1,000 million years ago [16]), but both species reproduce by passively releasing spores (conidia) that penetrate the cuticle of a suitable host. Mycelia then colonize the host's body tissue until the death of the host, when new spores are produced and released into the environment.
We used two aphid genotypes, both with and without Regiella present (5A, collected in the wild in 1999 near Madison, WI, and subsequently injected with Regiella symbionts from an aphid collected in Tompkins County, NY, in 2000; and LSR1, collected on alfalfa near Ithaca, NY, in 1998 with a natural Regiella infection and then artificially cleared of symbionts) (17). The use of two aphid genotypes, each with and without Regiella, allowed us to control for effects of aphid genotype on pathogen susceptibility. Before fungal infection, we maintained pea aphids asexually on fava bean (Vicia faba) plants in 16 h of light and 8 h of dark at 20°C. We exposed adults to fungus after their final molt (at 9 days of age), as we found that molting shortly after exposure strongly reduces infection probability. After exposure, aphids were kept individually for 4 days on fava bean plants under near 100% humidity, after which the humidity was reduced to 70%. This allowed enough time for fungal penetration of the aphid cuticle, which requires high humidity.
Zoophthora (specialist) infection.
We infected aphids with Zoophthora by placing them under a “spore shower” (based on references 9, 14, and 18–20). An isolate of Zoophthora was obtained from the USDA ARS Collection of Entomopathogenic Fungal Cultures and was grown for 2 weeks on SDAEY plates at 20°C (21). Approximately 15 h before infection, several small pieces of fungal mycelium (3 mm2) were cut with a sterile instrument and placed onto 1.5% tap water agar, which causes the fungus to sporulate. At the time of infection, the agar plates were inverted over a hollow tube for 60 min with aphids at the bottom of the chamber. The agar plates were rotated among treatment groups during infection to ensure that each treatment group was exposed to an equal dose of fungal spores (approximately 16.5 spores/mm2). We included a glass slide in this rotation so that spores could be counted under a light microscope to estimate spore density. Control aphids were handled similarly but were not exposed to fungus.
The Zoophthora infection was divided into three blocks conducted several days apart from one another. In each block, we exposed two-thirds of the individuals from each genotype to fungus and kept one-third under identical conditions as a control. Half of the aphids of each genotype harbored Regiella. Nine days after exposure, we recorded the survival of each aphid. We fit a logistic regression model (a generalized linear model [GLM] with quasibinomial error structure and logit link function) to aphid survival, with symbiont, fungal exposure, and block as fixed effects (22). We used R version 2.11 for our statistical analyses. As expected, aphids exposed to fungus had significantly lower survival than control aphids (odds ratio [OR] = 0.33, 95% confidence interval [CI] = 0.15 to 0.72, P = 5.8 × 10−3). There was also a significant interaction between exposure and symbiont infection on aphid survival (OR = 3.7, CI = 1.2 to 11, P = 0.021) but no effect of Regiella independently (OR = 0.70, CI = 0.28 to 1.73, P = 0.44). This means that in the absence of fungal infection, Regiella did not have a significant impact on aphid survival, but it increased survival of aphids exposed to fungus (Fig. 1). The trends were consistent across three blocks, but aphid survival differed across blocks (block 2, OR = 2.6, CI = 1.4 to 4.7, P = 2.5 × 10−3; block 3, OR = 3.4, CI = 1.8 to 6.7, P = 3.2 × 10−4). The trend was consistent across both aphid genotypes, and there was no significant effect of aphid genotype on survival; it was therefore removed from the model. More aphid genotypes will need to be tested to determine the effect, if any, of aphid genotype on fungal infection outcome. Using this same protocol and these same genotypes, we also confirmed the results of Scarborough et al. (9)—that Regiella protects pea aphids from Pandora, which, like Zoophthora, is an aphid-specific fungal pathogen (see Fig. SA1 in the supplemental material). We also conducted a repeat of the Zoophthora infection, using a second pathogen genotype, to ensure that our results were consistent across multiple experiments (see Fig. SA2).
Fig 1.
Results of infection with Zoophthora. Survival of each aphid was recorded 9 days after infection and is reported as the percent survival of each group. White bars represent aphids with no secondary symbionts (n = 138), and gray bars represent aphids that harbored Regiella (n = 131). Error bars are ± standard errors.
Beauveria (generalist) infection.
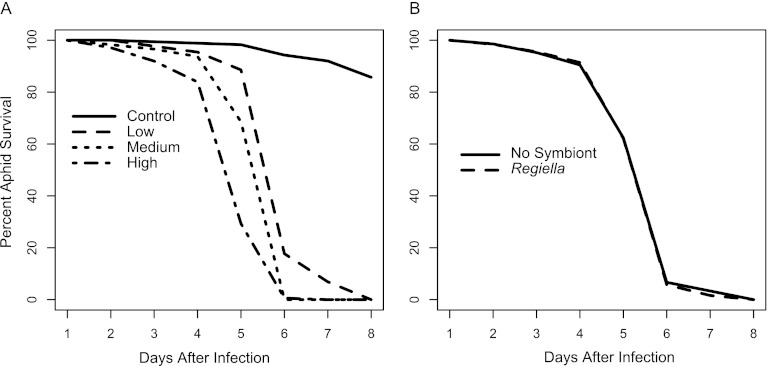
Cultures of Beauveria did not sporulate upon transfer to tap water agar, so we instead made up solutions of Beauveria spores (strain GHA, BotaniGard ES) in distilled water. Spores were washed and separated via centrifuge from inert ingredients, and 0.7 μl of the solution was pipetted onto the dorsal side of the abdomen of each aphid. Half of the aphids of each genotype harbored Regiella. We exposed aphids to four different spore doses (0, 25, 250, and 2,500 spores, estimated using a HYCOR KOVA Glasstic hemocytometer). Aphids became infected with Beauveria faster than with Zoophthora, so for the Beauveria infections we recorded the survival status of each aphid at 24-h intervals after exposure, and we analyze these data using a survival analysis (Fig. 2). For the Beauveria infection, we took the additional precaution of transferring the symbiont from the 5A line into the LSR1 aphid genotype. Symbiont-free first-instar LSR1 aphids were exposed to the hemolymph of 5A adult aphids that harbored Regiella via intrahemocoellic microinjection. Aphids were then kept for at least 10 generations to allow the symbiosis to stabilize and to allow the host lines to adapt to the presence of the bacteria (23). This allowed us to compare survival of genetically identical hosts with two different genotypes of symbionts. Survival data were analyzed using age-specific parametric survival models with a Weibull distribution using the Survival package in R version 2.11. The dose of fungal pathogen exposure had a significant impact on aphid survival (minimal model containing spore dose only, χ2 = 935.96 on 3 df, P < 0.0001). However, symbiont status had no significant effect on survival, had no interaction with dose, and was removed from the minimal model. This suggests that Regiella did not protect aphids from infection with Beauveria (Fig. 2). Aphid genotype had no significant effect on survival and was also removed from the minimal model. We repeated this experiment with aphids that harbored two other species of aphid secondary symbionts, Hamiltonella defensa and Serratia symbiotica, and again found no effect of symbionts on aphid susceptibility to Beauveria (see Fig. SB1 in the supplemental material). We also conducted a repeat of the Beauveria infection using different spore doses and found no effect of Regiella (see Fig. SB2). Lastly, to ensure that this negative result was not due to a sampling effect of Regiella genotypes, we conducted an additional experiment where we collected aphids with and without Regiella from several geographical locations and assayed their resistance to Beauveria. With this experimental design, we are not controlling for host genotype, as each strain used in the experiment will differ both in terms of symbiont and host genotypic background. However, we are able to determine if multiple wild-collected lines with Regiella are, on average, more resistant to Beauveria than wild-collected lines without symbionts. We found significant variation in resistance to Beauveria among aphid genotypes, but no effect of harboring Regiella symbionts (see Fig. SC in the supplemental material), suggesting that our results are not due to a lack of diversity in symbiont genotypes.
Fig 2.
Results of infection with Beauveria. Survival of each aphid was recorded every 24 h between 0 and 8 days after infection and is reported as survival curves measured in percent survival of each group. (A) Spore dose. Each line represents a different spore dose (control, 0 spores/aphid; low, 25; medium, 250; high, 2,500), n = 175 per dose. (B) Symbiont status. The solid line shows infected aphids without Regiella (n = 210). The dotted line shows infected aphids that harbored Regiella (n = 315).
Together, these data suggest that Regiella symbionts confer protection against several specialist fungal pathogens but not against a generalist pathogen. Regiella frequencies vary among aphid populations (24, 25), and researchers have tried to determine the factors that influence symbiont frequencies as a way of better understanding this model host-microbe interaction. Variation in Regiella frequency is explained in part as a balance between the benefits of protection from fungal pathogens and the costs of harboring bacteria. Our results suggest that several species of fungal pathogens may be driving this interaction but that Regiella is not beneficial against all species of fungi. In addition, these fungal pathogens are used in aphid biocontrol (11, 26, 27), and our results suggest that symbiont-mediated protection against pathogens may be an important consideration when selecting and developing biocontrol agents.
In general, researchers are working to develop an understanding of how evolution acts on the alternative defenses that organisms have to protect themselves from pathogens and parasites (28). One possible explanation for the pattern of symbiont-mediated protection observed here is that Regiella protection has evolved in response to pressure from individual species of fungal pathogens. A second possibility is that protection in this system has evolved in response to fungal pathogens that specialize on aphids but not to generalist insect pathogens, perhaps due to broad differences found in the infection strategies of generalists versus specialists (29, 30). Pathogen specificity has been shown to be an important factor influencing host-enemy interactions (31), and we therefore highlight the relative specificity of these pathogens as a potential explanation for the pattern of protection observed in this system, but clearly more data are needed. It is possible that close coevolution between aphid specialists and Regiella is needed to develop or maintain protection conferred by symbionts.
Supplementary Material
ACKNOWLEDGMENTS
Thierry Lefèvre, Alice Laughton, Eleanore Sternberg, Anand Bhardwaj, and four anonymous reviewers provided valuable assistance with the manuscript. We especially thank Richard Humber, Karen Hansen, and the USDA ARS Collection of Entomopathogenic Fungal Cultures, who provided cultures of Zoophthora.
This research was supported by NSF IOS-1025853 to N.M.G. B.A. was supported by a DFG grant (AL902/4-1), and B.J.P. is supported by an NSF Graduate Research Fellowship.
Footnotes
Published ahead of print 25 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03193-12.
REFERENCES
- 1. Haine ER. 2008. Symbiont-mediated protection. Proc. Biol. Sci. 275:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brownlie JC, Johnson KN. 2009. Symbiont-mediated protection in insect hosts. Trends Microbiol. 17:348–354 [DOI] [PubMed] [Google Scholar]
- 3. Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. 2010. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329:212–215 [DOI] [PubMed] [Google Scholar]
- 4. Oliver KM, Moran NA, Hunter MS. 2005. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. U. S. A. 102:12795–12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott JJ, Oh DC, Yuceer MC, Klepzig KD, Clardy J, Currie CR. 2008. Bacterial protection of beetle-fungus mutualism. Science 322:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6:e2 doi:10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vorburger C, Gehrer L, Rodriguez P. 2010. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 6:109–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lukasik P, van Asch M, Guo H, Ferrari J, Charles JGH, van der Putten W. 2013. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol. Lett. 16:214–218 [DOI] [PubMed] [Google Scholar]
- 9. Scarborough CL, Ferrari J, Godfray HC. 2005. Aphid protected from pathogen by endosymbiont. Science 310:1781. [DOI] [PubMed] [Google Scholar]
- 10. Pell JK, Eilenberg J, Hajek AE, Steinkraus DC. 2001. Biology, ecology and pest management potential of Entomophthorales, p 71–153 In Butt TM, Jackson C, Magan N. (ed), Fungi as biocontrol agents: progress, problems and potential. CAB International, Wallingford, England [Google Scholar]
- 11. Hajek AE, Delalibera I. 2010. Fungal pathogens as classical biological control agents against arthropods. Biocontrol 55:147–158 [Google Scholar]
- 12. Pell JK, Hannam JJ, Steinkraus DC. 2010. Conservation biological control using fungal entomopathogens. Biocontrol 55:187–198 [Google Scholar]
- 13. Nielsen C, Hajek AE. 2005. Control of invasive soybean aphid, Aphis glycines (Hemiptera: Aphididae), populations by existing natural enemies in New York State, with emphasis on entomopathogenic fungi. Environ. Entomol. 34:1036–1047 [Google Scholar]
- 14. Hatano E, Baverstock J, Kunert G, Pell JK, Weisser WW. 2012. Entomopathogenic fungi stimulate transgenerational wing induction in pea aphids, Acyrthosiphon pisum (Hemiptera: Aphididae). Ecol. Entomol. 37:75–82 [Google Scholar]
- 15. Vega FE, Goettel MS, Blackwell M, Chandler D, Jackson MA, Keller S, Koike M, Maniania NK, Monzon A, Ownley BH, Pell JK, Rangel DEN, Roy HE. 2009. Fungal entomopathogens: new insights on their ecology. Fungal Ecol. 2:149–159 [Google Scholar]
- 16. Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Lumbsch HT, Lutzoni F, Matheny PB, Mclaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Koljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schussler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N. 2007. A higher-level phylogenetic classification of the fungi. Mycol. Res. 111:509–547 [DOI] [PubMed] [Google Scholar]
- 17. International Aphid Genomics Consortium 2010. Genome sequence of the pea aphid Acyrthosiphon pisum. PloS Biol. 8:e1000313 doi:10.1371/journal.pbio.1000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baverstock J, Alderson PG, Pell JK. 2005. Pandora neoaphidis transmission and aphid foraging behaviour. J. Invertebr. Pathol. 90:73–76 [DOI] [PubMed] [Google Scholar]
- 19. Baverstock J, Baverstock KE, Clark SJ, Pell JK. 2008. Transmission of Pandora neoaphidis in the presence of co-occurring arthropods. J. Invertebr. Pathol. 98:356–359 [DOI] [PubMed] [Google Scholar]
- 20. Baverstock J, Roy HE, Clark SJ, Alderson PG, Pell JK. 2006. Effect of fungal infection on the reproductive potential of aphids and their progeny. J. Invertebr. Pathol. 91:136–139 [DOI] [PubMed] [Google Scholar]
- 21. Papierok B, Hajek AE. 1997. Fungi: Entomophthorales. In Lacey LA. (ed), Manual of techniques in insect pathology. Academic Press, San Diego, CA [Google Scholar]
- 22. Crawley MJ. 2007. The R book. John Wiley & Sons Ltd., West Sussex, United Kingdom [Google Scholar]
- 23. Koga R, Tsuchida T, Sakurai M, Fukatsu T. 2007. Selective elimination of aphid endosymbionts: effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol. Ecol. 60:229–239 [DOI] [PubMed] [Google Scholar]
- 24. Moran NA, Russell JA, Koga R, Fukatsu T. 2005. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 71:3302–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 11:2123–2135 [DOI] [PubMed] [Google Scholar]
- 26. Jackson MA, Dunlap CA, Jaronski ST. 2010. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. Biocontrol 55:129–145 [Google Scholar]
- 27. Michereff M, Oliveira SOD, de Liz RS, Faria M. 2011. Cage and field assessments of Beauveria bassiana-based mycoinsecticides for Myzus persicae Sulzer (Hemiptera: Aphididae) control in cabbage. Neotrop. Entomol. 40:470–476 [PubMed] [Google Scholar]
- 28. Parker BJ, Barribeau SM, Laughton AM, de Roode JC, Gerardo NM. 2011. Non-immunological defense in an evolutionary framework. Trends Ecol. Evol. 26:242–248 [DOI] [PubMed] [Google Scholar]
- 29. Barrett LG, Heil M. 2012. Unifying concepts and mechanisms in the specificity of plant-enemy interactions. Trends Plant Sci. 17:282–292 [DOI] [PubMed] [Google Scholar]
- 30. Schlenke TA, Morales J, Govind S, Clark AG. 2007. Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog. 3:1486–1501 doi:10.1371/journal.ppat.0030158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Antonovics J, Boots M, Ebert D, Koskella B, Poss M, Sadd BM. 2013. The origin of specificity by means of natural selection: evolved and nonhost resistance in host-pathogen interactions. Evolution 67:1–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.