Abstract
This is the first report to characterize the genotypes and subtypes of Cryptosporidium species infecting a geographically isolated population of feral Soay sheep (Ovis aries) on Hirta, St. Kilda, Scotland, during two distinct periods: (i) prior to a population crash and (ii) as host numbers increased. Cryptosporidium DNA was extracted by freeze-thawing of immunomagnetically separated (IMS) bead-oocyst complexes, and species were identified following nested-PCR-restriction fragment length polymorphism (RFLP)/PCR sequencing at two Cryptosporidium 18S rRNA loci. Two hundred fifty-five samples were analyzed, and the prevalent Cryptosporidium species in single infections were identified as C. hominis (11.4% of all samples tested), C. parvum (9%), C. xiaoi (12.5%), and C. ubiquitum (6.7%). Cryptosporidium parvum was also present with other Cryptosporidium species in 27.1% of all samples tested. Cryptosporidium parvum- and C. hominis-positive isolates were genotyped using two nested-PCR assays that amplify the Cryptosporidium glycoprotein 60 gene (GP60). GP60 gene analysis showed the presence of two Cryptosporidium genotypes, namely, C. parvum IIaA19G1R1 and C. hominis IbA10G2. This study reveals a higher diversity of Cryptosporidium species/genotypes than was previously expected. We suggest reasons for the high diversity of Cryptosporidium parasites within this isolated population and discuss the implications for our understanding of cryptosporidiosis.
INTRODUCTION
Cryptosporidium is an apicomplexan parasite that has been implicated in numerous waterborne and food-borne outbreaks associated with human and livestock disease. The disease is known as cryptosporidiosis, and the main symptom is severe diarrhea. Currently, there are approximately 25 valid Cryptosporidium species and greater than 60 genotypes. Cryptosporidium parvum and C. hominis cause the majority of human disease, with C. hominis being associated largely with human interactions while C. parvum can infect a wide range of mammals in addition to humans. In recent years, scientists have discovered other species/genotypes that can also infect humans. These include eight species, C. viatorum (humans), C. meleagridis (turkeys), C. canis (dogs), C. felis (cats), C. suis (pigs), C. muris (rodents), C. ubiquitum (sheep), C. cuniculus (rabbit), and six genotypes, C. hominis (monkey), C. andersoni-like, and Cryptosporidium chipmunk I, skunk, horse, and pig genotype II (1–6).
Because this is a zoonotic pathogen, much research has focused on the disease in cattle and sheep, which have the greatest capacity for shedding potentially infectious oocysts into the environment. Recent molecular studies have identified eight Cryptosporidium species and one genotype in sheep feces: C. parvum, C. hominis, C. bovis, C. andersoni, C. suis, C. xiaoi (previously known as C. bovis-like), C. fayeri (previously known as marsupial genotype), C. ubiquitum, and pig genotype II. Two novel genotypes have also been described (novel sheep and unique unknown genotypes), and after further investigation, these genotypes were identified as C. bovis, C. xiaoi, and C. ubiquitum (7, 8). Cryptosporidium parvum, C. xiaoi, and C. ubiquitum are the dominant species causing cryptosporidiosis in sheep (9). Cryptosporidium species such as C. fayeri, the pig genotype II, and in particular, C. hominis, C. andersoni, and C. suis may represent oocysts passing through the intestinal tract rather than those causing infection, as they have been identified only in a small number of sheep (10, 11).
Most studies in sheep involve domesticated, food production, or experimental animals. This study is the first to characterize the genetic diversity of Cryptosporidium in a completely unmanaged and geographically isolated population of sheep. The Soay sheep are a relic of primitive domestication, first brought to the remote archipelago of St. Kilda perhaps as early as the Bronze Age. Following the evacuation of humans and cattle from the main island of Hirta in 1932, the island has been grazed by a population of Soay sheep originally transferred from neighboring Soay. The Soay sheep population fluctuations and their isolation from human interference and other sheep have interested scientists since the 1950s, and much of the current research has focused on the parasites infecting the sheep (12). During an epidemiological study of protozoan parasites between 2001 and 2003, Cryptosporidium was first identified in the population by using Ziehl-Neelsen staining (13). This test selectively stains the oocysts but is limited in its ability to differentiate between morphologically cryptic yet genetically diverse species. In this study, molecular methods were used to characterize the genetic diversity of Cryptosporidium species infecting St. Kilda Soay sheep, and these results form the basis of discussion on the origins of infection in this isolated population.
MATERIALS AND METHODS
Study population and sample collection.
The selected study area was Village Bay (∼200 ha) on the island of Hirta (638 ha), part of the St. Kilda archipelago (57°49′N, 08°34′W), where the Soay sheep population has been monitored intensively since 1985 (12). The population is unmanaged and experiences intermittent winter population crashes when numbers exceed food supply. The total island count for sheep in the month of August in the years 2004, 2005, and 2006 gave estimates of 1,996, 1,332, and 1,794, respectively, indicating that the population decreased by 40% in early 2005 but had increased 15% by 2006. Fecal samples were collected from individually tagged sheep in the month of August in the years 2004 (n = 135) and 2006 (n = 141) from both sexes and four different age groups: lambs (∼4 months), yearlings (∼16 months), 2-year-olds (∼28 months), and adults (≥40 months). Fecal samples were stored at 4°C in small sealed plastic bags at the Scottish Parasite Diagnostic and Reference Laboratory (SPDRL) according to sheep age and sampling date. Two hundred fifty-five fecal samples were considered for analysis in this study.
AP microscopy.
Approximately 0.1 g of fecal material was emulsified with 200 μl of distilled water. One hundred microliters was transferred to a microscope slide to produce a fecal smear, which was air dried at room temperature (RT), methanol fixed, and stained with auramine-phenol (AP) (14). Slides were examined for the presence of oocysts using an Olympus BH-2 epifluorescence microscope equipped with a fluorescein isothiocyanate (FITC) UV filter (excitation, 355 nm; emission, 450 nm), and the presence of oocysts was determined under 20× and 40× objective lenses. Results were classified according to the number of oocysts observed per slide as follows: negative, no oocysts detected; 1+, 1 to 10 oocysts; 2+, 11 to 20 oocysts; 3+, 21 to 30 oocysts; and 4+, 31 to 50 oocysts.
IMS.
Prior to immunomagnetic separation (IMS), 0.4 g of each Cryptosporidium-positive fecal sample was mixed with 1 ml of distilled water. Five hundred microliters of the slurry and 9,500 μl of distilled water were added to Dynal L10 tubes to give a final volume of 10 ml (Dynal IMS kit; Invitrogen Ltd., Paisley, United Kingdom) according to the manufacturer's instructions. After the removal of the supernatant, 50 μl of lysis buffer (LB) containing 50 mM Tris-HCl (pH 8.5), 1 mM EDTA (pH 8), and 0.5% sodium dodecyl sulfate (SDS) was added to each tube containing the bead-oocyst complexes, and the DNA was extracted.
DNA extraction directly from the bead-oocyst complexes.
DNA extraction was performed by freeze-thawing to rupture oocysts and lyse the sporozoites to release Cryptosporidium DNA. IMS-prepared bead-oocyst complexes were suspended in LB before being subjected to 1 min of freezing in liquid nitrogen followed by 1 min at 65°C. This freeze-thaw cycle was repeated 15 times with vortexing every 5 cycles. Samples were then centrifuged at 3,000 × g for 10 s in a bench microcentrifuge (Micro-Centaur [MSE]; Sanyo, United Kingdom) and then placed in a Dynal MPC-S with a magnet strip inserted. The lysate was then transferred into a fresh tube containing 1.6 μl of 5 mg ml−1 proteinase K. Samples were incubated at 55°C for 3 h and then cooled on ice for 1 min, centrifuged at 3,000 × g for 10 s, and incubated at 90°C for 20 min to denature the proteinase K. Samples were then cooled on ice for 1 min and centrifuged at 10,000 × g for 5 min. Supernatants containing the DNA (approximately 50 μl each) were transferred to clean DNase/RNase-free prelabeled tubes and stored at −20°C for use in the PCR assays (15).
PCR-RFLP assays.
Two previously published nested PCR-restriction fragment length polymorphism (RFLP) assays were used to target the 18S rRNA gene at locus 1 (16, 17), i.e., modified forward primer, WR494F (18), and reverse primer, XR1 (19–21), and at locus 2 (19–24). Internal primers for locus 1 and locus 2 amplify secondary PCR amplicons of ∼435 bp and ∼826 bp, respectively. Each reaction was performed in either 50- or 100-μl mixtures containing premixed reagents at final concentrations of 200 μM (each) of the four deoxynucleoside triphosphates (dNTPs) (Invitrogen Ltd., United Kingdom), bovine serum albumin (BSA) at 400 μg ml−1 (Sigma-Aldrich, United Kingdom), Tween 20 (VWR, United Kingdom) at 2%, 200 nM (each) forward and reverse primers (MWG Biotech United Kingdom Ltd., United Kingdom), MgCl2 at concentrations varying from 2.0 to 6 mM depending on the PCR assay, and 2.5 U of Taq polymerase at the concentration specified for each assay in 1× PCR buffer IV (ABgene, United Kingdom). PCR amplifications were performed using a GeneAmp PCR Thermal Cycler, model 9700 (Applied Biosystems, United Kingdom). For locus 1 and locus 2, 3 μl of template was used for the first PCR amplification and 5 μl for the second PCR amplification. Positive and negative controls were included in every experiment. Ten microliters of the second-round PCR amplicons were visualized by UV transillumination after electrophoresis at 80 V for 1 h in 1.4% agarose gels stained with ethidium bromide (0.5 μg ml−1). Gels were photographed using the Gel Doc 2000 system (Bio-Rad, United Kingdom), equipped with QuantityOne software for gel documentation.
RFLP analysis.
RFLP analysis of both 18S rRNA assay PCR products were performed by digesting 20 μl of the secondary PCR amplicons with 20 U of each restriction enzyme. Digestion with AseI/DraI was performed on locus 1 PCR amplicons (16), whereas separate digestions with AseI and SspI (19–24) were performed on locus 2 amplicons. Further digestion with restriction enzyme MboII was used to determine the presence of C. xiaoi or C. ryanae. Fifty microliters of the RFLP products was visualized under UV transillumination on a 2% agarose gel run for 2 h at 100 volts. Gels were photographed as previously stated. Interpretation of banding patterns was made by comparison to the DNA marker (100-bp DNA ladder; Invitrogen Ltd.). The obtained profiles were matched with known banding patterns from published papers for locus 1 (16) and locus 2 (19–24) to identify the Cryptosporidium species/genotypes.
Genotyping of C. parvum- and C. hominis-positive samples.
Genotyping was performed using two nested-PCR assays targeting the glycoprotein 60 gene (GP60) for C. parvum or C. hominis at locus 3 (25) and locus 4 (26). Locus 3 and locus 4 amplify secondary PCR amplicons of ∼900 bp and ∼400 bp, respectively. PCRs consisted of premixed reagents as described above (PCR-RFLP), and 5 μl of template was used for both the primary and the secondary PCR amplifications in 100-μl final reaction volumes. Positive and negative PCR controls and gel image analysis of amplicons were as already described.
Sequencing analysis.
Sequencing was used to confirm Cryptosporidium species/genotypes from second-round PCR products that contain single species/genotypes as determined by locus 1 and 2 and PCR products that identify genotypes as determined by locus 3 and 4. Initially, sequencing was performed at the Scottish Microbiology Reference Laboratories, Stobhill Hospital, using the LI-COR L4200-L2 DNA sequencer (LI-COR Biosciences UK-Ltd., Cambridge, United Kingdom). PCR2 products that contained single C. parvum, C. hominis, C. xiaoi, C. ubiquitum, and possible C. andersoni isolates were sequenced in both directions using the appropriate second-round PCR forward and reverse primers for locus 1 and 2 (16, 19–24). The two sets of sequencing primers were respectively labeled with the 700 and 800 infrared dyes. PCR2 products that identify C. parvum and C. hominis genotypes were sequenced in both directions using the appropriate second-round PCR forward and reverse primers for locus 3 and 4 (25, 26). All modified oligonucleotides were purchased from MWG BioTech (United Kingdom).
Confirmation of the C. parvum and C. hominis genotypes was performed at the Sequencing Service, Dundee University, Scotland (http://www.dnaseq.co.uk/services.html). Cryptosporidium parvum and C. hominis amplicons were treated enzymatically with ExoSAP-IT (GE Healthcare) to remove excess dNTPs and primers according to the supplier's instructions. Bidirectional sequencing was performed in an ABI model 3730 sequencer using Big-Dye version 3.1, chemistry, and an automated capillary DNA sequencer.
For all sequenced samples, bidirectional sequences were aligned using the EMBL website (http://www.ebi.ac.uk/Tools/psa/emboss_needle/nucleotide.html) tools to obtain a consensus, which was manually edited by referring to the sequence chromatogram. The consensus sequence was used to search the GenBank database for similar sequences using the NCBI BLASTN tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The ClustalW2 alignment using the EMBL website (http://www.ebi.ac.uk/Tools/msa/clustalw2/) was used to compare sequences, and a phylogenetic tree was constructed using the MEGA 4 software (27, 28).
Nucleotide sequence accession numbers.
The 12 GP60 sequences from the St. Kilda samples were deposited in GenBank under accession numbers KC679684 to KC679695.
RESULTS
Microscopic analysis of AP-stained oocysts.
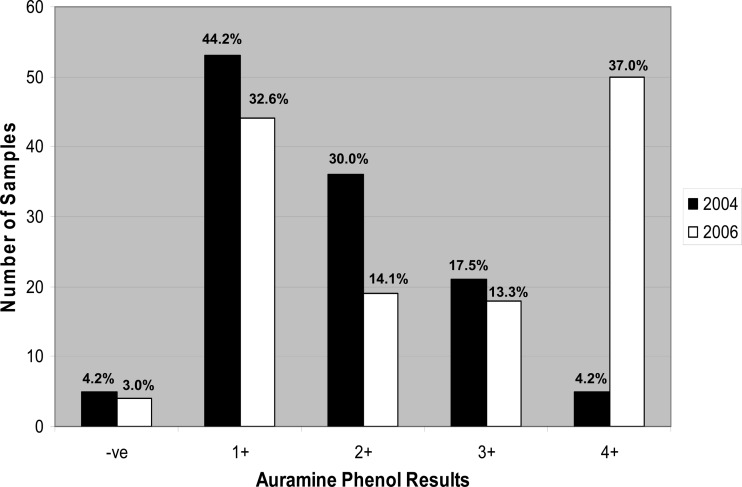
The fecal samples collected during 2006 showed a higher percentage of strong positives (4+) than did the fecal samples collected during 2004, which had a more aggregated distribution (Fig. 1). A significant proportion of the 2004 St. Kilda fecal samples (44.2%) contained 1 to 10 Cryptosporidium oocysts (1+) per slide when tested by AP. In comparison, a large proportion of the 2006 St. Kilda samples (37.0%) contained 31 to 50 Cryptosporidium oocysts (4+) per slide.
Fig 1.
Microscopic determination of the number of auramine phenol-stained Cryptosporidium oocysts in St. Kilda sheep fecal samples.
PCR amplification of the fecal sample DNA.
Two hundred forty-six AP-positive samples and nine AP-negative samples were analyzed by PCR (Table 1). Of the 2004 samples (n = 120), 64.2% and 42.5% were strong Cryptosporidium positives with loci 1 and 2, respectively, and 15.8% and 9.2% were weak Cryptosporidium positives with loci 1 and 2, respectively. Of the 2006 fecal samples (n = 135), 54.1% and 30.4% were strong Cryptosporidium positives with loci 1 and 2, respectively, and 38.5% and 0.7% were weak Cryptosporidium positives with loci 1 and 2, respectively (Table 1). Comparison of PCR results obtained with both loci showed that the nonspecific bands were observed frequently with both assays.
Table 1.
Frequency of Cryptosporidium species present in St. Kilda sheep fecal samples by DNA amplification of the 18S rRNA gene at locus 1 and locus 2
| PCR result for Cryptosporidium | No. of samples tested for indicated yr |
|||
|---|---|---|---|---|
| Locus 1 |
Locus 2 |
|||
| 2004 | 2006 | 2004 | 2006 | |
| Strong positivea | 77 | 73 | 51 | 41 |
| Weak positiveb | 19 | 52 | 11 | 1 |
| Negative | 24 | 10 | 58 | 93 |
| Total no. of samples tested | 120 | 135 | 120 | 135 |
The expected Cryptosporidium amplicons were detected by gel electrophoresis, i.e., ∼435 bp (locus 1) and ∼826 bp (locus 2).
Insufficient amplicon for enzymatic digestion.
Comparison between microscopy and PCR results.
A comparison of microscopy and PCR results is shown in Table 2. Microscopy of AP-stained oocysts is more sensitive at detecting Cryptosporidium than the PCR assay, but six of the nine AP-negative samples were PCR positive using locus 1, and two of these samples were also positive with locus 2.
Table 2.
Amplification of Cryptosporidium species DNA at loci 1 and 2 of the 18S rRNA gene on 255 samples stained by auramine phenol
| PCR assay target | No. (%) of AP test samples receiving score of: |
Total (%) | ||||
|---|---|---|---|---|---|---|
| Negative | 1+ | 2+ | 3+ | 4+ | ||
| Locus 1 | 6 (2.4) | 87 (34.1) | 42 (16.5) | 36 (14.1) | 50 (19.6) | 221 (86.7) |
| Locus 2 | 2 (0.8) | 32 (12.5) | 28 (11.0) | 19 (7.5) | 23 (9.1) | 104 (40.8) |
| Total no. analyzed | 9 | 97 | 55 | 39 | 55 | 255 |
RFLP analysis of the PCR-positive amplicons.
RFLP results for both loci were combined to give the overall result of species identification due to the fact that some of the samples were positive for only one of the PCR assays. RFLP results are shown in Table 3. From the 255 Soay sheep fecal samples, the predominant Cryptosporidium species and genotypes identified with 18S rRNA gene analysis were C. parvum (9%), C. hominis (11.4%), C. ubiquitum (6.7%), and C. xiaoi (12.5%). These Cryptosporidium species were present either as single species or as a mixture of species. Of the samples containing single Cryptosporidium species/genotypes, 36.7% (n = 120) and 47.4% (n = 135) were identified in the 2004 and 2006 samples, respectively. Of those containing mixed Cryptosporidium species, 38.3% (n = 120) and 13.3% (n = 135) were observed in the 2004 and 2006 samples, respectively (Table 3).
Table 3.
Species determination by restriction enzyme digestion of amplified Cryptosporidium DNA
| Cryptosporidium species/genotype | No. of samples |
% of total | ||
|---|---|---|---|---|
| 2004 | 2006 | Total | ||
| C. parvum | 9 | 14 | 23 | 9.0 |
| C. hominis | 14 | 15 | 29 | 11.4 |
| C. parvum/C. hominisa | 0 | 10 | 10 | 3.9 |
| C. xiaoi | 10 | 22 | 32 | 12.5 |
| Mixture of C. parvum + C. xiaoi | 3 | 1 | 4 | 1.6 |
| Mixture of C. parvum/C. hominis + C. xiaoi/C. ryanaeb | 4 | 3 | 7 | 2.7 |
| C. parvum/C. hominis/C. xiaoi/C. ryanaec | 1 | 2 | 3 | 1.2 |
| C. ubiquitum | 9 | 8 | 17 | 6.7 |
| Mixture of C. parvum + C. ubiquitum | 1 | 1 | 2 | 0.8 |
| Mixture of C. parvum + C. ubiquitum/Cryptosporidium ferret/mouse genotypes/C. meleagridisd | 1 | 0 | 1 | 0.4 |
| Mixture of C. ubiquitum + C. parvum + C. xiaoi | 1 | 2 | 3 | 1.2 |
| Mixture of C. ubiquitum + C. xiaoi | 5 | 3 | 8 | 3.1 |
| Mixture of C. ubiquitum + C. parvum/C. hominise | 0 | 1 | 1 | 0.4 |
| Mixture of C. ubiquitum + C. parvum/C. hominis/C. xiaoi/C. ryanaef | 31 | 7 | 38 | 14.9 |
| Cryptosporidium sp.g | 21 | 34 | 55 | 21.6 |
| No Cryptosporidium detected | 8 | 7 | 15 | 5.9 |
| Possibly C. andersoni | 2 | 5 | 7 | 2.7 |
| Total no. of samples tested | 120 | 135 | 255 | |
Locus 1 RFLP pattern identified C. parvum/C. hominis; sequencing of amplicons was too short for analysis, and locus 2 was either too weak to digest or PCR negative.
Locus 1 RFLP pattern identified C. parvum/C. hominis + C. xiaoi/C. ryanae, and locus 2 was either too weak to digest or PCR negative.
Locus 1 RFLP pattern identified C. parvum/C. hominis/C. xiaoi/C. ryanae; sequencing of amplicons was too short for analysis, and locus 2 was either too weak to digest or PCR negative.
Locus 1 RFLP pattern identified weak Cryptosporidium ferret/mouse genotypes/C. ubiquitum/C. meleagridis, and locus 2 RFLP pattern identified C. parvum.
C. parvum/C. hominis cannot be distinguished by digestion of locus 1 amplicons with restriction enzyme MboII.
Locus 1 and 2 PCR amplicons, which cannot be further digested because they contain mixtures of Cryptosporidium species.
Samples were too weak to digest but produced positive Cryptosporidium amplicons of the expected size.
Confirmation of species/genotypes by sequencing analysis.
Sequence analysis was performed to confirm the single species/genotypes detected with RFLP analysis on the following amplicons: C. ubiquitum (6/12, good consensus), C. xiaoi (4/8, reverse sequences only), and C. andersoni (3/7, reverse sequences only). Samples that gave 18S rRNA gene PCR-RFLP patterns conforming to C. parvum/C. hominis and C. parvum/C. hominis/C. bovis/C. ryanae were also sequenced. Of the 56 sequenced samples, 29 were identified as C. hominis (19 samples sequenced had good consensus, and 1 sample produced the forward sequence only, while 9 samples produced reverse sequences only). Twelve samples contained C. parvum (8 had good consensus, 1 produced the forward sequence only, and 3 produced reverse sequences only). Fifteen samples gave poor sequences, which were noninterpretable from sequencing analysis. Samples that were either C. parvum or C. hominis (from both collection dates) were considered for GP60 genotyping.
GP60 sequencing analysis of the C. parvum and C. hominis samples.
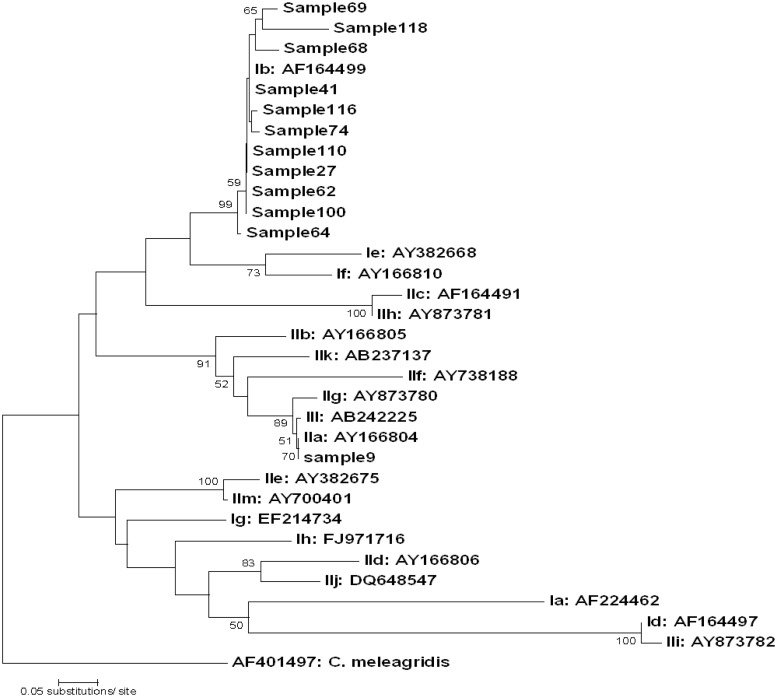
Initially, 128 samples that had 18S RFLP patterns corresponding to either C. parvum or C. hominis were amplified using the GP60 primers. An amplicon was produced in 33 samples. Further digestion of locus 2 amplicons with MboII restriction enzyme (29) revealed that 16 of the 33 samples had RFLP patterns conforming to C. parvum or C. hominis. The remaining 17 samples yielded banding patterns of mixed species in which C. parvum/C. ubiquitum/C. xiaoi/C. ryanae were present. Of the 16 GP60 PCR-positive samples that were sequenced, 12 gave good consensus (locus 3, n = 1; locus 4, n = 11). The 12 GP60 sequences from the St. Kilda samples were deposited in GenBank and aligned with the GP60 family I and II prototype sequences. Alignments confirmed the presence of two subtype families, namely, IIa and Ib (Table 4 and Fig. 2). BLASTN results confirmed that locus 4 sequences had high homology (95 to 99%) with IbA10G2 from a lamb (EU186152) (30) while locus 3 sequences had 99% homology with IIaA19G1R1, demonstrating two genetic clusters within the St. Kilda samples (Fig. 2).
Table 4.
Determination of Cryptosporidium genotypes in St. Kilda sheep DNA using a GP60-targeted approach
| Yr of sampling and sample no. (sheep tag) | Genotype |
|---|---|
| 2004 | |
| 9 (AR012) | IIaA19G1R1 |
| 27 (AY081) | IbA10G2 |
| 2006 | |
| 41 (AG102) | IbA10G2 |
| 62 (BY071) | IbA10G2 |
| 64 (BY161) | IbA10G2 |
| 68 (BY072) | IbA10G2 |
| 69 (BY096) | IbA10G2 |
| 74 (BY129) | IbA10G2 |
| 100 (BY160) | IbA10G2 |
| 110 (AW112) | IbA10G2 |
| 116 (AW073) | IbA10G2 |
| 118 (AW580) | IbA10G2 |
Fig 2.
Phylogenetic analysis of St. Kilda Cryptosporidium prototype sequences with those previously deposited in GenBank. A neighbor-joining tree was constructed by using Mega4, based on genetic distance calculated by the Kimura two-parameter model. Values on branches are percent bootstrap values using 1,000 replicates. Bootstrap values of <50% are shown, and the C. meleagridis sequence (accession number AF401497) was used as an out-group.
DISCUSSION
In this study, microscopy was chosen as the “gold standard” to determine the presence of oocysts in stools (14). The inability of AP staining to detect oocysts in six of the Cryptosporidium-positive samples may be due to the presence of debris masking stained oocysts and/or increasing background fluorescence of naked DNA, which cannot be seen by microscopy (31, 32). Oocysts are more readily detected in smears made from watery specimens than from formed stools, and in this study, 44% of the St. Kilda samples consisted of formed stools and 56% were unformed. Previous studies have shown PCR assays to be more sensitive than microscopy at detecting Cryptosporidium in stools (11, 33–35), which is in contrast to our findings. Failure to amplify oocyst DNA may have resulted from low IMS recovery due to the consistency of fecal samples. Indeed, the majority of the 2004 St. Kilda fecal samples contained low numbers of Cryptosporidium oocysts per slide (between 1 and 10) and low numbers in total (4 to 27 oocysts per sample). The condition of oocysts may influence recovery, as it is known that oocyst cell walls can deteriorate and epitopes that interact with the magnetic beads are stripped from the cell wall, resulting in fewer/no oocyst-bead complexes (36, 37). Greater sensitivity of detection was achieved by the amplification of locus 1, which is confirmed in previous studies showing a detection level of as few as two to three oocysts (16, 17). Assessment of primer sensitivity at loci 3 and 4 using 10-fold serial dilutions of C. parvum oocysts indicated a detection range of 20 to 200 oocysts (locus 3) and 2 to 20 oocysts (locus 4), which may contribute to the inability to successfully sequence certain samples. In addition, the high numbers of mixed species in this study prevented their sequencing due to sequence overlap and background signal interference.
In this study, we identified C. xiaoi, previously named C. bovis-like genotype (33), as the most prevalent species to be isolated from the Soay sheep population, and the possible presence of a cattle genotype, C. andersoni, was observed. This is intriguing since the sheep are an isolated population and no cattle have been living on the island since the 1930s. In our study, the C. xiaoi MboII RFLP banding patterns generated a weak band that was similar in size to the C. ryanae digested band. However, MboII can generate partial digestion, causing misinterpretations of generated RFLP patterns, and as no C. ryanae was found alone in the St. Kilda population, the generated weak band was considered to be the result of incomplete digestion and not to indicate the presence of C. ryanae.
Cryptosporidium hominis, the second most prevalent species to be isolated from St. Kilda sheep, is primarily associated with human contact and accounts for almost 50% of Cryptosporidium-positive human cases in the United Kingdom (38). This species has been identified in only a small number of other hosts including primates (39), an Australian marine animal and sheep (20, 40), Scottish cattle (41), and a lamb within the United Kingdom (30). The most prevalent C. hominis genotype in human cases within the United Kingdom is IbA10G2 (42, 43), which is identical to that isolated from St. Kilda sheep. This genotype was identified in the lamb from the United Kingdom and in drinking water (30, 44).
Cryptosporidium parvum infects a wide range of mammals as demonstrated by early transmission studies (45). Cryptosporidium parvum has been documented in domestic sheep (7), and Cryptosporidium species, presumed to be C. parvum, have been identified in Soay sheep by microscopic examination using modified Ziehl-Neelsen staining (13). C. parvum sequence heterogeneity is known to exist (46, 47), and variability was observed in all the St. Kilda locus 1 amplicons (46). This finding is similar to that from a study of lambs within an isolated geographical area in Spain (47). The IIaA19G1R1 genotype revealed in one of the 2004 St. Kilda isolates has been previously identified in a calf from the United Kingdom and in a human from Slovenia (48, 49).
Cryptosporidium ubiquitum is known to infect a wide range of hosts, including wild white-tailed deer, primates, antelope, squirrels, and sheep (11, 29, 35, 50, 51), demonstrating the zoonotic potential of this Cryptosporidium genotype (49, 52–55). It has also been shown to infect humans worldwide, including Canada, New Zealand, Slovenia, the United States, and the United Kingdom. The six St. Kilda samples confirmed as C. ubiquitum by sequencing all contained the two nucleotides AT at positions 691 and 692 bp in the hypervariable region of the 18S gene, which appears to be the most common C. ubiquitum DNA sequence (35, 49, 50, 54–57). Santín et al. (35) concluded that there were three variants of C. ubiquitum, which they named Cryptosporidium cervine 1 to 3. According to this classification, the St. Kilda samples appear to be the Cryptosporidium cervine 1 variant (35).
The diversity of Cryptosporidium species in the St. Kilda Soay sheep population may be due to a variety of reasons, including the consumption of oocysts by other sheep or their carriage by hosts such as birds, humans, invertebrates, and vertebrates in the sea surrounding St. Kilda, including fish and marine mammals (58, 59). Currently there is a lack of knowledge relating to Cryptosporidium transmission on St. Kilda, as few data using conventional or molecular analyses for Cryptosporidium are available.
To conclude, the main species of Cryptosporidium present in the St. Kilda sheep population are (i) C. parvum, (ii) C. hominis, (iii) C. ubiquitum, and (iv) C. xiaoi. An important outcome from this study is that C. xiaoi is the dominant Cryptosporidium species present in the St. Kilda sheep population despite other studies identifying C. ubiquitum as the dominant species in sheep. In addition, there is a high prevalence of diverse mixtures of Cryptosporidium species in the St. Kilda sheep population, and two genetic clusters are present, namely, IIaA19G1R1 and IbA10G2.
Further studies are required to determine a correlation between host age and sex and the different Cryptosporidium species detected in the Soay sheep population. In addition, molecular investigations to assess possible transmission routes would further our knowledge and understanding of cryptosporidiosis.
ACKNOWLEDGMENTS
This work is dedicated to the memory of Huw Smith, who died in October 2010.
We thank J. M. Pemberton and J. G. Pilkington from the Soay Sheep Project for collecting the samples, the National Trust for Scotland and Scottish Natural Heritage for permission to work on St. Kilda, and the MOD, QinetiQ, and Eurest staff on St. Kilda and Benbecula for logistical support.
REFERENCES
- 1.Cacciò SM, Thompson RCA, McLauchlin J, Smith HV. 2005. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 21:430–437 [DOI] [PubMed] [Google Scholar]
- 2.Elwin K, Hadfield SJ, Robinson G, Crouch ND, Chalmers RM. 2012. Cryptosporidium viatorum n. sp. (Apicomplexa: Cryptosporidiidae) among travellers returning to Great Britain from the Indian subcontinent, 2007–2011. Int. J. Parasitol. 42:675–682 [DOI] [PubMed] [Google Scholar]
- 3.Fayer R, Santín M, Macarisin D. 2010. Cryptosporidium ubiquitum n. sp. in animals and humans. Vet. Parasitol. 172:23–32 [DOI] [PubMed] [Google Scholar]
- 4.Kvác M, Kvetonová D, Sak B, Ditrich O. 2009. Cryptosporidium pig genotype II in immunocompetent man. Emerg. Infect. Dis. 15:982–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plutzer J, Karanis P. 2009. Genetic polymorphism in Cryptosporidium species: an update. Vet. Parasitol. 165:187–199 [DOI] [PubMed] [Google Scholar]
- 6.Smith H, Cacciò S, Cook N, Nichols R, Tait A. 2007. Cryptosporidium and Giardia as foodborne zoonoses. Vet. Parasitol. 149:29–40 [DOI] [PubMed] [Google Scholar]
- 7.Chalmers RM, Elwin K, Reilly WJ, Irvine H, Thomas AL, Hunter PR. 2002. Cryptosporidium in farmed animals: the detection of a novel isolate in sheep. Int. J. Parasitol. 32:21–26 [DOI] [PubMed] [Google Scholar]
- 8.Elwin K, Chalmers R. 2008. Contemporary identification of previously reported novel Cryptosporidium isolates reveals Cryptosporidium bovis and the Cervine genotype in sheep (Ovis aries). Parasitol. Res. 102:1103–1105 [Google Scholar]
- 9.Wang Y, Feng Y, Cui B, Jian F, Ning C, Wang R, Zhang L, Xiao L. 2010. Cervine genotype is the major Cryptosporidium genotype in sheep in China. Parasitol. Res. 106:341–347 [DOI] [PubMed] [Google Scholar]
- 10.Fayer R, Santín M. 2009. Cryptosporidium xiaoi n. sp. (Apicomplexa: Cryptosporidiidae) in sheep (Ovis aries). Vet. Parasitol. 164:190–200 [DOI] [PubMed] [Google Scholar]
- 11.Ryan UM, Bath C, Robertson I, Read C, Elliot A, Mcinnes L, Traub R, Besier B. 2005. Sheep may not be an important zoonotic reservoir for Cryptosporidium and Giardia parasites. Appl. Environ. Microbiol. 71:4992–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clutton-Brock TH, Pemberton J. 2004. The causes and consequences of instability, p 276–310 Clutton-Brock TH, Pemberton JM. (ed), Soay sheep: dynamics and selection in an island population. Cambridge University Press, Cambridge, England [Google Scholar]
- 13.Craig B, Pilkington J, Kruuk L, Pemberton J. 2007. Epidemiology of parasitic protozoan infections in Soay sheep (Ovis aries L.) on St Kilda. Parasitology 134:9–21 [DOI] [PubMed] [Google Scholar]
- 14.Casemore DP, Armstrong M, Sands RL. 1985. Laboratory diagnosis of cryptosporidiosis. J. Clin. Pathol. 38:1337–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols RAB, Smith HV. 2004. Optimization of DNA extraction and molecular detection of Cryptosporidium oocysts in natural mineral water sources. J. Food Prot. 67:524–532 [DOI] [PubMed] [Google Scholar]
- 16.Nichols RAB, Campbell BM, Smith HV. 2003. Identification of Cryptosporidium spp. oocysts in United Kingdom noncarbonated natural mineral waters and drinking waters by using a modified nested PCR-restriction fragment length polymorphism assay. Appl. Environ. Microbiol. 69:4183–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols RAB, Connelly L, Sullivan CB, Smith HV. 2010. Identification of Cryptosporidium species and genotypes in Scottish raw and drinking waters during a one-year monitoring period. Appl. Environ. Microbiol. 76:5977–5986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward P, Deplazes P, Regli W, Rinder H, Mathis A. 2002. Detection of eight Cryptosporidium genotypes in surface and waste waters in Europe. Parasitology 124:359–368 [DOI] [PubMed] [Google Scholar]
- 19.Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA. 1999. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 65:1578–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao L, Limor J, Li L, Morgan U, Thompson R, Lal A. 1999. Presence of heterogeneous copies of the small subunit rRNA gene in Cryptosporidium parvum human and marsupial genotypes and Cryptosporidium felis. J. Eukaryot. Microbiol. 46:44S–45S [PubMed] [Google Scholar]
- 21.Xiao L, Morgan UM, Limor J, Escalante A, Arrowood M, Shulaw W, Thompson RC, Fayer R, Lal AA. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L, Alderisio K, Limor J, Royer M, Lal AA. 2000. Identification of species and sources of Cryptosporidium oocysts in storm waters with small-subunit rRNA-based diagnostic and genotyping tool. Appl. Environ. Microbiol. 66:5492–5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao L, Limor J, Morgan UM, Sulaiman IM, Thompson RC, Lal AA. 2000. Sequence differences in the diagnostic target region of the oocyst wall protein gene of Cryptosporidium parasites. Appl. Environ. Microbiol. 66:5499–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao L, Limor JR, Sulaiman IM, Duncan RB, Lal AA. 2000. Molecular characterization of a Cryptosporidium isolate from a black bear. Parasitology 86:1166–1170 [DOI] [PubMed] [Google Scholar]
- 25.Glabermann S, Moore JE, Lowery CJ, Chalmers RM, Sulaiman I, Elwin K, Rooney PJ, Millar BC, Dooley JSG, Xiao L. 2002. Three drinking-water-associated cryptosporidiosis outbreaks, Northern Ireland. Emerg. Infect. Dis. 8:631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulaiman IM, Hira PR, Zhou L, Al-Ali FM, Al-Shelahi FA, Shweiki HM, Iqbal J, Khalid N, Xiao L. 2005. Unique endemicity of cryptosporidiosis in children in Kuwait. J. Clin. Microbiol. 43:2805–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Dudley J, Nei M, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Ortega Y, He G, Das P, Xu M, Zhang X, Fayer R, Gatei W, Cama V, Xiao L. 2007. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet. Parasitol. 144:1–9 [DOI] [PubMed] [Google Scholar]
- 30.Giles M, Chalmers R, Pritchard G, Elwin K, Mueller-Doblies D, Clifton-Hadley F. 2009. Cryptosporidium hominis in a goat and a sheep in the UK. Vet. Rec. 164:24–25 [DOI] [PubMed] [Google Scholar]
- 31.Weber R, Bryan RT, Juranek DD. 1992. Improved stool concentration procedure for detection of Cryptosporidium oocysts in fecal specimens. J. Clin. Microbiol. 30:2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster K, Smith H, Giles M, Dawson L, Robertson L. 1996. Detection of Cryptosporidium parvum oocysts in faeces: comparison of conventional coproscopical methods and the polymerase chain reaction. Vet. Parasitol. 61:5–13 [DOI] [PubMed] [Google Scholar]
- 33.Morgan U, Pallant L, Dwyer B, Forbes D, Rich G, Thompson R. 1998. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J. Clin. Microbiol. 36:995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santín M, Fayer R. 2007. Intragenotypic variations in the Cryptosporidium sp. cervine genotype from sheep with implications for public health. Parasitology 93:668–672 [DOI] [PubMed] [Google Scholar]
- 35.Santín M, Trout JM, Fayer R. 2007. Prevalence and molecular characterization of Cryptosporidium and Giardia species and genotypes in sheep in Maryland. Vet. Parasitol. 146:17–24 [DOI] [PubMed] [Google Scholar]
- 36.Ware MW, Wymer L, Lindquist HD, Schaefer FW. 2003. Third evaluation of an alternative IMS dissociation procedure for use with method 1622: detection of Cryptosporidium in water. J. Microbiol. Methods 55:575–583 [DOI] [PubMed] [Google Scholar]
- 37.Xiao L, Alderisio K, Jiang J. 2006. Detection of Cryptosporidium oocysts in water: effect of the number of samples and analytic replicates on test results. Appl. Environ. Microbiol. 72:5942–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols RAB, Moore J, Smith HV. 2006. A rapid method for extracting oocyst DNA from Cryptosporidium-positive human faeces for outbreak investigation. J. Microbiol. Methods 65:512–524 [DOI] [PubMed] [Google Scholar]
- 39.Spano F, Putignani L, Crisanti A, Sallicandro P, Morgan UM, Le Blancq SM, Tchack L, Tzipori S, Widmer G. 1998. Multilocus genotypic analysis of Cryptosporidium parvum isolates from different hosts and geographical origins. J. Clin. Microbiol. 36:3255–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan UM, Xiao L, Hill BD, O'Donoghue P, Limor J, Lal AA, Thompson RCA. 2000. Detection of the Cryptosporidium parvum “human” genotype in a dugong (Dugong dugong). J. Parasitol. 86:1352–1354 [DOI] [PubMed] [Google Scholar]
- 41.Smith HV, Nichols RAB, Mallon M, MacLeod A, Tait A, Reilly JW, Browning LM, Gray D, Reid SWJ, Wastling JM. 2005. Natural Cryptosporidium hominis infections in Scottish cattle. Vet. Rec. 156:710–711 [DOI] [PubMed] [Google Scholar]
- 42.Chalmers RM, Hadfield SJ, Jackson CJ, Elwin K, Xiao L, Hunter P. 2008. Geographic linkage and variation in Cryptosporidium hominis. Emerg. Infect. Dis. 14:496–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter PR, Hadfield SJ, Wilkinson D, Lake IR, Harrison FCD, Chalmers RM. 2007. Subtypes of Cryptosporidium parvum in humans and disease risk. Emerg. Infect. Dis. 13:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmers RM, Robinson G, Elwin K, Hadfield SJ, Thomas E, Watkins J, Casemore D, Kay D. 2010. Detection of Cryptosporidium species and sources of contamination with Cryptosporidium hominis during a waterborne outbreak in north west Wales. J. Water Health 8:311–325 [DOI] [PubMed] [Google Scholar]
- 45.O'Donoghue PJ. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139–195 [DOI] [PubMed] [Google Scholar]
- 46.Gibbons-Matthews C, Prescott A. 2003. Intra-isolate variation of Cryptosporidium parvum small subunit ribosomal RNA genes from human hosts in England. Parasitol. Res. 90:439–444 [DOI] [PubMed] [Google Scholar]
- 47.Quílez J, Torres E, Chalmers RM, Hadfield SJ, Del Cacho E, Sánchez-Acedo C. 2008. Cryptosporidium genotypes and subtypes in lambs and goat kids in Spain. Appl. Environ. Microbiol. 74:6026–6031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brook E, Christley R, French Hart C. 2008. Detection of Cryptosporidium oocysts in fresh and frozen cattle faeces: comparison of three methods. Lett. Appl. Microbiol. 46:26–31 [DOI] [PubMed] [Google Scholar]
- 49.Soba B, Petrovec B, Mioc V, Logar J. 2006. Molecular characterization of Cryptosporidium isolates from humans in Slovenia. Clin. Microbiol. Infect. 12:918–921 [DOI] [PubMed] [Google Scholar]
- 50.da Silva A, Cacciò S, Williams C, Won K, Nace E, Whittier C, Pieniazek N, Eberhard M. 2003. Molecular and morphologic characterization of a Cryptosporidium genotype identified in lemurs. Vet. Parasitol. 111:297–307 [DOI] [PubMed] [Google Scholar]
- 51.Perz JF, Le Blancq SM. 2001. Cryptosporidium parvum infection involving novel genotypes in wildlife from lower New York State. Appl. Environ. Microbiol. 67:1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Learmonth JJ, Ionas G, Ebbett KA, Kwan ES. 2004. Genetic characterization and transmission cycles of Cryptosporidium species isolated from humans in New Zealand. Appl. Environ. Microbiol. 70:3973–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leoni F, Amar C, Nichols G, Pedraza-Diaz S, McLauchlin J. 2006. Genetic analysis of Cryptosporidium from 2414 humans with diarrhea in England between 1985 and 2000. J. Med. Microbiol. 55:703–707 [DOI] [PubMed] [Google Scholar]
- 54.Ong CSL, Eisler DL, Alikhani A, Fung VWK, Tomblin J, Bowie WR, Isaax-Renton JL. 2002. Novel Cryptosporidium genotypes in sporadic cryptosporidiosis cases: first report of human infections with a orvine genotype. Emerg. Infect. Dis. 8:263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trotz-Williams LA, Martin DS, Gatei W, Cama V, Peregrine AS, Martin SW, Nydam DV, Jamieson F, Xiao L. 2006. Genotype and subtype analyses of Cryptosporidium isolates from dairy calves and humans in Ontario. Parasitol. Res. 99:346–352 [DOI] [PubMed] [Google Scholar]
- 56.Feltus DC, Giddings CW, Schneck BL, Monson T, Warshauer D, McEvoy JM. 2006. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J. Clin. Microbiol. 44:4303–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng Y, Alderisio KA, Yang W, Blancero LA, Kuhne WG, Nadareski CA, Reid M, Xiao L. 2007. Cryptosporidium genotypes in wildlife from a New York watershed. Appl. Environ. Microbiol. 73:6475–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graczyk TK, McOliver C, Silbergeld EK, Tamang L, Roberts JD. 2007. Risk of handling as a route of exposure to infectious waterborne Cryptosporidium parvum oocysts via Atlantic blue crabs (Callinectes sapidus). Appl. Environ. Microbiol. 73:4069–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reid A, Lymbery A, Ng J, Tweedle S, Ryan U. 2010. Identification of novel and zoonotic Cryptosporidium species in marine fish. Vet. Parasitol. 168:190–195 [DOI] [PubMed] [Google Scholar]