Abstract
In the United States, the 2009 pandemic influenza A (H1N1) virus (pH1N1) infected almost 20% of the population and caused >200,000 hospitalizations and >10,000 deaths from April 2009 to April 2010. On 24 April 2009, the CDC posted interim guidance on infection control measures in health care settings explicitly for pH1N1 and recommended using filtering face respirators (FFRs) when in close contact with a suspected- or confirmed-to-be-infected individual, particularly when performing aerosol-generating procedures. The persistence and infectivity of pH1N1 were evaluated on FFRs, specifically N95 respirators, under various conditions of absolute humidity (AH) (4.1 × 105 mPa, 6.5 × 105 mPa, and 14.6 × 105 mPa), sample matrices (2% fetal bovine serum [FBS], 5 mg/ml mucin, and viral medium), and times (4, 12, 24, 48, 72, and 144 h). pH1N1 was distributed onto N95 coupons (3.8 to 4.2 cm2) and extracted by a vortex-centrifugation-filtration process, and the ability of the remaining virus to replicate was quantified using an enzyme-linked immunosorbent assay (ELISA) to determine the log10 concentration of the infectious virus per coupon. Overall, pH1N1 remained infectious for 6 days, with an approximately 1-log10 loss of virus concentrations over this time period. Time and AH both affected virus survival. We found significantly higher (P ≤ 0.01) reductions in virus concentrations at time points beyond 24 to 72 h (−0.52-log10 reduction) and 144 h (−0.74) at AHs of 6.5 × 105 mPa (−0.53) and 14.6 × 105 mPa (−0.47). This research supports discarding respirators after close contact with a person with suspected or confirmed influenza infection due to the virus's demonstrated ability to persist and remain infectious.
INTRODUCTION
The 2009 H1N1 pandemic influenza A (H1N1) virus (pH1N1) outbreak affected >214 countries and caused at least 18,449 deaths worldwide (WHO, 6 August 2010). The estimated impact, as extrapolated from laboratory-confirmed hospitalizations in the United States from April 2009 to April 2010, was 60.8 million cases (range, 43.3 to 89.3 million), 274,304 hospitalizations (range, 195,086 to 402,719), and 12,469 deaths (range, 8,868 to 18,306) (1). The current Centers for Disease Control and Prevention (CDC) Prevention Strategies for Seasonal Influenza in Healthcare Settings: Guidelines and Recommendations states that face masks are a sufficient form of personal protective equipment (PPE) for hospital staff, associated workers, patients, and visitors when a person is suspected or known to be infected (3). During the pandemic, the first CDC interim guidance statement was posted on 24 April 2009 regarding infection control measures in health care settings specifically for pH1N1. Filtering facepiece respirators (FFRs) (i.e., N95) were recommended (in addition to standard precautions) in this guidance document as a conservative measure to protect health care personnel when patients are in isolation, particularly during aerosol-generating procedures, and for those in close contact with patients with suspected or confirmed pH1N1 infections (2, 4).
The number of N95 FFRs used during the 2009 pandemic period is unclear, and supply shortages were acknowledged in the CDC 2009 H1N1 Influenza Interim Guidance document (2). A study by the Institute of Medicine stated that 90 million respirators would be needed for a 42-day influenza pandemic (5). Meanwhile, Murray et al. (6) found that facial protective equipment (e.g., masks, respirators, and disposable eyewear) use more than doubled in the Vancouver Coastal Health service region during the 2009 pandemic. Specifically for respirators, the rate of use during the pandemic was 51% higher than the historical baseline; to estimate the supplies needed in the event of a pandemic, the authors suggested a 1:1 ratio of respirators to masks in acute care facilities where aerosol-generating medical procedures are performed (6). The numbers of FFRs used during influenza virus outbreaks are daunting due to the protocols (i.e., “donning and doffing” for every room), while minimal direct evidence on the exclusion of influenza A virus during FFR use and survival after deposition remains elusive.
N95s provide 99.5% filtration efficiency for particles >0.75 μm and ≥95% for particles between 0.1 to 0.3 μm (7). Influenza A virus is approximately 120 nm in diameter (8). Thus, with a proper seal, N95s deliver protection from infectious particles ranging from large droplets (>100 μm) to inhalable droplets (10 to 100 μm) and to nuclear aerosols (<10 μm) (9, 10). However, the main transmission route of influenza virus infection continues to be a topic of debate (9–12). Some contend that airborne transmission via small-particle aerosols is a feasible pathway that has not been given the appropriate attention (10, 11), while others cite evidence for close contact and large droplets as the cause of influenza infection (9, 12). Fomite transmission, particularly within the hospital setting, is another area for which data are limited. Regardless of deposition and transmission routes, knowledge about the survival and persistence of influenza A virus on the exterior of the facepiece is needed because of the repeated donning and doffing of FFRs and subsequent hand hygiene considerations.
Influenza A virus is an enveloped virus, and its lipid bilayer is a main determinant of survival, as viruses with higher lipid contents persist better under lower-humidity conditions (13). Research regarding influenza virus survival on surfaces has mostly focused on stainless steel (14–16). For the study of survival and interactions on respirators, MS2 coliphage, a single-stranded RNA [ss(+)RNA] virus that infects Escherichia coli, has been used as the surrogate (17–19). Previous studies provided insight into mostly older strains of influenza A virus, such as A/Brazil/11/78-like (16) and A/PR/8/34 (20), various materials (pajamas, tissue, soft toys, surgical masks, and hospital gowns) (16, 21), and a single absolute humidity (AH) (16, 20, 21). We used a robust design to evaluate the persistence and infectivity, as defined by the ability to infect tissue culture, of the pH1N1 virus deposited on N95 FFR materials under different conditions of AH, tested in various sample matrices (component of the sample besides pH1N1, such as mucus), and measured at time periods up to 6 days.
MATERIALS AND METHODS
The study evaluated the survival and infectivity of the pH1N1 virus within three matrices: viral medium (Dulbecco's modified Eagle medium [DMEM]) (Gibco, Grand Island, NY), 2% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), and 5 mg/ml mucin (MP Biomedicals, Soloni, OH) on coupons of N95 respirators (model no. 8210; 3M, St. Paul, MN). We studied survival under AH conditions of 4.1 × 105 mPa (18°C and 20% relative humidity [RH]), 6.5 × 105 mPa (25°C and 20% RH), and 14.6 × 105 mPa (21°C and 58.5% RH) for 0, 4, 12, 24, 48, 72, and 144 h time points. The experiments were performed three times for all conditions, with the exception of the 144-h time point at the 4.1 × 105 mPa AH, which was performed twice. All sample sizes were 9, with the following exceptions: FBS, 4.1 × 105 mPa for 12 h (n = 6) and 144 h (n = 3); viral medium, 14.6 × 105 mPa for 24 h (n = 8) and 144 h (n = 6); mucin, 4.1 × 105 mPa for 12 to 72 h (n = 6); mucin, 6.5 × 105 mPa for 72 and 144 h (n = 6); and mucin, 14.6 × 105 mPa for 24 and 48 h (n = 8).
Experiment parameters. (i) Influenza pH1N1 virus and propagation.
Influenza virus A/California/04/2009 H1N1 (influenza virus A [H1N1] pdm; CDC identification no. 2009712047; lot no. 08/13/2009) was obtained from the Influenza Division, CDC, and propagated in Madin-Darby canine kidney (MDCK) cells as described by Szretter et al. (22). The method is briefly detailed here. Confluent MDCK cells were washed twice with room temperature phosphate-buffered saline (PBS) (Gibco, Grand Island, NY) and once with complete DMEM (cDMEM)–7.5% bovine serum albumin (BSA) (Fisher, Fair Lawn, NJ). The virus, thawed in cool water, was diluted to obtain a multiplicity of infection (ratio of influenza virus to MDCK cells) of 1:100 with viral growth medium (diethyl maleate [DEM], 7.5% BSA), 2% penicillin-streptomycin (stock concentration, 10,000 units/ml penicillin G sodium and 10,000 μg/ml streptomycin sulfate) (Life Technologies, Carlsbad, CA), HEPES buffer (Gibco, Grand Island, NY), and tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (ThermoScientific, Rockford, IL). One milliliter of the diluted virus suspension was added to the MDCK cell monolayer. The suspension was rotated to thoroughly cover the entire monolayer and was incubated at 37°C for 45 min. Viral growth medium (20 ml) was added to the monolayer, and the flask (75 cm2) was not harvested until cytopathic effects (CPE) were detected in 75% of the monolayer. The monolayer supernatant was centrifuged for 15 min at 300 × g, and the supernatant was then divided into cryovials and stored at −80°C until the experiment. In an effort to prepare sufficient pH1N1 for the entire experiment, several flasks were prepared to propagate the virus at the same time. Once all the flasks showed the proper percentages of CPE, the virus was isolated from all the flasks and combined into one large population, and a stock concentration was rendered, averaging a 4.3 × 105 tissue culture infectious dose of 50% (TCID50) per ml. Infectious pH1N1 was quantified as TCID50, which refers to the number of pH1N1 that produced CPE in 50% of the cells inoculated.
(ii) Test matrices.
Viral medium, 2% FBS, and mucin (5 mg/ml) were used as the test matrices. Viral medium (detailed above) was used as a control matrix, while 2% FBS and mucin were proxies for sputum and mucus-like material generated during sneezing and coughing. The stock matrices of 4% FBS and mucin (10 mg/ml) were prepared and stored at −20°C, which were later combined during the experiment with equal volumes of virus suspension to achieve the desired 2% FBS and 5-mg/ml mucin concentrations. Viral medium was stored at 4°C until the experiment and also was combined with equal volumes of the virus suspension for the experiments.
(iii) N95 respirator coupons.
The 3M model no. 8210 N95 was chosen for evaluation because that respirator was listed in the Strategic National Stockpile (CDC, Atlanta, GA), approved for infection control in health care settings, and readily available. Additional details regarding the respirator can be found in a report by Fisher et al. (23). Circular coupons (3.8 to 4.2 cm2) were punched from N95 respirators using a grommet and hammer, placed in six-well plates with the exterior of the mask facing upwards for the outer shell to be exposed (Costar, Corning, NY), and UV sterilized for ≥15 min prior to the experiment.
(iv) Absolute humidity.
AH was defined by Shaman and Kohn as the “actual water vapor content of air irrespective of temperature” (24). This parameter reflects the relationship between percent relative humidity (% RH) and temperature, both of which are documented to influence the survival of influenza virus (24). Absolute humidity (AH) was calculated from measured temperature (°C) and % RH conditions. The vapor pressure (VP) of water used in the measurement for AH was VPw = % RH × ([SVP/100]%), where VPw is the vapor pressure of water vapor, % RH is percent relative humidity, and SVP is the saturated vapor pressure (in mPa), defined as SVP = (6.11 × 105 mPa) × e(0.067×T), where T is the temperature in degrees Celsius. The three AH conditions as measured via vapor pressure (VP), 4.1 × 105 mPa (18°C and 20% RH), 6.5 × 105 mPa (25°C and 20% RH), and 14.6 × 105 mPa (21°C and 58.5% RH), were maintained within an environmental chamber (model no. 6030; Caron, Marietta, OH) that was monitored with a temperature- and % RH-traceable sensor (Control Company, Friendswood, TX). The temperature (°C) and % RH were checked at least twice a day during the experimental time periods to ensure that the correct predetermined AH was attained within the environmental chamber.
(v) Time points.
Previous research on the survival of influenza A virus when suspended in viral medium on porous surfaces showed compelling reductions in viable viruses within approximately 24 to 48 h (16, 20). We studied additional time points within this 24- to 48-h period (4, 12, and 24 h) and also extended testing to 72 h. In our initial experiments, 72 h was the final time point at which we measured survival, similar to the procedure followed by Bean et al. (16). However, testing at 144 h (6 days) was added after the first two experiments at 4.1 × 105 mPa VP AH to detect if complete die-off occurred. In summary, triplicate coupons were processed for each VP value, matrix, and time point (0, 4, 12, 24, 48, 72, and 144 h), with the exception of the 144-h time point for the first two experiments at 4.1 × 105 mPa.
Sample processing. (i) Cell culture.
MDCK cells (CCL-3; ATCC, Manassas, VA) were maintained in tissue culture flasks (Corning, Corning, NY) until passage 90, at which time new cells were started. A modified procedure, as described by Szretter et al., was followed (22). The flasks (150 cm2) were seeded with 4 × 104 to 2.0 × 105 cells per ml, and cultures were grown to approximately 90 to 95% confluence under a 5% CO2 atmosphere at 37°C for 24 to 72 h. The medium for cell growth consisted of DMEM, containing fetal bovine serum (10% for growth and 2% for maintenance) and 2% penicillin-streptomycin.
(ii) N95 respirator and pH1N1 processing.
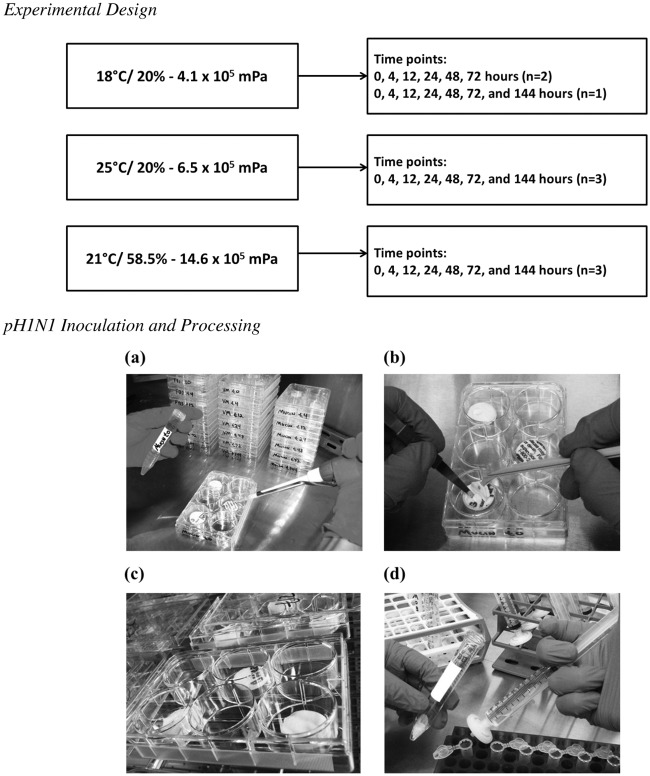
The UV-sterilized N95 respirator coupons and required sterilized supplies (forceps, cell spreaders, pipettes, pipette tips, etc.) were placed in a biosafety cabinet, in addition to the H1N1 stock inoculum and sample matrices (viral medium, 2% FBS, and 5 mg/ml mucin). The virus and sample matrices were prepared in equal parts and mixed. The virus-matrix suspension was inoculated (100 μl) onto individual respirator coupons in triplicate for each time point (see Fig. 1). The inoculated coupons dried in the biosafety cabinet for 1 h.
Fig 1.
Experimental design and photographs of the procedure for inoculating pH1N1 virus onto N95 respirators, where pH1N1 in the sample matrix was inoculated onto the exterior layer of three N95 coupons (a) and spread evenly for homogenous distribution and to aid in drying (b), inoculated coupons were stored in an environmental chamber at the defined parameters (c), and after vortexing and centrifugation, the sample was filtered through a syringe and stored at −80°C until ELISA processing (d).
Once the virus dried on the coupon, the inoculated coupon was placed inside a 15-ml conical tube (BD Falcon, Franklin Lakes, NJ) and 5 ml of 2% BSA–1× PBS (pH 8.5) was added. To separate pH1N1 from cell debris, the sample was vortexed for 20 min and centrifuged for 5 min at 3,000 × g to pellet the cell debris. To further purify the sample, the supernatant was removed and filtered through a premoistened (2% BSA–1× PBS) 0.22-μm syringe filter (Fisherbrand, Pittsburgh, PA; Millex-GS, Billerica, MA) into 1.5-ml Safe-Lock tubes (Eppendorf, Hauppauge, NY). The samples were labeled and stored at −80°C until processing.
(iii) ELISA.
MDCK cells at approximately 95% confluence were washed with 1× PBS, separated from the flask using trypsin-EDTA, concentrated by centrifugation at 500 × g for 10 min, and resuspended in viral medium (as described above). The sample (150 μl) underwent a 1:3 dilution (50 μl) in 96-well plates (Costar, Corning, NY) with 100 μl of viral culture medium (DMEM–1% BSA), for a total of 10 dilutions. MDCK cells (100 μl) were then pipetted into each well in the 96-well plates with the diluted samples. The plates were incubated under a 5% CO2 atmosphere at 37°C overnight. The range of detection for the experiments was 1.44 × 101 to 3.40 × 105 TCID50 per ml.
Using a BioTek ELx405 Select CW plate washer (BioTek Instruments, Winooski, VT), the plates were rinsed with 1× PBS. Manually, 80% acetone–1× PBS (cold) was added to every well and incubated for 8 min at room temperature. The acetone mixture was removed and the plates dried for 20 min. The BioTek plate washer was used for the remainder of the wash steps during immunostaining. Mouse anti-influenza A virus monoclonal antibody (Millipore, Temecula, CA) diluted 1:1,000 in 1× PBS–Tween 20–1% BSA was added (100 μl) to each well, incubated for an hour, and washed three times with 1× PBS–Tween 20 (200 μl). The secondary antibody, peroxidase-labeled affinity-purified goat anti-mouse IgG (KPL, Gaithersburg, MD), diluted 1:1,000 in 1× PBS–Tween 20–1% BSA, was then added to each well (100 μl), incubated at room temperature for 1 h, and washed three times with 1× PBS–Tween 20 (200 μl). A substrate development solution consisting of phosphate-citrate buffer with sodium (Sigma-Aldrich, St. Louis, MO), o-phenylenediamine dihydrochloride (ODP) tablets (10 mg) (Sigma-Aldrich, St. Louis, MO), and hydrogen peroxide (Sigma-Aldrich, St. Louis, MO) was added to each well and incubated at room temperature for 10 min, followed by the addition of 33 μl of sulfuric acid (1 N). The samples in the 96-well plates were read by a Synergy II plate reader (BioTek Instruments) with the Gen5 (v1.11 and 2.00) program set for reading a 96-well full plate at 490 nm absorbance. Data output was transferred to Microsoft Excel v14 (Redmond, WA) and the TCID50 for each sample was calculated using the method of Reed and Muench (25).
(iv) Data analysis.
Microsoft Excel v14 (Redmond, WA) was used for data formatting, log10 transformation, and averaging, while IBM SPSS v19 (Somers, NY) was used for descriptive statistics (median, mean, minimum, and maximum) and the box plot graphic. The virus concentration for each coupon was log10 transformed. The triplicate coupons for each time point were averaged, and the log10 change was calculated by subtracting the log10 virus per coupon from the sample's respective time zero log10 per coupon. The log10 change relative to the zero time point was used for statistical analysis.
SAS v9.2 (Cary, NC) was used to create general linear models that assessed the potential relationships between the mean log10 change of virus concentration and the three independent parameters under study: viral medium, absolute humidity, and die-off time. For these analyses, the data points were used individually and not averaged. Maximum likelihood estimates (MLEs) and standard errors were analyzed to determine the statistical differences within the levels of each parameter (i.e., sample matrix, absolute humidity, and time points). To account for the correlation of the mean log10 differences due to clustering of replicates over time, the method of generalized estimating equations (GEE) with a compound symmetrical correlation structure was implemented. GEE parameter estimates and robust empirical standard errors were obtained using a P value of 0.01 as the significance level for staying in the model.
RESULTS
The average starting inoculum was 4.3 × 105 pH1N1 TCID50 per ml (n = 27), or 4.3 × 104 pH1N1 TCID50 per coupon (100 μl), and the average recovery concentration was 1 × 103 pH1N1 TCID50 per ml, or 1 × 102 pH1N1 TCID50 per coupon, at time zero after 1 h of drying. The loss in recovery is attributed to desiccation and/or attachment to the N95 coupon. The infectivity of pH1N1 TCID50 per coupon is represented by its respective log10 concentration in Fig. 2. The overall trend shows a decrease in infectivity over the 6 days for each matrix and AH. For pH1N1 in viral medium, the recovered median log10 per coupon concentration started at 1.80, 2.40, and 1.20 for 4.1 × 105 mPa, 6.5 × 105 mPa, and 14.6 × 105 mPa, respectively, and decreased to 0.00, 0.94, and 0.16, respectively, at the 144-h endpoint. In FBS (2%), the recovered TCID50 log10 per coupon concentrations started at 1.36, 1.49, and 1.35 for 4.1 × 105 mPa, 6.5 × 105 mPa, and 14.6 × 105 mPa, respectively, and decreased to 0.00, 0.77, and 0.42, respectively, at the 144-h endpoint. In mucin (5 mg/ml), the recovered concentrations started at 1.04, 2.12, and 2.28 for 4.1 × 105 mPa, 6.5 × 105 mPa, and 14.6 × 105 mPa, respectively, and decreased to 0.16, 0.16, and 0.72, respectively, by the 144-h endpoint (except for the 4.1 × 105 mPa condition, where the last time point included was 72 h).
Fig 2.
The pH1N1 virus TCID50 log10 concentration per coupon over time (6 days) for different matrices and absolute humidity (AH) levels, where the horizontal line in the middle mark of each bar represents the median, the top and bottom of the bars represent the 25th and 75th percentiles, respectively, and the error bars represent the 95% confidence intervals. The first, second, and third bars within each time point are the results from the 4.1 × 105 mPa, 6.5 × 105 mPa, and 14.6 × 105 mPa AH conditions, respectively. Each box represents 3 coupons.
For each time point, the log10 change, compared to the zero time point, similarly illustrates a reduction in infectivity over time (Table 1). The range of the log10 change (lowest to highest) for 4.1 × 105 mPa was 0.01 to −1.33, −0.06 to −0.56, and −0.13 to −0.59 for viral medium, FBS, and mucin, respectively. The range of the log10 change for 6.5 × 105 mPa was −0.85 to −1.34, 0.00 to −1.40, and −0.70 to −1.72 for viral medium, FBS, and mucin, respectively. The range of the log10 change for 14.6 × 105 mPa was 0.07 to −0.97, −0.14 to −1.10, and −0.77 to −1.99 for viral medium, FBS, and mucin, respectively.
Table 1.
Mean TCID50 log10 change per coupon of the infectivity of pH1N1 virus on N95 coupons in relation to time zero for each matrix and absolute humidity (VP in mPa) over time
| Sample matrix | Time point (h) | Mean TCID50 log10 change (SD) at an absolute humidity ofa: |
||
|---|---|---|---|---|
| 4.1 × 105 mPa | 6.5 × 105 mPa | 14.6 × 105 mPa | ||
| Viral medium | 4 | −0.09 (1.1) | −1.11 (1.2) | 0.07 (1.3) |
| 12 | −0.25 (1.5) | −0.94 (1.0) | −0.56 (0.3) | |
| 24 | −0.68 (0.6) | −0.89 (1.5) | −0.43 (0.5) | |
| 48 | 0.01 (1.9) | −0.98 (0.8) | −0.38 (0.7) | |
| 72 | −0.70 (0.8) | −0.85 (1.0) | −0.52 (0.6) | |
| 144 | −1.33 (0.0) | −1.34 (0.8) | −0.97 (0.0) | |
| FBS (2%) | 4 | −0.06 (0.8) | 0.00 (2.5) | −0.21 (1.2) |
| 12 | −0.25 (0.5) | −0.94 (1.3) | −0.14 (1.4) | |
| 24 | −0.32 (0.1) | −0.88 (1.3) | −0.54 (0.6) | |
| 48 | −0.28 (0.8) | −1.32 (0.6) | −1.10 (0.0) | |
| 72 | −0.56 (0.2) | −1.36 (0.4) | −0.77 (0.6) | |
| 144 | −0.48 (0.0) | −1.40 (0.9) | −0.66 (0.9) | |
| Mucin (5 mg/ml) | 4 | −0.13 (0.6) | −0.85 (1.2) | −0.94 (1.6) |
| 12 | −0.17 (0.7) | −0.70 (1.4) | −0.95 (1.5) | |
| 24 | −0.58 (0.0) | −1.01 (1.1) | −1.77 (0.3) | |
| 48 | −0.54 (−0.7) | −0.70 (1.6) | −1.99 (0.1) | |
| 72 | −0.59 (0.3) | −0.92 (1.4) | −0.85 (1.8) | |
| 144 | No data | −1.72 (0.4) | −0.77 (1.9) | |
The values represent the range of the log10 change for each absolute humidity level. All sample sizes were 9 (see the exceptions listed in Materials and Methods).
The MLE univariate analysis revealed significant differences within each of the parameter groups in regard to the log10 change of pH1N1 influenza A virus (Table 2). The higher VP AHs, 14.6 × 105 mPa (P < 0.01) and 6.5 × 105 mPa (P < 0.0001), resulted in significantly greater pH1N1 log10 reductions relative to the reference VP AH of 4.1 × 105 mPa. This translates into an overall model estimate of a −0.69- and −0.75-log10 reduction for 14.6 × 105 mPa and 6.5 × 105 mPa, respectively, when AH is evaluated alone regarding pH1N1 survival on N95 (Table 2). pH1N1 had a significantly larger reduction in viral medium (P < 0.0001), the reference matrix, than in FBS or mucin. However, the log10 changes of pH1N1 in FBS (P = 0.08) and mucin (P = 0.07) were not significantly different from one another. Reductions in pH1N1 infectivity at the tested time points significantly increased from the reference point of 4 h, with the exception of the 12-h time point (P = 0.31), where the significance levels were as follows: 24 h, P < 0.01; 48 h, P < 0.01; 72 h, P < 0.01; and 144 h, P < 0.0001. This means that there was subsequently greater loss in survival over time, where the log10 reductions at 4 h, 48 h, and 144 h (6 days) estimated by the model were −0.35, −0.71, and −0.97, respectively (Table 2, where the intercept was added with MLE for the individual parameter).
Table 2.
Maximum likelihood estimates univariate analysis of the infectivity of pH1N1 virus on N95 couponsa
| Parameter | Cumulative MLE log10 change | Estimate (SE) | Confidence limits | P value (α) |
|---|---|---|---|---|
| Absolute humidity (vapor pressure in mPa) | ||||
| 4.1 × 105b | −0.40 | −0.40 (0.07) | −0.27 to −0.53 | <0.0001 |
| 6.5 × 105 | −0.75 | −0.35 (0.09) | −0.18 to −0.53 | <0.0001 |
| 14.6 × 105 | −0.69 | −0.29 (0.09) | −0.11 to −0.47 | <0.01 |
| Matrix | ||||
| Viral mediumb | −0.63 | −0.63 (0.06) | −0.51 to −0.75 | <0.0001 |
| Mucin (5 mg/ml) | −0.80 | −0.17 (0.09) | 0.01 to −0.34 | 0.07 |
| FBS (2%) | −0.48 | 0.15 (0.09) | 0.32 to −0.02 | 0.08 |
| Time point (h) | ||||
| 4b | −0.35 | −0.35 (0.08) | −0.19 to −0.51 | <0.0001 |
| 12 | −0.47 | −0.12 (0.12) | 0.11 to −0.36 | 0.31 |
| 24 (1 day) | −0.66 | −0.31 (0.12) | −0.07 to −0.54 | <0.01 |
| 48 (2 days) | −0.71 | −0.36 (0.12) | −0.13 to −0.60 | <0.01 |
| 72 (3 days) | −0.70 | −0.35 (0.12) | −0.12 to −0.58 | <0.01 |
| 144 (6 days) | −0.97 | −0.62 (0.13) | −0.36 to −0.88 | <0.0001 |
The significance limit is P < 0.01, as determined by a regression model.
This individual parameter was the referent group for their respective parameter groups.
The GEE multivariate analysis data, shown in Table 3, demonstrated the overall log10 change of pH1N1 for each parameter when the data were simultaneously modeled, taking into account the potential correlations between the triplicate experimental runs. The parameters that had a significant impact on the TCID50 log10 change of pH1N1 under the given conditions were the VP AHs of 14.6 × 105 mPa (P < 0.01) and 6.5 × 105 mPa (P < 0.01), as well as the time periods of 144 h (P < 0.01) and 24 to 72 h (P < 0.01). The GEE model illustrates that the log10 reduction of pH1N1 of −0.53 and −0.47 was attributed to the 6.5 × 105 mPa and 14.6 × 105 mPa VP AH values, respectively, while the log10 reduction of −0.74 and −0.52 might be credited to the 144-h and 24- to 72-h time periods (Table 3, where the intercept was added with GEE for the individual parameter). The matrices (viral medium, FBS, and mucin) did not have a significant impact on the survival of pH1N1.
Table 3.
Generalized estimated equation analysis of the infectivity of pH1N1 on N95 couponsa
| Sample matrix | Cumulative log10 change | Estimate (SE) | Confidence limits | P value (α) |
|---|---|---|---|---|
| Intercept | — | −0.22 (0.10) | −0.03 to −0.41 | 0.02 |
| 4.1 × 105 mPab | −0.22 | — | — | — |
| 6.5 × 105 mPa | −0.53 | −0.31 (0.09) | −0.13 to −0.49 | <0.01 |
| 14.6 × 105 mPa | −0.47 | −0.25 (0.08) | −0.08 to −0.41 | <0.01 |
| Viral mediumb | −0.22 | — | — | — |
| Mucin (5 mg/ml) | −0.38 | −0.17 (0.09) | −0.34 to 0.02 | 0.08 |
| 2% FBS | −0.05 | 0.17 (0.10) | 0.36 to −0.02 | 0.08 |
| 4 to 12 hb | −0.22 | — | — | — |
| 24 to 72 h | −0.52 | −0.30 (0.08) | −0.14 to −0.46 | <0.01 |
| 144 h | −0.74 | −0.53 (0.14) | −0.25 to −0.80 | <0.01 |
The parameter estimates can be used to calculate mean log10 change (cumulative generalized estimated equation [GEE]) by adding the estimate for an individual parameter with the intercept value. A further model estimation can be obtained by combining the intercept (−0.22) with the parameters in a given scenario; for example, at 14.6 × 105 mPa absolute humidity estimate (−0.25) in 5 mg/ml mucin estimate (−0.17) for 144 h (−0.53) results in a −1.17 mean log10 reduction of influenza A virus H1N1 on N95 coupons. The significance limit is P < 0.01.
This group is the referent group and is reflective of the intercept.
DISCUSSION
Overall, pH1N1 (A/California/04/2009) remained infectious for 6 days with an approximately 1-log10 loss when deposited onto coupons of N95 respirators under the given conditions. While AH impacted survival in our experiments, the GEE multivariate analysis of factors suggested that the main component affecting survival at 6 days was elapsed time, which contributed to an overall −0.74 TCID50 log10 reduction. Although the concentration of influenza virus that is potentially transferred from a respirator to hands and fingers is unknown, understanding that influenza virus might remain infectious for 6 days on the exterior side of a respirator (i.e., there might be a risk for transmission) is vital for health care personnel, patients, and visitors. Health care personnel who are in constant contact with confirmed or suspected cases of influenza should dispose of their respirators prior to leaving a patient's room.
There are three papers that have been published specifically on the persistence of the influenza virus on respirators or porous surfaces. Bean et al. researched the survival of an A/Brazil/11/78 (H1N1)-like virus on pajamas, tissues, magazines, and handkerchiefs (16). Greatorex et al. examined the survival of A/Cambridge/AHO4/2009 (H1N1) virus on various household materials, including the porous surfaces of a J Cloth, silver-containing cloth, and a soft toy (21). Sakaguchi et al. studied the survival of A/PR/8/34 (H1N1) virus on N95 respirators (Hi-Luck 350), surgical masks, and hospital gowns (20). Bean et al. and Sakaguchi et al. used cell cultures to measure infectivity with an approximate AH of 14.6 × 105 mPa (16, 20), while Greatorex et al. set AH conditions around 5 × 105 mPa (21). All three studies came to a similar conclusion that influenza A viruses show reductions in infectivity within 24 to 48 h. An additional study examined the persistence of various influenza A viruses on bank notes and found that influenza virus A/Moscow/10/99 (H3N2) remained infectious for up to 3 days and had increased survival in respiratory secretions (26).
The results from this study conflict with those of Bean et al. (16) and Sakaguchi et al. (20), which used A/Brazil/11/78 (H1N1)-like and A/PR/8/34 (H1N1) viruses, respectively. The two studies can be compared with our study in terms of influenza virus survival within viral medium at ∼14.6 × 105 mPa (27.8°C to 28.3°C and 35% to 40% RH [16] and 25.2°C and 55% RH [20]). A 3-log10 reduction in A/PR/8/34 infectivity was observed after 24 h on N95 respirators (20), while A/Brazil/11/78 (H1N1)-like was undetectable (∼3-log10 loss) within 8 to 12 h on porous surfaces (16). We studied the pH1N1 A/California/04/2009 virus and found only an overall 0.43-log10 reduction at 24 h and a 0.97-log10 reduction after 6 days at the 14.6 × 105 mPa VP AH (Table 1). A main component of persistence and infectivity is the interaction between the viral envelope and AH, as viruses with greater lipid content persist better under lower humidity conditions (13). Viral mutations and reassortments in the year-to-year strains provide the virus with new survival capabilities. While all three studies used cell culture infectivity methods and the three strains are all descendants of the 1918 pandemic virus, the pH1N1 virus is a reassortment of the North American swine (H3N2 and H1N2) and Eurasian H1N1 viruses (27, 28). This reassortment might be responsible for the greater persistence of pH1N1 virus than the A/Brazil/11/78 (H1N1)-like and A/PR/8/34 (H1N1) viruses under the given conditions. The unusual constellation of genes from multiple lineages (28) was a factor in its greater persistence due to the new unevaluated structural components.
As discussed previously, AH significantly impacts the survival of aerosolized influenza virus (24, 29). Besides the present study, only one other study evaluated the survival of H1N1 on respirators (20). As noted previously, Sakaguchi et al. found a marked loss of infectivity at only 24 to 48 h, and although this might largely reflect differences in the HIN1 strains, the only conditions they studied consisted of a relatively high AH of ∼14.6 × 105 mPa (25.2°C and 55% RH) (20). We evaluated three different AHs and found that log10 reductions were significantly higher under both the 6.5 × 105 mPa (P < 0.01) and 14.6 × 105 mPa (P < 0.0001) conditions than under the lowest condition of 4.1 × 105 mPa (Table 2). The multivariate GEE analysis, where all parameters are simultaneously evaluated, further confirms the important role of increased AH in overall decreased pH1N1 survival (Table 3). Humidity is controlled in health care facilities, and the Ventilation Standard for Health Care Facilities lists the recommended humidity levels for a variety of health care spaces (i.e., trauma room and wound intensive care), ranging from 30 to 60% for relative humidity and 20 to 24°C for temperature (30). This equates to an absolute humidity as measured by vapor pressure at approximately 7 × 105 mPa to 18.3 × 105 mPa, of which this study examined two conditions (4.1 × 105 mPa and 6.5 × 105 mPa) at the lower end and one condition (14.6 × 105 mPa) at the higher end of the scale. Hence, our study approximated AH levels typically present in many health care settings. Extra caution, however, should be taken in U.S. temperate regions during the wintertime to properly adjust the humidity settings to stay within this approved range.
Because influenza is dispersed via small or large respiratory droplets, mucus and saliva are the most likely matrices by which the virus is deposited on surfaces. Mucus and saliva mainly consist of water, with mucous glycoproteins, free proteins, and other electrolytes as the remaining constituents (37, 38). The substrates we used to suspend the virus were meant to simulate key constituents of phlegm or saliva and did not appear to have an important impact on the overall persistence of pH1N1, as determined by the GEE analysis. This suggests that similar precautions for preventing fomite transmission of influenza virus should be taken regardless of the presence or absence of visible respiratory secretions. The Strategic National Stockpile contained a variety of National Institute of Occupational Safety and Health (NIOSH)-approved particulate N95 respirators (3M, Moldex, Moldex-Metrix, Kimberly Clark, and Gerson) that were authorized for release for emergency use during the 2009 H1N1 pandemic (31). The CDC's Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnel recommended the use of a fit-tested N95 respirator (NIOSH-approved) for health care personnel who are within 6 feet of a patient or within a small enclosed airspace with a suspected or confirmed H1N1 patient (2). The 3M N95 respirator meets the 95% efficiency level and N series tests with NaCl, hence the name N95, and is comprised of three layers. The details of the layers are proprietary; however, the electrostatically charged polypropylene fibers play an important role in protecting the user from viruses. There are publications citing potential disinfection methods for contaminated respirators (32–35) that maintain their integrity (36), although decontamination of disposable FFRs for the purpose of reuse has not been recommended by the CDC.
The pH1N1 pandemic stressed the health care and public health infrastructures with challenges, such as how to effectively disseminate vaccines and respirators in a rapid manner to health care personnel and facilities. Although heroic efforts were put forth in addressing these challenges, it is fortunate that the 2009 H1N1 strain was a less-virulent strain than was initially anticipated. Particulate respirators (i.e., N95s) were incorporated into the CDC's Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnel as a cautionary approach during the pandemic (2). However, as the pandemic progressed and supplies were exhausted, questions about the persistence of influenza on porous media and respirators were posed due to a desire to increase respirator supply through extended wear and reuse. This research supports discarding respirators after close contact with a person having a suspected or confirmed influenza infection due to the virus's demonstrated ability to persist for 6 days on the outer side of the FFR with only an approximate 1-log10 loss in infectivity. While this study examined the impact of AH on pH1N1 on the exterior of FFRs, it is worth noting that a person's respiration and water vapor on the inner side of the respirator might also play a role in its persistence and infectivity. The starting concentration of influenza on respirators, the transmission rate from fomites to hands, and the human infectious dose remain unclear. Further research is needed to determine the risk of transmission from influenza-contaminated respirators.
ACKNOWLEDGMENTS
This study was made possible by the CDC Pandemic Influenza Scientific Agenda funding.
We thank our CDC colleagues Ryan A. Fitzgerald from the Division of Healthcare Quality and Promotion, Clinical and Environmental Microbiology Laboratory, for laboratory assistance and Jorn Winter from the Influenza Division for providing protocols and consultation. We are also appreciative of the guidance from Jeffery Shaman from the Department of Environmental Health Sciences and the Mailman School of Public Health, Columbia University, on the topic of absolute humidity.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 18 January 2013
REFERENCES
- 1. Shrestha SS, Swerdlow DL, Borse RH, Prabhu VS, Finelli L, Atkins CY, Owusu-Edusei K, Bell B, Mead PS, Biggerstaff M, Brammer L, Davidson H, Jernigan D, Jhung MA, Kamimoto LA, Merlin TL, Nowell M, Redd SC, Reed C, Schuchat A, Meltzer MI. 2011. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010). Clin. Infect. Dis. 52:S75–S82 [DOI] [PubMed] [Google Scholar]
- 2. CDC 2010. Interim guidance on infection control measures for 2009 H1N1 influenza in healthcare settings, including protection of healthcare personnel. Centers for Disease Control and Prevention. http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm [PubMed]
- 3. CDC 2010. Prevention strategies for seasonal influenza in healthcare settings: guidelines and recommendations. Centers for Disease Control and Prevention. http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm
- 4. OSHA 2009. Guidance on preparing workplaces for an influenza pandemic (OSHA 3327-05R 2009). Occupational Safety, Health Administration, U.S. Department of Labor, US Department of Human and Health Services, Washington, DC [Google Scholar]
- 5. The Institute of Medicine of the National Academies 2006. Reusability of facemasks during an influenza pandemic: facing the flu. Committee on the Development of Reusable Facemasks for Use During an Influenza Pandemic, The Institute of Medicine of the National Academies, The National Academies Press, Washington, DC [Google Scholar]
- 6. Murray M, Grant J, Bryce E, Chilton P, Forrester L. 2010. Facial protective equipment, personnel, and pandemics: impact of the pandemic (H1N1) 2009 virus on personnel and use of facial protective equipment. Infect. Control Hosp. Epidemiol. 31:1011–1016 [DOI] [PubMed] [Google Scholar]
- 7. Qian Y, Willeke K, Grinshpun SA, Donnelly J, Coffey CC. 1998. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am. Ind. Hyg. Assoc. J. 59:128–132 [DOI] [PubMed] [Google Scholar]
- 8. Richman DD, Whitley RJ, Hayden FG. (ed). 2009. Clinical virology, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 9. Hall CB. 2007. The spread of influenza and other respiratory viruses: complexities and conjectures. Clin. Infect. Dis. 45:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weber TP, Stilianakis NI. 2008. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J. Infect. 57:361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tellier R. 2006. Review of aerosol transmission of influenza A virus. Emerg. Infect. Dis. 12:1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. 2007. Transmission of influenza A in human beings. Lancet Infect. Dis. 7:257–265 [DOI] [PubMed] [Google Scholar]
- 13. Assar SK, Block SS. 2001. Survival of microorganisms in the environment, p 1221–1242 In Block SS. (ed), Disinfection, sterilization, and preservation, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 14. McDevitt JJ, Rudnick S, First M, Spengler J. 2010. Role of absolute humidity in the inactivation of influenza viruses on stainless steel surfaces at elevated temperatures. Appl. Environ. Microbiol. 76:3943–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noyce JO, Michels H, Keevil CW. 2007. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 73:2748–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH. 1982. Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 146:47–51 [DOI] [PubMed] [Google Scholar]
- 17. Fisher E, Shaffer R. 2010. Survival of bacteriophage MS2 on filtering facepiece respirator coupons. Appl. Biosaf. 15:71–76 [Google Scholar]
- 18. Rengasamy S, Fisher E, Shaffer R. 2009. Evaluation of the survivability of MS2 viral aerosols deposited on filtering face piece respirator samples incorporating antimicrobial technologies. Am. J. Infect. Control. 38:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher EM, Richardson AW, Harpest SD, Hofacre KC, Shaffer RE. 2012. Reaerosolization of MS2 bacteriophage from an N95 filtering facepiece respirator by simulated coughing. Ann. Occup. Hyg. 56:315–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakaguchi H, Wada K, Kajioka J, Watanabe M, Nakano R, Hirose T, Ohta H, Aizawa Y. 2010. Maintenance of influenza virus infectivity on the surfaces of personal protective equipment and clothing used in healthcare settings. Environ. Health Prev. Med. 15:344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greatorex JS, Digard P, Curran MD, Moynihan R, Wensley H, Wreghitt T, Varsani H, Garcia F, Enstone J, Nguyen-Van-Tam JS. 2011. Survival of influenza A(H1N1) on materials found in households: implications for infection control. PLoS One 6:e27932 doi:10.1371/journal.pone.0027932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Szretter KJ, Balish AL, Katz JM. 2006. Influenza: propagation, quantification, and storage, p 15G.11.11–15G.11.22 In Coico R. (ed), Current protocols in microbiology. John Wiley & Sons, Inc., Hoboken, NJ: [DOI] [PubMed] [Google Scholar]
- 23. Fisher E, Rengasamy S, Viscusi D, Vo E, Shaffer R. 2009. Development of a test system to apply virus-containing particles to filtering facepiece respirators for the evaluation of decontamination procedures. Appl. Environ. Microbiol. 75:1500–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaman J, Kohn M. 2009. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. U. S. A. 106:3243–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 26. Thomas Y, Vogel G, Wunderli W, Suter P, Witschi M, Koch D, Tapparel C, Kaiser L. 2008. Survival of influenza on banknotes. Appl. Environ. Microbiol. 74:3002–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taubenberger JK, Morens DM. 2010. Influenza: the once and future pandemic. Public Health Rep. 125:15–26 [PMC free article] [PubMed] [Google Scholar]
- 28. Trifonov V, Khiabanian H, Rabadan R. 2009. Geographic dependence, surveillance, and origins of the 2009 influenza A (H1N1) virus. N. Engl. J. Med. 361:115–119 [DOI] [PubMed] [Google Scholar]
- 29. Myatt TA, K MH, Allen JG, Macintosh DL, Fabian MP, McDevitt JJ. 2010. Modeling the airborne survival of influenza virus in a residential setting: the impacts of home humidification. Environ. Health 9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ANSI/ASHRAE/ASHE 2008. Standard 170-2008: ventilation of health care facilities. American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc., Atlanta, GA [Google Scholar]
- 31. Sharfstein JM. 2009. Letter of authorization: emergency use of disposable N95 respirators from strategic national stockpile. U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 32. Bergman MS, Viscusi DJ, Heimbuch BK, Wander JD, Sambol AR, Shaffer RE. 2010. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J. Eng. Fiber. Fabr. 5:33–41 [Google Scholar]
- 33. Viscusi DJ, Bergman MS, Eimer BC, Shaffer R. 2009. Evaluation of five decontamination methods for filtering facepiece respirators. Ann. Occup. Hyg. 53:815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heimbuch BK, Wallace WH, Kinney K, Lumley AE, Chang-Yu W, Woo MH, Wander JD. 2011. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control 39:e1–e9 [DOI] [PubMed] [Google Scholar]
- 35. Lore MB, Heimbuch BK, Brown TL, Wander JD, Hinrichs SH. 2012. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann. Occup. Hyg. 56:92–101 [DOI] [PubMed] [Google Scholar]
- 36. Saltar WB, Kinney K, Wallace WH, Lumley AE, Heimbuch BK, Wander JD. 2010. Analysis of residual chemicals on filtering facepiece respirators after decontamination. J. Occup. Environ. Hyg. 7:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fabian TK, Fejerdy P, Csermely P. 2008. Saliva in health and disease (chemical biology of), p 1–9 In Wiley encyclopedia of chemical biology. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 38. Creeth JM. 1978. Constituents of mucus and the separation. Br. Med. Bull. 34:17–24 [DOI] [PubMed] [Google Scholar]