Abstract
Basidiomycetes that cause brown rot of wood are essential biomass recyclers in coniferous forest ecosystems and a major cause of failure in wooden structures. Recent work indicates that distinct lineages of brown rot fungi have arisen independently from ligninolytic white rot ancestors via loss of lignocellulolytic enzymes. Brown rot thus proceeds without significant lignin removal, apparently beginning instead with oxidative attack on wood polymers by Fenton reagent produced when fungal hydroquinones or catechols reduce Fe3+ in colonized wood. Since there is little evidence that white rot fungi produce these metabolites, one question is the extent to which independent lineages of brown rot fungi may have evolved different Fe3+ reductants. Recently, the catechol variegatic acid was proposed to drive Fenton chemistry in Serpula lacrymans, a brown rot member of the Boletales (D. C. Eastwood et al., Science 333:762-765, 2011). We found no variegatic acid in wood undergoing decay by S. lacrymans. We found also that variegatic acid failed to reduce in vitro the Fe3+ oxalate chelates that predominate in brown-rotting wood and that it did not drive Fenton chemistry in vitro under physiological conditions. Instead, the decaying wood contained physiologically significant levels of 2,5-dimethoxyhydroquinone, a reductant with a demonstrated biodegradative role when wood is attacked by certain brown rot fungi in two other divergent lineages, the Gloeophyllales and Polyporales. Our results suggest that the pathway for 2,5-dimethoxyhydroquinone biosynthesis may have been present in ancestral white rot basidiomycetes but do not rule out the possibility that it appeared multiple times via convergent evolution.
INTRODUCTION
Most wood decay basidiomycetes are white rot fungi that use oxidative enzymes to degrade lignin (1), which otherwise protects the underlying cellulose and hemicelluloses these organisms require for growth (2). However, another mode of wood decay, termed brown rot, is a central route for biomass recycling in coniferous forest ecosystems and has arisen multiple times in the basidiomycete lineage via loss of ligninolytic enzymes and other enzymes (1, 3). Brown rot fungi in divergent lineages are thought to disrupt lignocellulose with nonenzymatic oxidants during incipient wood decay, removing little lignin in the process, and then apparently employ polysaccharidases later to consume most of the cellulose and hemicelluloses (4–6).
The characteristics of brown rot oxidants have been the subject of much research, because it remains unclear how so minimal a system can increase wood porosity enough to permit enzymatic access to the polysaccharides. However, it is generally agreed that biodegradative hydroxyl radicals produced via extracellular Fenton chemistry (H2O2 + Fe2+ + H+ → H2O + Fe3+ + ·OH) are important oxidants in incipient brown rot (5–8). The requisite Fe2+ and H2O2 are produced in part by secreted fungal hydroquinones or catechols, and by more-reactive semiquinone radicals derived from them, which together drive one-electron reductions of Fe3+ and O2 in the wood (9–12). In some cases, this chemistry is apparently also promoted by fungal laccases that oxidize hydroquinones or catechols to generate semiquinones (13).
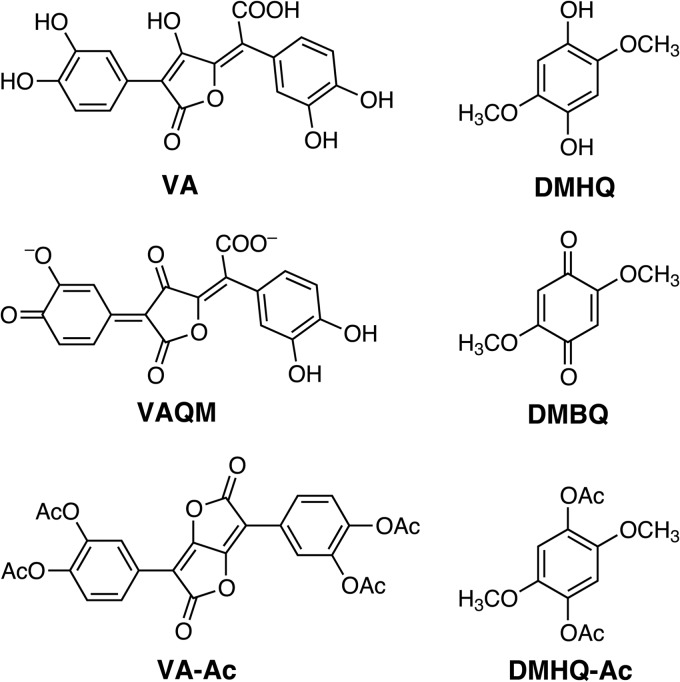
Recently, a genome sequence was reported for Serpula lacrymans, a brown rot fungus in the Boletales that causes a so-called dry rot and is one of the most destructive decayers of wooden structures in the Northern Hemisphere. Eastwood et al. (3) propose that S. lacrymans uses the catechol variegatic acid (VA) (Fig. 1) to drive Fenton chemistry. Their hypothesis is based on the observations that VA occurred in S. lacrymans mycelium grown on nutrient agar and that it reduced Fe3+ triacetate in vitro. The expected product from VA oxidation is a quinone methide (VAQM) that exhibits an intense blue color (Fig. 1). VA occurs in many members of the Boletales, and its oxidation to VAQM is responsible for the bluing reaction that results when their fruiting bodies are bruised (14–16).
Fig 1.
Chemical structures of metabolites discussed in the text. Potential reactions of VA or DMHQ with Fe3+ or laccase and of their semiquinones with Fe3+ or O2 are omitted for brevity. For details, see Fig. 1 in reference 13. Ac, acetyl.
However, a different hypothesis for the source of extracellular Fenton chemistry in S. lacrymans was presented earlier. Shimokawa et al. (17) found 2,5-dimethoxy-1,4-benzoquinone (DMBQ) (Fig. 1) in the extracellular medium of cultures grown on defined liquid medium and also observed that harvested fungal mycelium reduced DMBQ to 2,5-dimethoxyhydroquinone (DMHQ) (Fig. 1). They propose that DMHQ is the extracellular reductant of Fe3+ in S. lacrymans, basing their hypothesis on previous evidence that DMHQ drives Fenton chemistry by reducing Fe3+ and O2 in members of another brown rot lineage, the Gloeophyllales, when they are grown in liquid media (9–12). More-recent work conducted with biodegrading wood supports a physiological role for DMHQ in brown rot of natural substrates within the Gloeophyllales (18), and also the Polyporales (13).
Both of the above hypotheses assume that S. lacrymans maintains a steady-state pool of VA or DMHQ, which continuously reacts with Fe3+ and O2 in the colonized wood, either nonenzymatically or with the assistance of a laccase. However, neither mechanism has been demonstrated for this fungus in a natural lignocellulosic substrate.
If one of the proposed metabolites has a physiological role, the following three results should be obtainable using wood undergoing decay by S. lacrymans. First, the reactive, reduced form of the metabolite (VA or DMHQ) should be detected with rapid sampling and analysis. Second, when the aqueous phase containing the metabolite, Fe3+ chelates, and fungal enzymes is separated from the wood and attached mycelium, after a short time the metabolite should appear in its oxidized quinonoid form instead (VAQM or DMBQ). Third, when the hydroquinone or catechol is combined in vitro with Fe3+ chelates that mimic physiological conditions or with a laccase, it should become oxidized to the quinone with production of reactive oxygen species as a consequence. Here we have used these criteria to obtain evidence for or against the two hypotheses.
MATERIALS AND METHODS
Reagents and organism.
Variegatic acid was synthesized from 3,4-dimethoxyphenylacetic acid in eight steps using a Suzuki cross-coupling method and purified by chromatography on silica gel as described previously (19). The 1H nuclear magnetic resonance (NMR), 13C NMR, and mass spectra of the final product were identical to those reported previously (19). 2,5-Dimethoxyhydroquinone was prepared by reducing 2,5-dimethoxy-1,4-benzoquinone with sodium dithionite and recrystallized from chloroform as described earlier (10). All other chemicals were reagent grade.
Serpula lacrymans dikaryotic strain Hamburg S7 (ATCC MYA-656) was maintained on malt agar. For wood decay experiments, the fungus was first grown on malt agar in 15-cm-diameter petri plates. Once a confluent lawn of mycelium was obtained, sterile thin nylon mesh was placed atop the lawn, and autoclaved wafers of aspen wood (cut transversely; approximately 2 by 10 by 20 mm; weight [dry weight], 97 ± 10 mg) were placed in a single layer on the mesh. The cultures were incubated at 22°C, and the plates were harvested at intervals for analysis of the colonized wood.
Metabolite and iron analyses.
To identify and quantify fungal hydroquinones, catechols, and quinones in the aqueous phase of colonized wood, 6 to 12 aspen wafers were stacked, placed in a folded polypropylene sheet, and crushed in a large vise. Liquid pressed from the wood was then taken up in a syringe and injected as rapidly as possible, without filtration, onto a high-performance liquid chromatography (HPLC) column for analysis. The HPLC system consisted of a Phenomenex Luna C18(2) reversed-phase column (150 by 4.6 mm; 5-μm particle size; Torrance, CA), eluted at ambient temperature with water-acetonitrile-formic acid (900:100:1) for 10 min, followed by a 20-min linear gradient to acetonitrile-formic acid (1,000:1). Chromatographic peaks were monitored spectrophotometrically using a photodiode array detector. Metabolites were quantified against external standards.
To trap, identify, and quantify fungal hydroquinones or catechols as stable acetylated derivatives, individual colonized aspen wafers were rapidly immersed in excess acetic anhydride-pyridine (1:1) and stored for 1 week. For each wafer, the remaining acetic anhydride was then quenched with excess methanol, the liquid fraction was collected, the wood was extracted with additional methanol, and the liquid fractions were combined. The by-products (acetic acid, methanol, methyl acetate, and pyridine) were removed by vacuum evaporation followed by azeotropic distillation in the presence of toluene under reduced pressure. The resulting acetylated extracts, containing at least 0.5 mg of material, were redissolved in 1.0 ml of deuterochloroform (CDCl3).
These solutions were analyzed by 1H NMR spectroscopy using a 500-MHz Bruker Biospin Avance 500 instrument (Billerica, MA) equipped with a cryogenically cooled 5-mm gradient probe with inverse geometry. Spectra were processed using Bruker Topspin 3.1 software. The CDCl3 was then evaporated from the samples, which were redissolved in acetonitrile. Portions were then diluted 10-fold in water, filtered, and analyzed by HPLC as described above for nonacetylated samples. External standards were used for metabolite quantification.
Metabolite levels for the 8- and 14-day acetylation experiments are reported per gram (dry weight) of wood, the average weights (dry weights) having been determined on six other colonized wafers harvested at the same times. Nominal concentrations of the metabolites are also reported, using average water contents obtained by weighing three other harvested wafers at 8 and 14 days, drying them to constant weight at 60°C in a vacuum oven, and reweighing them to take the differences. For the concentrations given, the variance of each quotient was calculated as the square root of the sum of the two squared variances for metabolite levels and water contents.
To analyze soluble oxalate in colonized wood, individual day 8 and day 14 wafers were weighed and stirred overnight in 1.0 ml of distilled, deionized water, after which the extracts were analyzed by ion exclusion HPLC as described previously (20). The extracted wafers were then dried and reweighed to determine their original water contents by difference, after which the oxalate concentrations were corrected for dilution.
To analyze total soluble iron in colonized wood, two sets of four wafers were harvested on day 8 and weighed, after which each set was extracted overnight with 3.0 ml of distilled, deionized water. Dissolved iron concentrations in the extracts were then determined by reduction with ascorbate followed by spectrophotometric measurement of Fe2+ as the complex with ferrozine at 562 nm (ε = 27.9 mM−1 cm−1) as described earlier (21), using an Agilent 8453 diode array UV/visible spectrophotometer (Santa Clara, CA). The wafers were then dried and reweighed to obtain their original water contents, after which the iron concentrations were corrected for dilution.
Assays of metabolite oxidation and reactive oxygen production.
Laccase-catalyzed oxidation mixtures contained 100 μM VA or DMHQ and 100 units of chromatographically purified Trametes villosa laccase (Novozymes, Davis, CA) in 1.0 ml of 20 mM sodium tartrate, pH 4.1, at 22°C in an unstoppered cuvette with a 10-mm path length. One unit of laccase oxidizes 1 μmol of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) per min under these conditions. Repeated spectra were obtained using the diode array spectrophotometer, and once the results showed that the oxidation of VA to VAQM or of DMHQ to DMBQ was complete, the reaction mixtures were assayed for H2O2 by adding 1 unit of chromatographically purified Phanerochaete chrysosporium lignin peroxidase isozyme H2 and 400 μM final concentration of vacuum distilled veratryl alcohol (22). The UV absorbances of the reactions were then monitored at 310 nm for the formation of veratraldehyde (ε = 9.3 mM−1 cm−1). One unit of lignin peroxidase oxidizes 1 μmol of veratryl alcohol per min under these conditions, and one molecule of veratraldehyde is produced per H2O2 consumed (22).
Reactions to monitor the oxidation of DMHQ by Fe3+ contained 50 to 200 μM concentrations of the hydroquinone and 50 to 200 μM FeCl3 in 720 μl of 9 mM sodium oxalate, pH 4.1, at 22°C in an unstoppered 2-mm-path-length cuvette. DMHQ was added as a stock solution in dioxane (final concentration of 2.5% [vol/vol]), which served not only as a vehicle for reductant addition but also as a radical trap to minimize destruction of DMHQ and DMBQ by Fenton reagent formed during the reactions. Repeated spectra were obtained using the UV/visible spectrophotometer and were deconvoluted into pure component spectra, which were then fit to the kinetic model described previously to obtain rate constants for the reaction of DMHQ with Fe3+ under these reaction conditions (18). Attempted reactions to monitor the oxidation of VA by Fe3+ were performed under the same conditions, except that a 10-mm-path-length cuvette was used.
Assays for Fenton chemistry contained 9 mM sodium oxalate (pH 4.1), 10 mM sodium [α-14C]benzoate (0.023 mCi−1 mmol−1; pH 4.1; Sigma/Aldrich, St. Louis, MO), 0.3 mM FeCl3, and 3 mM DMHQ or VA as the reductant in a final volume of 2.0 ml, using a reaction vial large enough that O2 in the headspace would not be limiting. DMHQ or VA was added last as a weighed solid, after which the reaction vial was promptly stoppered and stirred in the dark at 22°C. At three 1-h intervals, the headspace of each vial was flushed through an alkaline cocktail, and trapped 14CO2 was assayed by scintillation counting (23). As a control, a reaction without reductant was included. A control without FeCl3 was not included, as previous work has already shown that iron is required for DMHQ-driven Fenton oxidations of organic target molecules (10).
RESULTS AND DISCUSSION
Biodegradative competence.
We grew Serpula lacrymans on autoclaved wafers of aspen wood placed on spacers over malt agar and harvested specimens at intervals to determine weight (dry weight) losses. The losses and standard deviations (n = 6) were as follows: 0.5% ± 0.5% on day 8, 4.0% ± 2.7% on day 14, 7.4% ± 4.0% on day 21, and 26.2% ± 9.2% on day 2. The high extent of weight loss between 14 and 28 days establishes that our cultures degraded the wood. The low rate of weight loss during the first few weeks is typical of brown rot fungi, including S. lacrymans (24, 25), and indicates an incipient phase during which wood polysaccharides are oxidatively cleaved before substantial material is removed from the wood.
Dissolved fungal metabolites in the wood.
To look for metabolites with a potential biodegradative role during incipient decay, we began by crushing stacks of colonized day 8 aspen wafers in a vise, after which samples of the liquid pressed out were analyzed by HPLC. We performed these analyses as rapidly as possible because, in past work with other brown rot fungi, we found that DMHQ oxidized rapidly in such squeezates of colonized wood (13, 18). However, we found that aspen colonized by S. lacrymans was too dry to yield much liquid, which usually resulted in delays of nearly a minute before enough sample could be collected for injection onto the HPLC column.
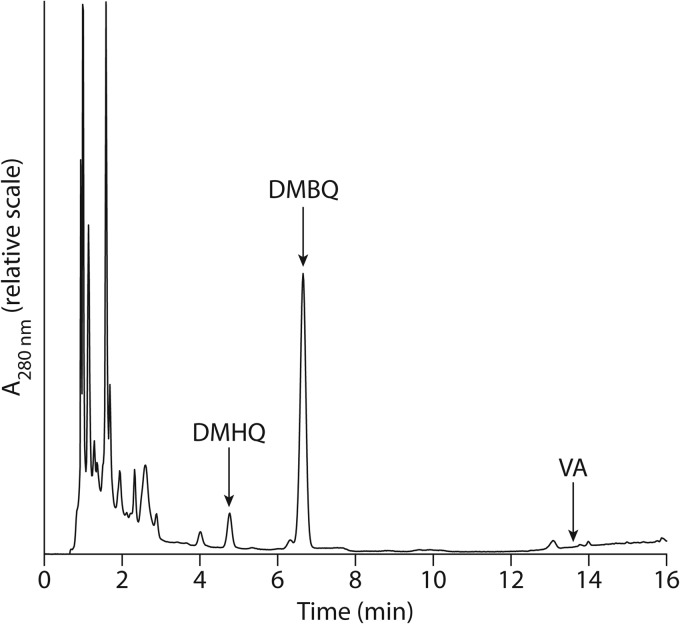
The HPLC results showed that VA was not detected in these squeezate samples (Fig. 2). The presence or absence of oxidized VA (VAQM) could not be ascertained, because injection of an authentic standard prepared from VA using laccase (16) showed that this blue quinone methide did not elute from the HPLC column. It is likely that VAQM was in fact absent, because the extracts exhibited no detectable blue color. However, these results do not rule out the possibility that it had been present in the colonized wood at some time prior to our analysis, because quinone methides are chemically so reactive that VAQM might not persist long enough to be detected.
Fig 2.
HPLC analysis of the aqueous phase obtained from 12 pooled, crushed aspen wafers 8 days after inoculation with S. lacrymans. The y axis shows absorbance at 280 nm. Elution positions of the standards are indicated by black arrows.
DMHQ was also absent in most analyses of liquid from crushed day 8 wood, but DMBQ, the product expected from DMHQ oxidation, was invariably present, as shown not only by the presence of a correctly eluting HPLC peak but also by the visible absorption spectrum of the peak (data not shown). In just one of these experiments, we were able to inject a sample rapidly after crushing 12 pooled aspen wafers and found HPLC peaks eluting at the correct position for both the hydroquinone and the quinone (Fig. 2). These results suggested that DMHQ was in fact present in the wood but was rapidly oxidized to DMBQ once extracted. The apparent average concentrations of the two metabolites in the squeezates from day 8 colonized wood were 92 μM for DMHQ and 100 μM for DMBQ, but the identity of the presumed hydroquinone peak remained uncertain.
Trapping of reduced fungal metabolites in the wood.
It was evident from the foregoing results that the relative importance of VA and DMHQ as redox-active agents in the colonized wood could be assessed only if the samples were rapidly derivatized in situ to produce less reactive products. We harvested three colonized wood wafers at intervals, acetylated them separately with excess acetic anhydride/pyridine, and worked up the resulting liquid extracts. We then looked for the acetylated derivatives of VA and DMHQ by 1H NMR spectroscopy and HPLC.
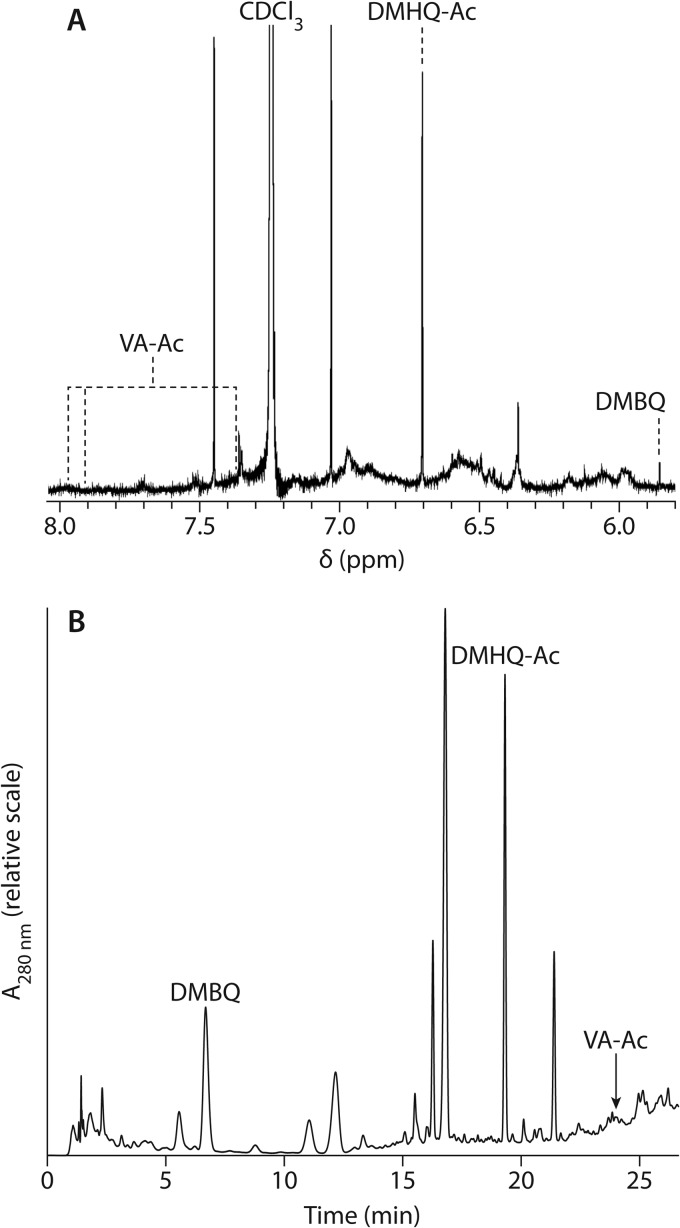
1H NMR spectra in CDCl3 of unfractionated extracts after acetylation showed that none of the colonized wafers contained detectable levels of acetylated variegatic acid (3,3′,4,4′-tetraacetoxypulvinic lactone [VA-Ac]) (Fig. 1), as shown by the absence of signals at 7.91 and 7.97 ppm (Fig. 3A), which were present in the NMR spectrum of our authentic VA-Ac standard and have also been previously reported (14). By contrast, a prominent signal corresponding to the two identical ring hydrogens in acetylated DMHQ (1,4-diacetoxy-2,5-dimethoxybenzene [DMHQ-Ac]) (Fig. 1) was present at 6.70 ppm in all samples at 8 and 14 days. As expected, given the HPLC results obtained with nonacetylated extracts (Fig. 2), a signal at 5.86 ppm corresponding to the two identical ring hydrogens in DMBQ was also present in all day 8 and day 14 samples.
Fig 3.
(A) 1H NMR spectrum of an acetylated day 8 extract of aspen wood undergoing decay by S. lacrymans. The vertical dashed lines indicate chemical shifts (δ) for hydrogens in standards of the indicated compounds. (B) HPLC analysis of an acetylated day 8 extract of decaying wood. Elution positions of standards are indicated.
Analysis of the acetylated extracts by HPLC (Fig. 3B) confirmed that VA-Ac was not detected in any sample but that all derivatized aspen wafers contained DMHQ-Ac and DMBQ at 8 and 14 days. HPLC/mass spectrometric analysis with negative ionization likewise confirmed the result (data and methods not shown; M+ + 1 = 255 for DMHQ-Ac; M+ + 1 = 169 for DMBQ). The levels of the acetylated hydroquinone greatly exceeded the levels of the quinone (Table 1), as also indicated by the 1H NMR spectra (Fig. 3A).
Table 1.
Concentrations of metabolites and iron in aspen wood undergoing decay by S. lacrymans
| Time (days) | 2,5-DMHQ-Ac concna |
2,5-DMBQ concna |
Oxalate concnb (mM) (soluble) | Iron concnc (mM) (soluble) | ||
|---|---|---|---|---|---|---|
| μg g−1 (dry wt) of wood | mM (nominal) | μg g−1 (dry wt) of wood | mM (nominal) | |||
| 8 | 2.60 ± 1.43 | 4.06 ± 2.37 | 0.46 ± 0.29 | 0.71 ± 0.47 | 8.7 ± 3.5 | 0.46, 0.47 |
| 14 | 0.52 ± 0.20 | 0.60 ± 0.26 | 0.11 ± 0.04 | 0.12 ± 0.05 | 12.5 ± 6.0 | NDd |
Values are from acetylated wood specimens. Average values ± standard deviations (SD) for three replicate wafers are shown. On day 21, 2,5-DMHQ-Ac and 2,5-DMBQ were detected in two out of three replicate wafers analyzed. On day 28, both metabolites were detected in one out of three replicates analyzed (data not shown).
Average values ± SD for three replicate wafers are shown. An average pH value of 4.1 was determined for the extracts after correction for dilution.
Eight colonized wafers were pooled into two groups of four to obtain duplicate values.
ND, not determined.
Average nominal concentrations of the two metabolites (Table 1), based on our determinations of the average water content in the wood, were much higher than those we found in the water obtained from wood squeezates. We infer that most of the DMHQ and DMBQ was adsorbed on the wood during fungal decay and desorbed only when the wafers were extracted with pyridine/acetic anhydride—since both metabolites are sufficiently hydrophobic to be retained on a reversed-phase HPLC column in 10% acetonitrile (Fig. 2), they probably also adhere to lignin in water.
Routes for oxidation of VA and DMHQ.
Given our result that DMHQ but not VA could be trapped in wood undergoing brown rot by S. lacrymans, we could envisage only one situation in which VA could nevertheless be present and have a biodegradative role: it would have to oxidize much faster than DMHQ in the colonized wood, and then the resulting VAQM would have to undergo rapid subsequent reactions so that its characteristic blue color would disappear. There are two possibilities for the initial oxidative step in this sequence: it might be catalyzed by an oxidative enzyme, or it might proceed via direct reaction of VA with Fe3+ as proposed by Eastwood et al. (3). We consider these two routes separately below.
Feasibility of an enzyme-catalyzed route.
If the colonized wood were to contain a fungal laccase or peroxidase, VA would be expected to undergo an initial one-electron oxidation to produce VA semiquinone radicals. These semiquinones might then promote Fenton chemistry by reducing O2 to produce superoxide as described earlier for some other hydroquinones (13, 26). The S. lacrymans genome likely encodes laccases and peroxidases (3), and a laccase is generally thought responsible for the oxidation of VA to VAQM that occurs when fruiting bodies of VA-producing boletes are bruised (15, 16). However, we consider rapid VA removal by this route unlikely for two reasons.
First, we were unable to detect any laccase or peroxidase activity in day 8 aqueous extracts of S. lacrymans-colonized wood using ABTS as the substrate. This finding contrasts with the result we obtained earlier with a brown rot fungus in the Polyporales, Postia placenta, which produced easily detected ABTS-oxidizing laccase activity in wood, and apparently uses this enzyme to generate DMHQ semiquinone radicals that promote Fenton chemistry (13).
Second, we found that the laccase-catalyzed oxidation of VA produced no detectable H2O2, an expected product of superoxide dismutation if the VA semiquinone were reactive enough to reduce O2 (27). By contrast, we found that the laccase-catalyzed oxidation of DMHQ under the same conditions generated 0.3 equivalent of H2O2 per DMHQ supplied, which indicates the production of 0.6 equivalent of superoxide, in good agreement with earlier work (13). These results indicate that, even if S. lacrymans were to produce a laccase to oxidize VA in biodegrading wood, the resulting VA semiquinone radical would fail to promote Fenton chemistry via superoxide production.
Feasibility of a direct reaction with Fe3+.
If VA were rapidly consumed in the wood via direct one-electron oxidations by Fe3+, a complete Fenton system could ensue. A key requirement for this chemistry is that VA would need to have a sufficiently negative reduction potential to react with the Fe3+ chelates that predominate under physiological conditions. The principal Fe3+ chelator in wood undergoing brown rot is oxalate, which we found to have a concentration around 9 mM (pH 4.1) in our day 8 S. lacrymans cultures (Table 1). At this concentration and pH, almost all of the Fe3+ in colonized wood will be present as the Fe3+ trioxalate and dioxalate complexes, which have standard reduction potentials of −120 mV and +181 mV, respectively (28, 29). These values are far more negative than the ca. +400 mV reduction potential reported for Fe3+ triacetate at pH 4.5 (30), which is the relatively reducible chelate used by Eastwood et al. for their in vitro reaction of VA with Fe3+ (3).
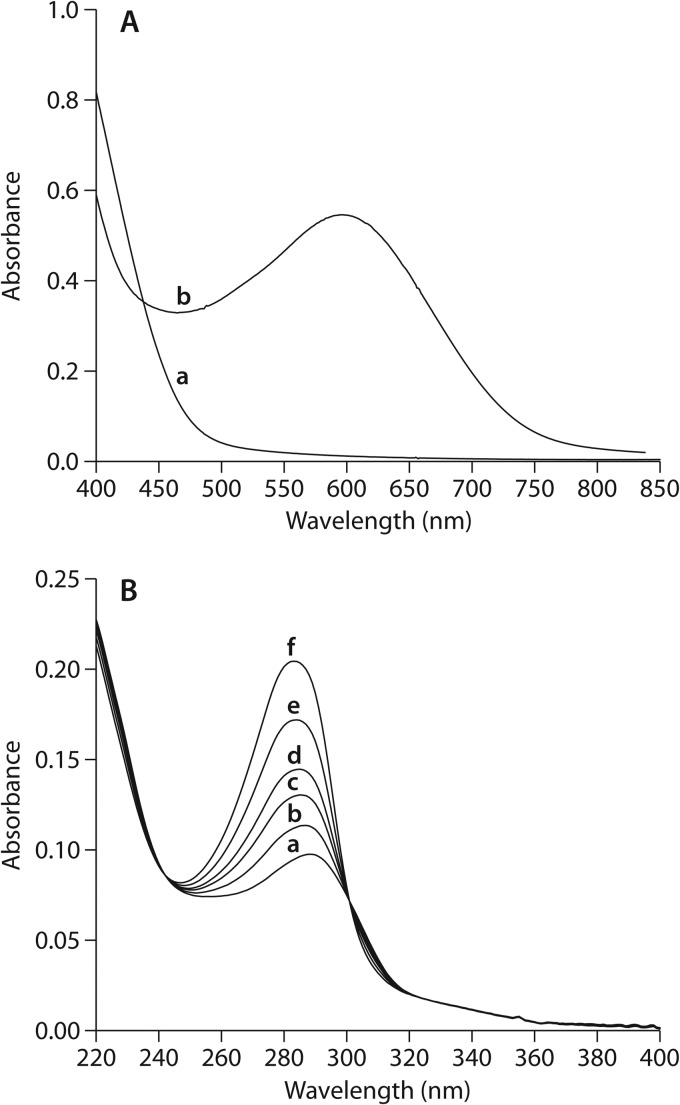
Since the one-electron reduction potentials for VA, DMHQ, and their semiquinones are apparently unknown, we proceeded empirically by comparing the abilities of VA and DMHQ to react with Fe3+ in 9 mM sodium oxalate, pH 4.1. VA was not detectably oxidized to VAQM under these conditions even after 24 h (Fig. 4A), from which we draw two conclusions. First, VA was not present at a significant concentration in our wood cultures, because its very low rate of reaction with Fe3+ in physiological oxalate and also the lack of laccase or peroxidase activity in the wood indicate that it would have been successfully trapped with acetic anhydride/pyridine if it had been present. Second, the direct reaction of VA with Fe3+ in physiological oxalate is too slow to drive Fenton chemistry in wood colonized by S. lacrymans.
Fig 4.
(A) Curve a shows an absorption spectrum of 100 μM VA after 24 h in the presence of 1.0 mM FeCl3 in physiological oxalate. For comparison, curve b shows the absorption spectrum of VAQM produced by the reaction of 100 μM VA with laccase as described in Materials and Methods. (B) Absorption spectra obtained from the reaction of 50 μM DMHQ with 50 μM FeCl3 in physiological oxalate. Spectra are shown for the following reaction times (in minutes): 0.2 (curve a), 7.0 (curve b), 20.0 (curve c), 49.0 (curve d), 110.0 (curve e), and 225.0 (curve f).
By contrast, DMHQ was oxidized to DMBQ by Fe3+ in physiological oxalate (Fig. 4B). By obtaining spectrophotometric time courses of the DMHQ-dependent reaction at various initial concentrations of the two reactants and then deconvoluting the resulting spectra as described earlier (18), we determined that DMHQ and Fe3+ in 9 mM oxalate (pH 4.1, 22°C) react with apparent first-order kinetics and a rate constant of 2.5 M−1 s−1 (95% confidence interval, 1.9 to 4.1 M−1 s−1). Although this rate constant is only about 6% of the one we reported earlier for the same reaction at the relatively low oxalate concentration (ca. 1 mM) typical of wood undergoing early decay by the brown rot fungus Gloeophyllum trabeum (18), we found that our S. lacrymans-colonized wood samples contained about 40 times the soluble Fe3+ concentration at day 8 that we reported earlier for G. trabeum-colonized wood at a similar stage of decay (Table 1) and that our wood samples also contained a higher concentration of soluble DMHQ (ca. 90 μM versus 20 μM).
Consequently, the steady-state rate of DMHQ-dependent Fe3+ reduction in S. lacrymans-colonized wood likely has the same order of magnitude that it does in G. trabeum-colonized wood, where a role for DMHQ-driven Fenton chemistry is already well established (18). The rates for both fungi may actually be higher, since it is now apparent that most of the DMHQ remains adsorbed to the wood and is thus underestimated in squeezate experiments. However, it is prudent not to base rate calculations on high nominal DMHQ concentrations observed in acetylated samples, because it is unclear how much of the adsorbed hydroquinone may be available to react with Fe3+.
Measurements of Fenton chemistry.
As a further test of whether DMHQ or VA has a likely biodegradative role in S. lacrymans, we compared their abilities to drive Fenton chemistry in vitro via Fe3+ reduction in physiological oxalate. Since ·OH is a highly nonselective oxidant, much of it is lost in side reactions during such assays, but relative measurements comparing potential Fenton reductants can nevertheless be obtained by performing reactions with an excess of a radical trap that yields a diagnostic product after oxidation. We used α-14C-labeled benzoic acid as the trap, because decarboxylation is one route for benzoic acid oxidation by ·OH (31), and the resulting 14CO2 is easily trapped in an alkaline scintillation cocktail and quantified (23).
In a reaction mixture containing DMHQ as the reductant, FeCl3, physiological oxalate, and [α-14C]benzoate as described in Materials and Methods, 14CO2 was evolved at an initial linear rate of 1.61% h−1 (R2 = 0.95). Exogenous H2O2 was not required, i.e., DMHQ alone was able to generate a complete Fenton system. By contrast, Fenton chemistry did not ensue when VA was supplied instead as the reductant (0.02% h−1; R2 = 1.00) or when no reductant was provided (0.02% h−1; R2 = 0.97). These results are consistent with our Fe3+ reduction data (Fig. 4) and point to a role for DMHQ rather than VA in oxidative attack on lignocellulose during incipient wood decay by S. lacrymans. The efficacy of DMHQ and its semiquinone in reducing Fe3+ and O2 is likely attributable to the presence of electron-donating methoxyl groups on the aromatic ring (27). VA lacks such substituents.
Conclusion.
Evidence has now accumulated to support the involvement of DMHQ in wood biodegradation by brown rot fungi that belong to three divergent lineages: the Gloeophyllales (18), the Polyporales (13), and now the Boletales. Although the pathway for DMHQ biosynthesis has not been elucidated, it must entail multiple enzymatic steps. The existence of complex biosynthetic sequences yielding a single biodegradative metabolite in distantly related brown rot fungi is noteworthy, considering that the extensive scientific literature on fungal natural products makes no mention of DMHQ in white rot fungi. However, there is an old report of DMBQ, the oxidized form of the metabolite, in cultures of Polyporus fumosus (32), which is now known as the white rot fungus Bjerkandera fumosa. If the isolate used was correctly identified, this observation could indicate that the pathway for DMHQ/DMBQ production does occur in some white rot fungi, which would be consistent with its presence in the white rot ancestors from which brown rot fungi independently evolved. Alternatively, DMHQ biosynthesis may have appeared independently in remarkable instances of convergent evolution, each time that a brown rot lineage appeared via the loss of lignocellulolytic white rot genes.
ACKNOWLEDGMENTS
We thank Yuki Tobimatsu for advice on variegatic acid synthesis, Ali Azarpira for assistance with mass spectrometric analysis, John Ralph for advice on NMR analysis, and Karen Nakasone for advice on fungal phylogeny.
This work was supported by grant DE-SC0006929 (K.E.H and C.J.H.) from the U.S. Department of Energy, Office of Biological and Environmental Research.
Footnotes
Published ahead of print 1 February 2013
REFERENCES
- 1.Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Gorecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TKA, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Duenas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719 [DOI] [PubMed] [Google Scholar]
- 2.Boerjan W, Ralph J, Baucher M. 2003. Lignin biosynthesis. Annu. Rev. Plant Biol. 54:519–546 [DOI] [PubMed] [Google Scholar]
- 3.Eastwood DC, Floudas D, Binder M, Majcherczyk A, Schneider P, Aerts A, Asiegbu FO, Baker SE, Barry K, Bendiksby M, Blumentritt M, Coutinho PM, Cullen D, de Vries RP, Gathman A, Goodell B, Henrissat B, Ihrmark K, Kauserud H, Kohler A, LaButti K, Lapidus A, Lavin JL, Lee YH, Lindquist E, Lilly W, Lucas S, Morin E, Murat C, Oguiza JA, Park J, Pisabarro AG, Riley R, Rosling A, Salamov A, Schmidt O, Schmutz J, Skrede I, Stenlid J, Wiebenga A, Xie XF, Kües U, Hibbett DS, Hoffmeister D, Hogberg N, Martin F, Grigoriev IV, Watkinson SC. 2011. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 333:762–765 [DOI] [PubMed] [Google Scholar]
- 4.Cowling EB. 1961. Comparative biochemistry of the decay of sweetgum sapwood by white-rot and brown-rot fungi. USDA Tech. Bull. 1258. US Government Printing Office, Washington, DC [Google Scholar]
- 5.Eriksson KE, Blanchette RA, Ander P. 1990. Microbial and enzymatic degradation of wood and wood components. Springer, Berlin, Germany [Google Scholar]
- 6.Hatakka A, Hammel KE. 2010. Fungal biodegradation of lignocelluloses, p 319–340 In Hofrichter M. (ed), Mycota, 2nd ed, vol 10 Springer, Berlin, Germany [Google Scholar]
- 7.Arantes V, Jellison J, Goodell B. 2012. Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 94:323–338 [DOI] [PubMed] [Google Scholar]
- 8.Goodell B, Jellison J, Liu J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. 1997. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J. Biotechnol. 53:133–162 [Google Scholar]
- 9.Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. 2001. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl. Environ. Microbiol. 67:2705–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerem Z, Jensen KA, Hammel KE. 1999. Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven Fenton reaction. FEBS Lett. 446:49–54 [DOI] [PubMed] [Google Scholar]
- 11.Newcombe D, Paszczynski A, Gajewska W, Kroger M, Feis G, Crawford R. 2002. Production of small molecular weight catalysts and the mechanism of trinitrotoluene degradation by several Gloeophyllum species. Enzyme Microb. Technol. 30:506–517 [Google Scholar]
- 12.Paszczynski A, Crawford R, Funk D, Goodell B. 1999. De novo synthesis of 4,5-dimethoxycatechol and 2,5-dimethoxyhydroquinone by the brown rot fungus Gloeophyllum trabeum. Appl. Environ. Microbiol. 65:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei DS, Houtman CJ, Kapich AN, Hunt CG, Cullen D, Hammel KE. 2010. Laccase and its role in production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycete Postia placenta. Appl. Environ. Microbiol. 76:2091–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besl H, Bresinsky A, Kopanski L, Steglich W. 1978. Pilzpigmente. XXXV. 3-O-Methylvariegatsäure und verwandte Pulvinsäurederivate aus Kulturen von Hygrophoropsis aurantiaca (Boletales). Z. Naturforsch. C 33:820–825 [Google Scholar]
- 15.Gill M, Steglich W. 1987. Pigments of fungi (macromycetes). Prog. Chem. Org. Nat. Prod. 51:1–317 [DOI] [PubMed] [Google Scholar]
- 16.Steglich W, Furtner W, Prox A. 1968. Neue Pulvinsäure-Derivate aus Xerocomus chrysenteron (Bull. ex St. Amans) Quél. und Untersuchungen zur Frage des Vorkommens von Anthrachinonpigmenten bei Boletaceen. Z. Naturforsch. B 23:1044–1050 [PubMed] [Google Scholar]
- 17.Shimokawa T, Nakamura M, Hayashi N, Ishihara M. 2004. Production of 2,5-dimethoxyhydroquinone by the brown-rot fungus Serpula lacrymans to drive extracellular Fenton reaction. Holzforschung 58:305–310 [Google Scholar]
- 18.Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE. 2006. Fungal hydroquinones contribute to brown rot of wood. Environ. Microbiol. 8:2214–2223 [DOI] [PubMed] [Google Scholar]
- 19.Ahmed Z, Langer P. 2005. Synthesis of natural pulvinic acids based on a ‘[3+2] cyclization-Suzuki cross-coupling’ strategy. Tetrahedron 61:2055–2063 [Google Scholar]
- 20.Hunt C, Kenealy W, Horn E, Houtman C. 2004. A biopulping mechanism: creation of acid groups on fiber. Holzforschung 58:434–439 [Google Scholar]
- 21.Dominik P, Kaupenjohann M. 2000. Simple spectrophotometric determination of Fe in oxalate and HCl soil extracts. Talanta 51:701–707 [DOI] [PubMed] [Google Scholar]
- 22.Tien M, Kirk TK. 1988. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 161:238–249 [Google Scholar]
- 23.Kirk TK, Connors WJ, Bleam RD, Hackett WF, Zeikus JG. 1975. Preparation and microbial decomposition of 14C-lignins. Proc. Natl. Acad. Sci. U. S. A. 72:2515–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curling SF, Clausen CA, Winandy JE. 2002. Relationships between mechanical properties, weight loss, and chemical composition of wood during incipient brown-rot decay. Forest Prod. J. 52:34–39 [Google Scholar]
- 25.Ortiz R, Jamet A, Herrera P, Vindigni G, Pereira A. 2011. Influencia del deterioro incipiente producido por el hongo de pudrición parda Serpula lacrymans, sobre las propiedades mecánicas de compresión normal y paralela a la fibra en madera de Pinus radiata D. Don. Inf. Constr. 63:69–74 [Google Scholar]
- 26.Guillén F, Muñoz C, Gómez-Toribio V, Martínez AT, Martínez MJ. 2000. Oxygen activation during oxidation of methoxyhydroquinones by laccase from Pleurotus eryngii. Appl. Environ. Microbiol. 66:170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien PJ. 1991. Molecular mechanisms of quinone cytotoxicity. Chem.-Biol. Interact. 80:1–41 [DOI] [PubMed] [Google Scholar]
- 28.Hyde SM, Wood PM. 1997. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe(III) reduction by cellobiose dehydrogenase and Fe(II) oxidation at a distance from the hyphae. Microbiology 143:259–266 [DOI] [PubMed] [Google Scholar]
- 29.Varela E, Tien M. 2003. Effect of pH and oxalate on hydroquinone-derived hydroxyl radical formation during brown rot wood degradation. Appl. Environ. Microbiol. 69:6025–6031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaelis L, Friedheim E. 1931. Potentiometric studies on complex iron systems. J. Biol. Chem. 91:343–353 [Google Scholar]
- 31.Sagone AL, Decker MA, Wells RM, Democko C. 1980. A new method for the detection of hydroxyl radical production by phagocytic cells. Biochim. Biophys. Acta 628:90–97 [DOI] [PubMed] [Google Scholar]
- 32.Bu'Lock JD. 1955. Constituents of the higher fungi. Part IV. A quinone from Polyporus fumosus. J. Chem. Soc. 1955:575–576 [Google Scholar]