Abstract
In Listeria monocytogenes serotype 4b isolates from sporadic listeriosis, heavy metal resistance was primarily encountered in certain clonal groups (ECI, ECII, and ECIa). All arsenic-resistant isolates harbored the arsenic resistance cassette previously identified in pLI100; ECIa harbored additional arsenic resistance genes and a novel cadmium resistance determinant in a conserved chromosomal locus.
TEXT
Listeria monocytogenes is the etiological agent of listeriosis, which occurs rarely but exhibits a relatively high fatality rate (approximately 16%) (1, 2). Listeriosis outcomes include sepsis, meningitis, stillbirths, and abortions. At high risk are neonates, the elderly, pregnant women, and immunocompromised individuals (1). Strains of certain serotypes (1/2a, 1/2b, and 4b) are responsible for the majority (over 95%) of human clinical cases (3, 4). Serotype 4b strains have been implicated in numerous outbreaks and in a significant portion of sporadic cases (4, 5). Three serotype 4b clonal groups (ECI, ECII, and ECIa [also designated ECIV]) have been responsible for multiple outbreaks (6). Members of these clonal groups have also been frequently isolated from food processing environments and foods (7–13).
While conducting a longitudinal study of 136 serotype 4b isolates obtained from sporadic human listeriosis in the United States between 2003 and 2008 (to be presented separately), we identified 45 isolates with resistance to cadmium and/or arsenic. Resistance of L. monocytogenes to these heavy metals has long been recognized and has also even been utilized as a subtyping tool (14, 15), but limited information is available on the genetic determinants mediating such resistance in strains from human listeriosis. The few available reports have investigated the distribution of cadmium resistance determinants among isolates of environmental or food origin (11, 16); determinants associated with arsenic resistance have remained elusive.
The panel of 45 heavy metal-resistant clinical isolates investigated here consisted primarily of the three previously recognized clonal groups: ECI (n = 26), ECII (n = 7), and ECIa (n = 8) (Table 1). DNA hybridizations and PCR were employed as previously described (17) with the DNA probes and primers listed in Table 2.
Table 1.
L. monocytogenes strains used in this study
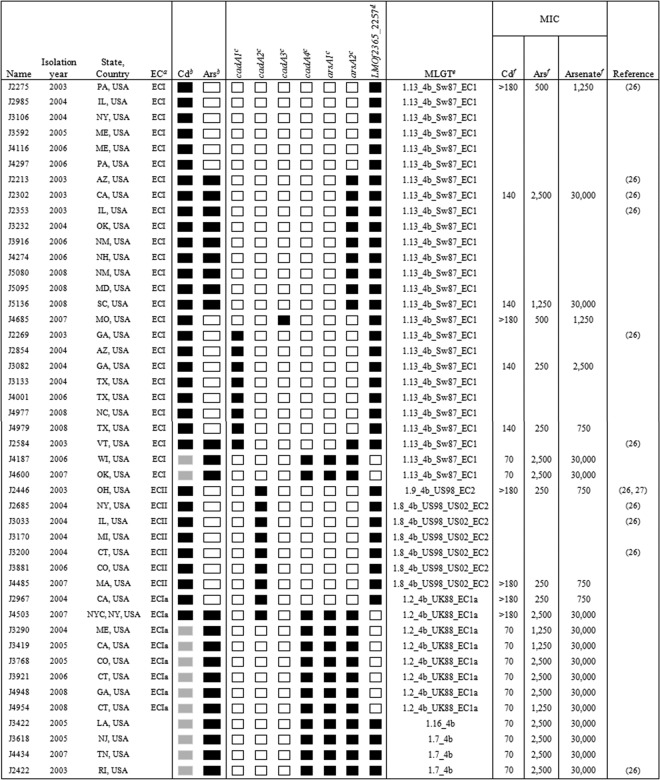
The EC designation was determined with the DNA-DNA hybridization results for previously described genetic markers (30) and confirmed by multilocus genotyping (MLGT) (28).
For cadmium (Cd), black and gray boxes indicate MICs of ≥140 μg/ml (growth at 70 μg/ml) and 70 μg/ml (growth at 35 μg/ml but not at 70 μg/ml), respectively. Tolerance levels were determined based on growth in the presence of the indicated amount of cadmium chloride on Iso-Sensitest agar, as described previously (23). For arsenic (Ars), black and white boxes indicate resistance and susceptibility, respectively, based on the presence or absence of growth in the presence of sodium (meta)arsenite (500 μg/ml) on Iso-Sensitest agar, as described previously (23).
Black and white squares represent the presence and absence, respectively, of signal in the hybridization experiment using the probe targeting the designated resistance gene or of the amplicon derived from the primers targeting the relevant DNA probe.
Black and white squares signify the presence and absence, respectively, of the amplicon when PCR was conducted with primers P4F and P4R (Fig. 1 and Table 2). The extension time was set at 1 min.
MLGT was conducted as described previously (28).
MICs for cadmium (Cd), arsenic (Ars, arsenite), and arsenate (another chemical form of arsenic, which is reduced to arsenite and then extruded by arsenic resistance cassettes [25]) were determined as previously described (23), using Iso-Sensitest agar plates containing different heavy metal concentrations: 10, 35, 70, 140, and 180 μg/ml of cadmium chloride (Fischer Scientific, Pittsburg, PA); 50, 125, 250, 500, 750, 1,250, 2,500, and 5,000 μg/ml of sodium (meta)arsenite (Sigma, St. Louis, MO); and 50, 125, 250, 500, 750, 1,250, 2,500, 5,000, 10,000, 12,000, 15,000, and 30,000 μg/ml of sodium arsenate dibasic heptahydrate (Sigma).
Table 2.
Probes and primers employed in this study
| Probe | Primer (alternative name) | Sequence (5′ to 3′) | Target | GenBank accession no. | Reference |
|---|---|---|---|---|---|
| cadA1 | cadA1-Tn5422F | CAGAGCATTTACTGACCATCAATCGTT | Tn5422-associated cadA (cadA1) | L28104 | 16 |
| cadA1-Tn5422R | TCTTCTTCATTTAACGTTCCAGCAAAAA | Tn5422-associated cadA (cadA1) | L28104 | 16 | |
| cadA2 | cadA2-pLM80F | ACAAGTTAGATCAAAAGAGTCTTTTATT | cadA (LMOh7858_pLM80_0083) on pLM80 (cadA2) | AADR01000058 | 16 |
| cadA2-pLM80R | ATCTTCTTCATTTAGTGTTCCTGCAAAT | cadA (LMOh7858_pLM80_0083) on pLM80 (cadA2) | AADR01000058 | 16 | |
| cadA3 | cadA3-EGDeF | TGGTAATTTCTTTAAGTCATCTCCCATT | cadA (lmo1100) in EGD-e (cadA3) | AL591977 | 16 |
| cadA3-EGDeR | GCGATGATTGATAATGTCGATTACAAAT | cadA (lmo1100) in EGD-e (cadA3) | AL591977 | 16 | |
| LMOSA_2330F (P1F) | GCATACGTACGAACCAGAAG | cadA (LMOSA_2330) on the chromosome of Scott A (cadA4) | AFGI01000005.1 | This study | |
| LMOSA_2330R (P1R) | CAGTGTTTCTGCTTTTGCTCC | cadA (LMOSA_2330) on the chromosome of Scott A (cadA4) | AFGI01000005.1 | This study | |
| LMOSA_2220F (P2F) | CAACTTTGACCCTGTGGAG | arsA (LMOSA_2220) on the chromosome of Scott A (arsA1) | AFGI01000005.1 | This study | |
| LMOSA_2220R (P2R) | CTTTCCATTCAATCACTGCG | arsA (LMOSA_2220) on the chromosome of Scott A (arsA1) | AFGI01000005.1 | This study | |
| pLI37_F (P3F) | CAACCAGATCAGTTACCATTAAC | arsA on pLI100 (pli0037) and the chromosome of Scott A (LMOSA_2260) (arsA2) | NC_003383 | This study | |
| pLI37_R (P3R) | TGCTTCTCCAGAGATTTCTTCTG | arsA on pLI100 (pli0037) and the chromosome of Scott A (LMOSA_2260) (arsA2) | NC_003383 | This study | |
| F2365_2257F (P4F) | ACATTGCGAGAACACCTTGG | LMOf2365_2257 | NC_002973.6 | This study | |
| F2365_2257R (P4R) | GATTTATCGGCGCAATGACG | LMOf2365_2257 | NC_002973.6 | This study |
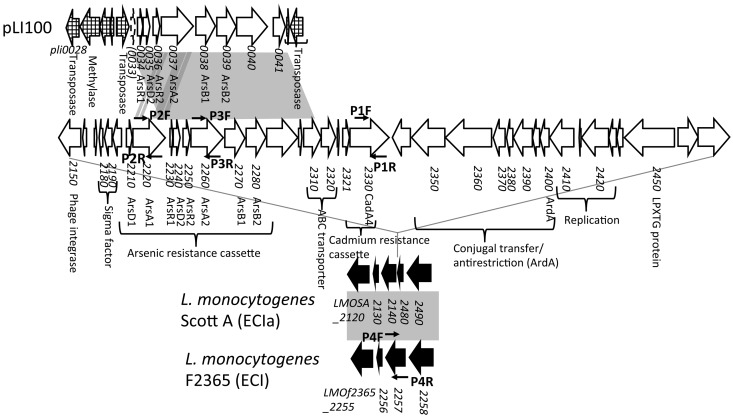
We assessed the presence of three cadmium resistance determinants previously employed in the analysis of environmental and food isolates: cadA1, associated with the plasmid-borne transposon Tn5422 (14, 18); cadA2, harbored by large plasmids such as pLM80 (19, 20); and cadA3, associated with an integrative conjugative element on the chromosome of L. monocytogenes EGDe (21). We also included a novel putative cadmium resistance determinant, cadA4, recently identified on the chromosome of the ECIa strain L. monocytogenes Scott A (22). A putative arsenic resistance cassette (arsR1D2R2A2B1B2 in Fig. 1) was identified on pLI100, harbored by L. innocua CLIP 11262 (19, 21). An extended arsenic resistance cassette, which includes arsR1D2R2A2B1B2 and two additional upstream genes (arsD1A1), was recently identified on the Scott A chromosome, where it is part of a 35-kb genomic island (with a GC content of 34%—lower than the L. monocytogenes average of 38%) (20, 22). This island harbors several additional genes, including the putative cadmium resistance determinant cadA4 mentioned previously (Fig. 1) (22).
Fig 1.
Genomic organization of the ca. 35-kb island harboring arsenic and cadmium resistance cassettes in ECIa strain Scott A. The DNA sequence of Scott A genome contig 5 (accession no. AFGI01000005.1) was retrieved from the NCBI database, annotated with the xBASE bacterial genome annotation service (http://www.xbase.ac.uk/annotation/) using F2365 as the reference genome (29–34), and analyzed with DNA and protein BLAST (35) and NCBI's Conserved Domain Search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (36). This annotation was later updated based on that available in NCBI (accession no. CM001159.1). The Scott A genomic region was compared with pLI100 and F2365 using the online Artemis Comparison Tool (WebACT; http://www.webact.org/WebACT/home) (37). Genes and pseudogenes are shown as arrows and stippled arrows, respectively. The conserved flanking genes in L. monocytogenes are shown in black, and other genes in the 35-kb insertion are in white. Flanking open reading frames (ORFs) on pLI100 are in a grid pattern. Homologous regions are represented in gray. Arrows above and below selected ORFs indicate the locations and orientations of primers (Table 2).
Association of cadmium resistance determinants with clonal groups and lower cadmium tolerance levels in strains harboring the novel determinant cadA4.
The determinants cadA1, cadA2, and cadA4 were frequently detected among the 45 cadmium-resistant clinical isolates, while only one strain (J4685) was found to harbor cadA3 (Table 1). Multiple determinants were also only detected in one strain (J4503), which harbored both cadA2 and cadA4. Several isolates (n = 15) lacked any of the four cadmium resistance determinants (Table 1), suggesting the presence of one or more as-yet-unidentified cadmium resistance determinants.
Associations were noted between resistance determinants and clonal groups: cadA1 was only encountered in ECI, accounting for 8 (ca. 33%) of the cadmium-resistant ECI isolates, while none of the ECI isolates harbored cadA2; cadA2 and cadA4 were primarily encountered in ECII and ECIa, respectively (P < 0.0001 for both when analyzed with Fisher's exact test using SAS [SAS Institute, Inc., Cary, NC]) (Fig. 2). All 15 isolates negative for cadA1 to cadA4, and presumably harboring as-yet-unidentified determinants, were ECI, constituting >50% of this clonal group (Fig. 2 and Table 1).
Fig 2.
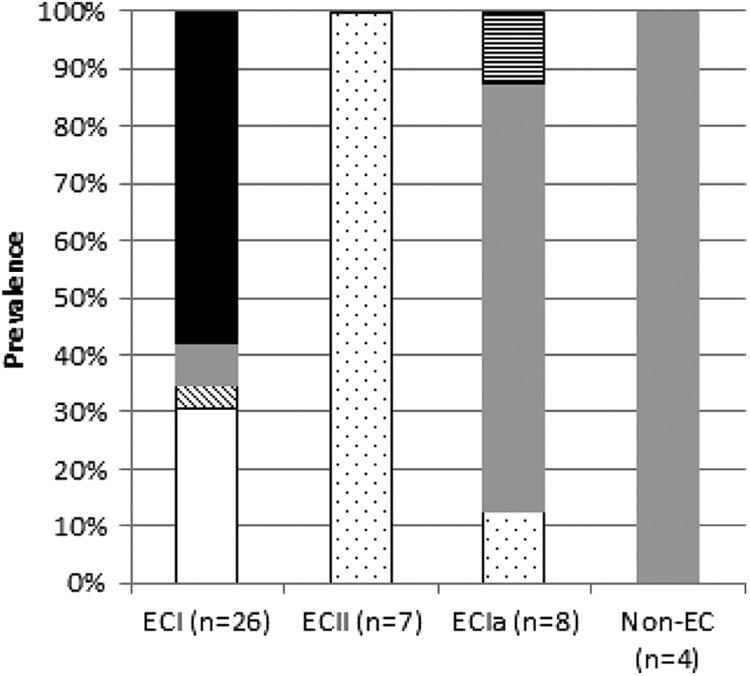
Prevalence of the different cadmium determinants within epidemic clones. Different cadmium resistance determinants are indicated as follows: white, cadA1; dots, cadA2; diagonal lines, cadA3; gray, cadA4; horizontal lines, copresence of cadA2 and cadA4; and black, unidentified determinants.
The absence of cadA2 among the clinical ECI isolates was in contrast to findings for serotype 4b isolates from foods and food processing plants, where ECI isolates were found to be equally likely to harbor cadA1 or cadA2 (11). The reasons for this discrepancy are unknown but may reflect differential pathogenicity: ECI strains harboring cadA1 or those with an unknown cadmium resistance determinant(s) may constitute subsets of ECI more likely to be involved in human illness than those with cadA2. Further studies of ECI strains from different sources are needed to identify possible relationships between determinant types and source.
The presence of cadA4 was found to be associated with a lower level of resistance to cadmium regardless of the clonal group. With the exception of strain J4503, which harbored both cadA2 and cadA4 and had a cadmium MIC of >180 μg/ml, the cadmium MIC for all other cadA4-harboring isolates was 70 μg/ml regardless of their clonal group (Table 1). Even though these isolates grew poorly or not at all at the previous resistance threshold of 70 μg/ml (11, 15, 23), they grew well at 35 μg/ml. In contrast, other cadmium-susceptible isolates of L. monocytogenes typically have a cadmium MIC of 10 μg/ml (24). All other cadmium-resistant isolates had MICs of ≥140 μg/ml, regardless of their clonal group or determinant type (Table 1). The reasons for the association of cadA4 with relatively reduced cadmium resistance remain to be determined; however, we suspect that this finding could be related to the fact that the protein encoded by cadA4 is more divergent from those encoded by cadA1 to cadA3, although highly conserved regions were still observed (data not shown). To illustrate, in the pairwise comparisons of CadA1 to CadA3, identities ranged from 68 to 74%, whereas the identities between CadA4 and other CadA proteins were 35 to 36%.
All arsenic-resistant strains harbor pLI100-associated arsA2, while a subset additionally harbor arsA1 in a conserved chromosomal locus.
As previously mentioned, a putative arsenic resistance cassette along with cadA4 and several other genes was part of a 35-kb island in the ECIa strain Scott A (22) (Fig. 1). In Scott A, this genomic island appears to be inserted in a gene (homolog of LMOf2365_2257 in F2365) that is uninterrupted in F2365 and other strains with sequenced genomes (Fig. 1).
Two arsA genes, arsA1 and arsA2, were selected as genetic markers representing arsenic resistance genes either unique to Scott A (arsA1) or shared between Scott A and pLI100 (arsA2) (Fig. 1 and Table 2). PCR employing primers derived from arsA1 and arsA2 revealed that all 23 arsenic-resistant isolates were positive for arsA2. However, a subset (13/23) of the arsA2-positive isolates also harbored arsA1. None of the arsenic-susceptible isolates was positive for either arsA1 or arsA2 (Table 1). All arsenic-resistant ECIa isolates harbored both arsA1 and arsA2, as did two of the 12 ECI arsenic-resistant isolates and all four non-ECI, -ECII, or -ECIa isolates. All isolates harboring both arsA1 and arsA2 also harbored cadA4 (Table 1). None of the isolates harbored arsA1 in the absence of arsA2 (Table 1), suggesting that the additional arsenic resistance genes found in Scott A were acquired by a genetic element that already harbored the arsenic resistance cassette previously detected in pLI100.
As mentioned earlier, the LMOf2365_2257 homolog in the genome of Scott A appears to have been interrupted by the insertion of the 35-kb genomic island harboring the arsenic resistance cassette (including both arsA1 and arsA2) and cadA4 (Fig. 1). PCR with primers P4F and P4R annealing to the flanking region (Fig. 1 and Table 2) revealed that this gene (i.e., the LMOf2365_2257 homolog) was intact in all arsenic-susceptible isolates as well as in those resistant isolates that only harbored arsA2 (Table 1 and Fig. 3A). Of the 13 resistant isolates that harbored both arsA1 and arsA2, the expected PCR product (1,083 bp) was not obtained from the two ECI isolates or the seven ECIa isolates, suggesting that the gene was interrupted by a large insertion; however, the expected PCR product was obtained from the four remaining isolates (all four outside ECI, ECII, or ECIa), suggesting that they possessed the arsenic resistance determinants in a locus different from the LMOf2365_2257 homolog (Table 1 and Fig. 3A). The locations of the resistance determinants in these isolates or in those only harboring arsA2 remain to be identified.
Fig 3.
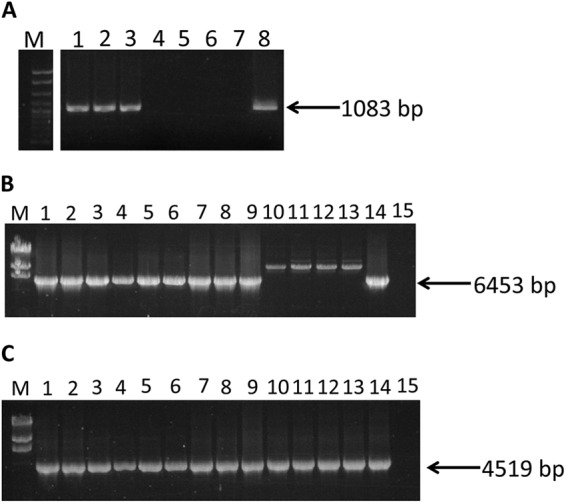
Locations of the arsenic resistance cassette in arsenic-resistant isolates. (A) PCR with primers P4F and P4R. Lanes: 1 to 3, J5080, J5095, and J5136, respectively, harboring only arsA2; 4 to 7, ECIa isolates J3290, J3419, J3768, and J3921, respectively; 8, F2365 (positive control); and M, exACTGene cloning DNA ladder (Fisher Scientific). (B) PCR with primers P4F and P2R. Lanes: 1 and 2, J4187 and J4600, respectively (ECI isolates positive for both arsA1 and arsA2); 3 to 9, ECIa isolates J3290, J3419, J3768, J3921, J4503, J4948, and J4954, respectively; 10 to 13, non-EC isolates J3422, J2422, J3618, and J4434, respectively; 14, Scott A (positive control); 15, negative control; and M, DNA molecular mass marker II (Roche Diagnostics, Indianapolis, IN). The larger bands in lanes 10 to 13 represent unspecific PCR products. (C) PCR with primers P2F and P3R. Lanes are as in panel B. For negative controls, sterile water was used as the template. The size of the intended amplicon is indicated by an arrow.
The locations of arsenic resistance cassettes were further examined by PCR using primers P4F and P2R, annealing to LMOf2365_2257 and arsA1, respectively (Fig. 1 and Table 2). The expected PCR product (6,453 bp) was obtained with ECI and ECIa isolates positive for arsA1 and arsA2, confirming that, like Scott A, these isolates harbored the arsenic resistance cassette in the LMOf2365_2257 homolog (Table 1 and Fig. 3B). In contrast, the four non-EC isolates positive for both arsA genes failed to yield the expected amplicon, in agreement with the presence of an uninterrupted LMOf2365_2257 homolog and suggesting the presence of the resistance genes in other, currently unidentified locations (Table 1 and Fig. 3B). PCR of isolates harboring both arsA1 and arsA2 using primers P2F and P3R, annealing to arsA1 and arsA2, respectively (Fig. 1 and Table 2), revealed that, regardless of genomic location, the two arsenic resistance genes were close to each other, with the PCR product having the size expected (4,519 bp) based on the gene arrangement in Scott A (Fig. 3C).
To examine whether the presence of different arsenic resistance genes was associated with different levels of tolerance to arsenic, MICs of selected isolates were determined for arsenite (also initially employed to determine resistance to arsenic) and arsenate (another chemical form of arsenic which is reduced to arsenite and pumped out by ArsB transporters in the arsenic resistance cassettes) (25) (Table 1). As expected, all tested arsenic-resistant isolates showed a higher arsenite MIC (1,250 to 2,500 μg/ml) compared to the susceptible isolates, whose MICs ranged from 250 to 500 μg/ml (Table 1). A similar trend was observed for arsenate (30,000 μg/ml for arsenic-resistant isolates versus 750 to 2,500 μg/ml for those susceptible to arsenic) (Table 1). Arsenite or arsenate MICs were similar, regardless of whether an isolate harbored arsA2 only or both arsA1 and arsA2 (Table 1).
In conclusion, we have described unexpected associations between heavy metal resistance determinants and clonal groups of serotype 4b L. monocytogenes from sporadic human listeriosis in the United States. Further studies are warranted regarding novel cadmium resistance determinants, most likely to be found among cadmium-resistant ECI isolates. The identification of a chromosomal island harboring the arsenic resistance cassette in several arsenic-resistant isolates, including all those of ECIa, agrees with earlier conclusions (based on plasmid curing outcomes) that arsenic resistance in L. monocytogenes was not associated with plasmids (15).
Our findings reflect a complex and diverse repertoire of heavy metal resistance genes within serotype 4b L. monocytogenes from human listeriosis that are likely to be acquired horizontally from various gene pools. The arsenic and cadmium resistance determinants examined here were predominantly found among isolates of clonal groups that have been repeatedly implicated in outbreaks. We can hypothesize that the association of epidemic clones with human illness may reflect the acquisition by these strains of a complex combination of accessory genes that facilitate their proliferation in different environments and may enhance their virulence potential. The complex repertoire of heavy metal resistance genes (and the complicated evolutionary history suggested by their distribution) is consistent with this hypothesis. Further studies on cadmium and arsenic resistance genes will enhance our understanding of the evolution and function of these accessory genes and are needed to elucidate the possible contributions of such genes to the frequent involvement of epidemic clones in food contamination and human food-borne disease.
ACKNOWLEDGMENTS
This study was partially supported by USDA grant 2006-35201-17377 and the U.S. Department of Agriculture's Agricultural Research Service.
We thank all members of our laboratory for discussions, encouragement, and support in the course of the project.
Footnotes
Published ahead of print 1 February 2013
REFERENCES
- 1. Painter J, Slutsker L. 2007. Listeriosis in humans, p 85–110 In Ryser ET, Marth EH. (ed), Listeria, listeriosis, and food safety, 3rd ed CRC Press, Boca Raton, FL [Google Scholar]
- 2. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17: 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kathariou S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65: 1811–1829 [DOI] [PubMed] [Google Scholar]
- 4. Swaminathan B, Gerner-Smidt P. 2007. The epidemiology of human listeriosis. Microbes Infect. 9: 1236–1243 [DOI] [PubMed] [Google Scholar]
- 5. Varma JK, Samuel MC, Marcus R, Hoekstra RM, Medus C, Segler S, Anderson BJ, Jones TF, Shiferaw B, Haubert N, Megginson M, McCarthy PV, Graves L, Gilder TV, Angulo FJ. 2007. Listeria monocytogenes infection from foods prepared in a commercial establishment: a case-control study of potential sources of sporadic illness in the United States. Clin. Infect. Dis. 44: 521–528 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Zhang W, Knabel SJ. 2007. Multi-virulence-locus sequence typing identifies single nucleotide polymorphisms which differentiate epidemic clones and outbreak strains of Listeria monocytogenes. J. Clin. Microbiol. 45: 835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chenal-Francisque V, Lopez J, Cantinelli T, Caro V, Tran C, Leclercq A, Lecuit M, Brisse S. 2011. Worldwide distribution of major clones of Listeria monocytogenes. Emerg. Infect. Dis. 17: 1110–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. den Bakker HC, Fortes ED, Wiedmann M. 2010. Multilocus sequence typing of outbreak-associated Listeria monocytogenes isolates to identify epidemic clones. Foodborne Pathog. Dis. 7: 257–265 [DOI] [PubMed] [Google Scholar]
- 9. Eifert JD, Curtis PA, Bazaco MC, Meinersmann RJ, Berrang ME, Kernodle S, Stam C, Jaykus LA, Kathariou S. 2005. Molecular characterization of Listeria monocytogenes of the serotype 4b complex (4b, 4d, 4e) from two turkey processing plants. Foodborne Pathog. Dis. 2: 192–200 [DOI] [PubMed] [Google Scholar]
- 10. Franciosa G, Scalfaro C, Maugliani A, Floridi F, Gattuso A, Hodzic S, Aureli P. 2007. Distribution of epidemic clonal genetic markers among Listeria monocytogenes 4b isolates. J. Food Prot. 70: 574–581 [DOI] [PubMed] [Google Scholar]
- 11. Ratani SS, Siletzky RM, Dutta V, Yilidrim S, Osborne JA, Lin W, Hitchins AD, Ward TJ, Kathariou S. 2012. Heavy metal and disinfectant resistance of Listeria monocytogenes from foods and food processing plants. Appl. Environ. Microbiol. 78: 6938–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ward TJ, Evans P, Wiedmann M, Usgaard T, Roof SE, Stroika SG, Hise K. 2010. Molecular and phenotypic characterization of Listeria monocytogenes from U.S. Department of Agriculture Food Safety and Inspection Service surveillance of ready-to-eat foods and processing facilities. J. Food Prot. 73: 861–869 [DOI] [PubMed] [Google Scholar]
- 13. Yildirim S, Lin W, Hitchins AD, Jaykus LA, Altermann E, Klaenhammer TR, Kathariou S. 2004. Epidemic clone I-specific genetic markers in strains of Listeria monocytogenes serotype 4b from foods. Appl. Environ. Microbiol. 70: 4158–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lebrun M, Audurier A, Cossart P. 1994. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are similar to cadA and cadC of Staphylococcus aureus and are induced by cadmium. J. Bacteriol. 176: 3040–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McLauchlin J, Hampton MD, Shah S, Threlfall EJ, Wieneke AA, Curtis GD. 1997. Subtyping of Listeria monocytogenes on the basis of plasmid profiles and arsenic and cadmium susceptibility. J. Appl. Microbiol. 83: 381–388 [DOI] [PubMed] [Google Scholar]
- 16. Mullapudi S, Siletzky RM, Kathariou S. 2010. Diverse cadmium resistance determinants in Listeria monocytogenes isolates from the turkey processing plant environment. Appl. Environ. Microbiol. 76: 627–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee S, Ward TJ, Siletzky RM, Kathariou S. 2012. Two novel type II restriction-modification systems occupying genomically equivalent locations on the chromosomes of Listeria monocytogenes strains. Appl. Environ. Microbiol. 78: 2623–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebrun M, Audurier A, Cossart P. 1994. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are present on Tn5422, a novel transposon closely related to Tn917. J. Bacteriol. 176: 3049–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuenne C, Voget S, Pischimarov J, Oehm S, Goesmann A, Daniel R, Hain T, Chakraborty T. 2010. Comparative analysis of plasmids in the genus Listeria. PLoS One 5: e12511 doi:10.1371/journal.pone.0012511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson KE, Fouts DE, Mongodin EF, Ravel J, DeBoy RT, Kolonay JF, Rasko DA, Angiuoli SV, Gill SR, Paulsen IT, Peterson J, White O, Nelson WC, Nierman W, Beanan MJ, Brinkac LM, Daugherty SC, Dodson RJ, Durkin AS, Madupu R, Haft DH, Selengut J, Van Aken S, Khouri H, Fedorova N, Forberger H, Tran B, Kathariou S, Wonderling LD, Uhlich GA, Bayles DO, Luchansky JB, Fraser CM. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32: 2386–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, Charbit A, Chetouani F, Couve E, de Daruvar A, Dehoux P, Domann E, Dominguez-Bernal G, Duchaud E, Durant L, Dussurget O, Entian KD, Fsihi H, Garcia-del Portillo F, Garrido P, Gautier L, Goebel W, Gomez-Lopez N, Hain T, Hauf J, Jackson D, Jones LM, Kaerst U, Kreft J, Kuhn M, Kunst F, Kurapkat G, Madueno E, Maitournam A, Vicente JM, Ng E, Nedjari H, Nordsiek G, Novella S, de Pablos B, Perez-Diaz JC, Purcell R, Remmel B, Rose M, Schlueter T, Simoes N, Tierrez A, Vazquez-Boland JA, Voss H, Wehland J, Cossart P. 2001. Comparative genomics of Listeria species. Science 294: 849–852 [DOI] [PubMed] [Google Scholar]
- 22. Briers Y, Klumpp J, Schuppler M, Loessner MJ. 2011. Genome sequence of Listeria monocytogenes Scott A, a clinical isolate from a food-borne listeriosis outbreak. J. Bacteriol. 193: 4284–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mullapudi S, Siletzky RM, Kathariou S. 2008. Heavy-metal and benzalkonium chloride resistance of Listeria monocytogenes isolates from the environment of turkey-processing plants. Appl. Environ. Microbiol. 74: 1464–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katharios-Lanwermeyer S, Rakic-Martinez M, Elhanafi D, Ratani S, Tiedje JM, Kathariou S. 2012. Coselection of cadmium and benzalkonium chloride resistance in conjugative transfers from nonpathogenic Listeria spp. to other listeriae. Appl. Environ. Microbiol. 78: 7549–7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaur S, Kamli MR, Ali A. 2011. Role of arsenic and its resistance in nature. Can. J. Microbiol. 57: 769–774 [DOI] [PubMed] [Google Scholar]
- 26. Sperry KE, Kathariou S, Edwards JS, Wolf LA. 2008. Multiple-locus variable-number tandem-repeat analysis as a tool for subtyping Listeria monocytogenes strains. J. Clin. Microbiol. 46: 1435–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ward TJ, Usgaard T, Evans P. 2010. A targeted multilocus genotyping assay for lineage, serogroup, and epidemic clone typing of Listeria monocytogenes. Appl. Environ. Microbiol. 76: 6680–6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee S, Ward TJ, Graves LM, Wolf LA, Sperry K, Siletzky RM, Kathariou S. 2012. Atypical Listeria monocytogenes serotype 4b strains harboring a lineage II-specific gene cassette. Appl. Environ. Microbiol. 78: 660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chaudhuri RR, Loman NJ, Snyder LA, Bailey CM, Stekel DJ, Pallen MJ. 2008. xBASE2: a comprehensive resource for comparative bacterial genomics. Nucleic Acids Res. 36: D543–D546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Delcher AL, Bratke KA, Powers EC, Salzberg SL. 2007. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23: 673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5: R12 doi:10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35: 3100–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25: 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410 [DOI] [PubMed] [Google Scholar]
- 36. Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39: D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J. 2005. ACT: the Artemis Comparison Tool. Bioinformatics 21: 3422–3423 [DOI] [PubMed] [Google Scholar]