Abstract
Using in situ subtropical aquatic mesocosms, fecal source (cattle manure versus sewage) was shown to be the most important contributor to differential loss in viability of fecal indicator bacteria (FIB), specifically enterococci in freshwater and Escherichia coli in marine habitats. In this study, sunlight exposure and indigenous aquatic microbiota were also important contributors, whose effects on FIB also differed between water types.
TEXT
The fecal indicator bacteria (FIB), enterococci and Escherichia coli, are used for the assessment of sanitary quality of recreational waters worldwide. A reasonable correlation between the incidence of gastrointestinal illness in recreational bathers and levels of FIB established in the earlier epidemiological studies (1, 2) continues to be supported by more recent data (3). Despite their long history of use, many uncertainties remain concerning the fate of FIB upon release into the environment and hence their role as a useful predictor of recreator health effects when released from various fecal sources (4).
While sources of FIB are well described and include many mammals and birds, as well as environmental sources (e.g., soils, sediments, and aquatic vegetation) (5–11), few studies have explored survival rates of FIB from animal sources (12). In general, human fecal pollution sources (e.g., sewage and septage) have been investigated more extensively than others (such as domestic farm animals, wildlife, etc.) because of the risk entailed by enteric viral pathogens (13), which are largely assumed to be human specific. However, recent data suggest that risks posed to human health by fresh cattle feces may not be substantially different than those from human sources (14), warranting the need for further research in this area, particularly in relation to the fate of bovine FIB versus those of sewage origin.
Earlier studies report that exposure to ambient sunlight and biotic factors (competition and predation by indigenous aquatic microbiota) are important contributors to FIB decay in ambient waters (15–19). In general, the detrimental effect of sunlight is more pronounced in marine waters than in freshwater (12, 16, 20, 21), while the opposite is the case for biotic interactions (22, 23). However, the majority of FIB decay studies use laboratory-grown control strains and conduct experiments under artificial conditions (18, 24–27), which cannot accurately depict the behavior of FIB originating from “natural” sources and the complexity of interactions in aquatic ecosystems.
The objective of this study was to investigate the effect of select environmental parameters, including (i) water type, (ii) exposure to ambient sunlight, and (iii) the presence of indigenous aquatic microbiota, on the viability (i.e., culturability by standard methods) of E. coli and enterococci originating from cattle manure or primary municipal wastewater effluent. Field-deployable submersible mesocosms allowed the assessment of environmental stressors on the decay of FIB by closely mimicking the release of these organisms into a recreational water body.
Two submersible mesocosms measuring approximately 50.8 cm (height) by 101.6 cm (width) by 101.6 cm (depth) were constructed using 1-in. and -in. polyvinyl chloride (PVC) pipes. Small holes were drilled into the commercial-grade, white PVC pipe frame to allow the device to submerge upon deployment. The mesocosm allowed for both light (top half) and dark (bottom half) treatments. In the dark treatment, the lower half was covered by a heavy-duty opaque sheet that effectively blocked sunlight. Sample mixtures with a total volume of 200 ml were contained using 75-mm-flat-width, 13- to 14-kDa-molecular-mass-cutoff regenerated cellulose dialysis tubing (Spectrum Laboratories, Rancho Dominguez, CA). Fishing line (24-lb test line) was tied directly onto the PVC mesocosm frames, and dialysis bags containing samples were attached using -in. fishing snap-swivels and 6-lb test line. The sides of the rectangular frame were wrapped in plastic wire mesh to prevent accidental puncture of the bags from floating debris.
The two submersible mesocosms were deployed for a 7-day period at a freshwater site (Riverfront Park at Hillsborough River, Tampa, FL; 28°04′11″N, 82°22′38″W) and at a marine water site (Fort De Soto Park at Gulf of Mexico, Tierra Verde, FL; 27°38′17″N, 82°43′07″W). Study sites were selected based on accessibility and the availability of structures that would allow each mesocosm to be secured (dock or pier) in waters where recreation was considered acceptable.
Hourly light intensity and temperature measurements were recorded by a Hobo data logger (Bourne, MA). Light intensity was measured approximately 2 to 3 cm below the surface of the water (i.e., at the level of the sunlight-exposed dialysis bags), and it averaged 3,545 and 4,530 lm m−2 for freshwater and marine sites, respectively. Average temperatures (± standard deviation [SD]) for the duration of the experiment were 20.8 ± 2.22°C (freshwater site) and 21.2 ± 2.10°C (marine site). Average solar insolation incident on a horizontal surface for the study month of February (5.01 kWh m−2 day−1) and clearness index 0.67 (cloud cover normalized on a scale from 0 to 1) data for the region where the mesocosms were installed were obtained from the NASA Langley Atmospheric Science Data Center (http://eosweb.larc.nasa.gov/cgi-bin/sse/sse.cgi).
Primary wastewater effluent from a local municipal wastewater treatment plant (Howard F. Curren Advanced Wastewater Treatment Plant, Tampa, FL) was collected on the day of the experiment (time 0 [T0]), and fresh cattle manure was collected from 10 different animals on the day prior to the experiment and held at 4°C overnight to minimize changes in microbial populations. Dialysis bags representing a “human” source were filled with 100 ml of primary effluent and 100 ml of either untreated (e.g., not filter-sterilized) or filter-sterilized ambient water, depending on the biotic treatment (described below). A composite source (“cattle”) was created by mixing 10 g of cattle manure from each animal, followed by resuspension (1:10 ratio) in sterile phosphate-buffered water (0.0425 g · liter−1 KH2PO4 and 0.4055 g · liter−1 of MgCl2; pH 7.2) (28). Fecal suspensions were vigorously vortexed until there were no visible clumps, and solids were allowed to settle for 5 min. One hundred milliliters of the supernatant was then mixed with ambient water as described above.
In order to determine the effect of environmental variables on the loss of viability of FIB, treatments included (i) exposure to ambient sunlight and aquatic indigenous microbiota (treatment a), (ii) exposure to ambient sunlight only (treatment b), (iii) exposure to aquatic indigenous microbiota only (treatment c), and (iv) exposure to neither variable (treatment d) (see Table 2). This experimental design was replicated for both FIB sources (primary effluent and cattle manure) and water types (freshwater and marine water). For treatments that contained no aquatic indigenous microbiota (i.e., treatments b and d), ambient water was successively filter sterilized by 0.45-μm and 0.22-μm nitrocellulose membrane filters and finally through a NanoCeram filter (Argonide, Stanford, FL) to remove virus-sized particles. The removal efficiency for bacteria was evaluated using tryptic soy agar and mEI and mTEC media for aerobic/facultatively anaerobic heterotrophs, enterococci, and E. coli, respectively. All three types of media demonstrated negligible concentrations of bacteria (i.e., <5 CFU/100 ml). Triplicate dialysis bags per treatment were collected at the beginning of the experiment (T0), after 24 h (T1), and every other day for 7 days.
Table 2.
The effect of bovine versus sewage fecal source on decay of enterococci and E. coli in two water types under four treatment regimensa
| FIB | Water type | Source of variation | % of total variation | P valueb |
|---|---|---|---|---|
| Enterococci | Freshwater | Fecal source | 70.8 | <0.0001 |
| Treatment | 5.00 | 0.33 | ||
| Interaction | 2.35 | 0.64 | ||
| Marine | Fecal source | 3.31 | 0.085 | |
| Treatment | 56.9 | <0.0001 | ||
| Interaction | 24.0 | 0.0016 | ||
| E. coli | Freshwater | Fecal source | 10.8 | 0.015 |
| Treatment | 39.3 | 0.0009 | ||
| Interaction | 26.9 | 0.0052 | ||
| Marine | Fecal source | 69.1 | <0.0001 | |
| Treatment | 11.2 | 0.021 | ||
| Interaction | 5.78 | 0.13 |
The treatments were with or without sunlight and with or without aquatic microbiota. See Table 3 for individual effects.
Two-way factorial ANOVA.
Harvested dialysis bags were placed into marked ziplock bags filled with fresh ambient water (to avoid sample desiccation) and were transported on ice back to the lab for analysis. Dialysis bags were mixed by being inverted several times to evenly mix the contents prior to sample analyses. In the early stages of the experiment (e.g., T0 and T1), decimal dilution series were performed, and FIB were enumerated by culture on mEI and mTEC media using standard membrane filtration protocols (29, 30). In the later stages of the experiment (e.g., T5 and T7), dilutions were not performed as levels of FIB decreased; 10 ml of undiluted sample was processed instead. All FIB densities were log10 transformed and normalized to a 100-ml volume. “Decay” (loss in viability) is expressed as log10 reduction over 7 days, calculated by subtracting the density on day 7 from that at the start of the experiment (T0).
Prior to beginning the experiment, power analysis was conducted using pilot data to determine the appropriate number of replicates needed (e.g., dialysis bags per treatment) using GraphPad (StatMate version 2.00 for Windows; GraphPad, San Diego CA). The effect of fecal source (cattle manure or primary effluent), water type (freshwater or marine) and treatments (exposure to ambient sunlight and indigenous aquatic microbiota) was assessed by two-way factorial analysis of variance (ANOVA) (GraphPad Prism software version 5.00 for Windows).
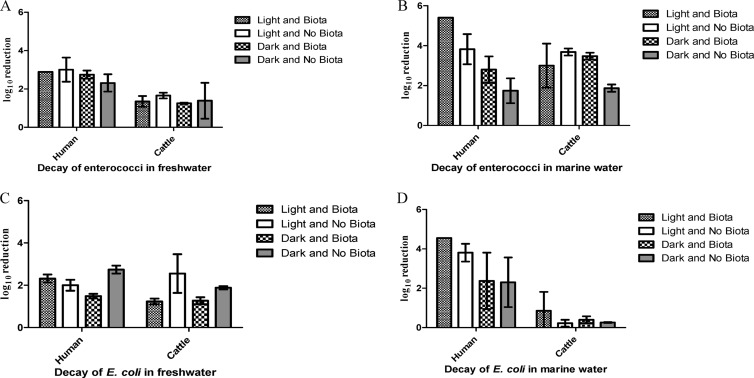
In general, cattle manure FIB persisted considerably longer than their sewage counterparts, irrespective of the water type or treatment, and the difference was generally significant (Fig. 1 and Table 1). This finding is consistent with previous reports showing long-term persistence and even initial growth of E. coli and enterococci in cow fecal patties (31) and with bovine manure incorporated into soil (32). Extended persistence of FIB in cattle manure was especially evident for enterococci in freshwater and E. coli in marine waters, where densities decreased 2 to 3 orders of magnitude less than that of sewage FIB (Fig. 1A and D). Fecal source was the most important determinant of survival for enterococci in freshwater and for E. coli in marine water (P < 0.0001), contributing ∼70% to the total observed variation (Table 2). Interaction of variables was not significant for either one of these data sets (P > 0.05), suggesting that the influence of fecal source is not dependent on the treatment effects (i.e., presence/absence of indigenous aquatic microbiota or light/dark condition) but rather is a result of intrinsic properties of the FIB from different fecal sources.
Fig 1.
Log10 reduction over 7 days of FIB from sewage and cattle manure in marine (B and D) and freshwater (A and C) mesocosms. Shown are the effects of fecal source and treatments (±sunlight and ±aquatic microbiota).
Table 1.
Schematic of the experimental design
| Source | Sunlight | Indigenous microbiota | Treatment designation |
|---|---|---|---|
| Human | Present | Present | a |
| Absent | b | ||
| Absent | Present | c | |
| Absent | d | ||
| Cattle manure | Present | Present | a |
| Absent | b | ||
| Absent | Present | c | |
| Absent | d |
At least one study suggested that decay rates of FIB are not source dependent, when the sources considered were limited to sewage influent/effluent and urban storm drain runoff (33). In contrast, our data indicate that FIB from bovine manure may exhibit markedly different decay patterns compared to sewage-derived FIB, presumably because of the nature of fecal sources (e.g., higher particulate matter content of cattle manure, providing potential nutrients and surface for attachment and different selective pressures on FIB in host gastrointestinal systems) as well as likely differences in the strains involved. This finding agrees with the differential survival of E. coli isolates from dog, sewage, and soil sources found by Anderson et al. (12); however, to the best of our knowledge, direct comparisons of FIB decay from cattle manure and sewage effluents have not previously been reported. This finding is important for several reasons, including the possibility that zoonotic (bacterial) pathogens from cattle manure may share the ability to survive in secondary habitats. Conversely, longer persistence of bovine FIB compared to likely zoonotic pathogens (4) would make them a more conservative marker of health risk.
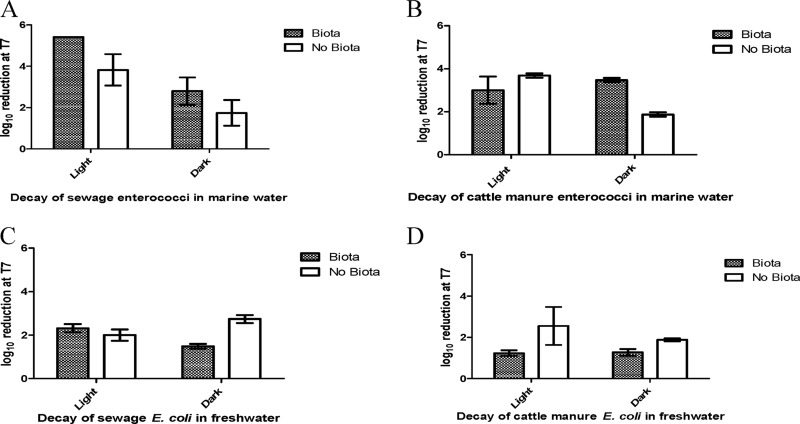
This observed trend of extended survival of FIB from cattle manure was also evident for enterococci in marine waters and for E. coli in freshwater, which also included pronounced treatment effects (Fig. 1B and C and Fig. 2). Specifically, the presence/absence of indigenous aquatic microbiota and exposure to light/dark conditions contributed nearly 60% and 40% to observed variations in enterococcal (P < 0.0001) and E. coli (P = 0.0009) densities, respectively (Table 2). Interestingly, for enterococci originating from cattle manure, neither treatment variable was independently significant, but the presence of indigenous marine aquatic microbiota enhanced decay under the dark conditions (Fig. 2B and Table 3). In contrast, enterococci originating from sewage were impacted by both treatment variables (indigenous aquatic microbiota and sunlight), although exposure to sunlight was a more important determinant than it was for isolates from cattle (Fig. 2A and Table 3). Irrespective of the fecal source, the presence of indigenous aquatic microbiota was the only significant contributor to decay of E. coli in freshwater (Fig. 2C and D and Table 3). Many studies have reported the detrimental effect of sunlight on FIB survival (19, 34, 35), but the impact of indigenous aquatic microbiota is rarely considered in the same context. Our findings suggest that the commonly overlooked influence of biological interactions may bias results, leading to inaccurate assessments of the complex interplay of environmental variables on the fate of FIB (and possibly other zoonotic pathogens). In support of our conclusion, a recent FIB decay study conducted in Florida indicated that both predation and competition are important contributors to E. coli decay in subtropical freshwater systems (27).
Fig 2.
Log10 reduction over 7 days of enterococci in marine waters (A and B) and E. coli in freshwater (C and D) mesocosms. Shown are the effects of sunlight exposure and the presence of indigenous aquatic microbiota.
Table 3.
The effect of light/dark treatments and the presence/absence of indigenous microbiota on decay of enterococci in marine and E. coli in freshwater habitats
| FIB | Water type | Fecal source | Source of variation | % of total variation | P valuea |
|---|---|---|---|---|---|
| Enterococci | Marine | Human | Light vs dark | 66.7 | 0.0001 |
| Presence of biota | 21.2 | 0.0048 | |||
| Interaction | 0.83 | 0.47 | |||
| Cattle | Light vs dark | 15.8 | 0.077 | ||
| Presence of biota | 7.50 | 0.20 | |||
| Interaction | 45.8 | 0.0087 | |||
| E. coli | Freshwater | Human | Light vs dark | 0.22 | 0.70 |
| Presence of biota | 23.4 | 0.0031 | |||
| Interaction | 65.7 | 0.0001 | |||
| Cattle | Light vs dark | 5.78 | 0.23 | ||
| Presence of biota | 53.1 | 0.0076 | |||
| Interaction | 7.23 | 0.23 |
Two-way factorial ANOVA.
In summary, we employed submersible in situ mesocosms to estimate the role of fecal pollution source, exposure to ambient light, and influence of microbial predation and competition on FIB decay. Although exposure to sunlight and the presence of aquatic microbiota under certain conditions were influential on FIB decay, the fecal source seemed to be the more consistent factor influencing FIB survival under the conditions employed in this study. Hence the extrapolation of health risks (implied by FIB) from sewage impacted recreational waters to those impacted by cattle manure requires additional comparisons to potential pathogens before it can be deemed valid.
ACKNOWLEDGMENTS
The United States Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. It has been subjected to Agency's administrative review and approved for publication. The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Published ahead of print 1 February 2013
REFERENCES
- 1.Cabelli VJ, Dufour AP, Levin MA, McCabe LJ, Haberman PW. 1979. Relationship of microbial indicators to health effects at marine bathing beaches. Am. J. Public Health 69:690–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. 1982. Swimming-associated gastroenteritis and water quality. Am. J. Epidemiol. 115:606–616 [DOI] [PubMed] [Google Scholar]
- 3.Wade TJ, Sams E, Brenner KP, Haugland R, Chern E, Beach M, Wymer L, Rankin CC, Love D, Li Q, Noble R, Dufour AP. 2010. Rapidly measured indicators of recreational water quality and swimming-associated illness at marine beaches: a prospective cohort study. Environ. Health 9:66 doi:10.1186/1476-069X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoen ME, Soller JA, Ashbolt NJ. 2011. Evaluating the importance of faecal sources in human-impacted waters. Water Res. 45:2670–2680 [DOI] [PubMed] [Google Scholar]
- 5.Badgley BD, Thomas FI, Harwood VJ. 2011. Quantifying environmental reservoirs of fecal indicator bacteria associated with sediment and submerged aquatic vegetation. Environ. Microbiol. 13:932–942 [DOI] [PubMed] [Google Scholar]
- 6.Byappanahalli MN, Fujioka RS. 1998. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci. Technol. 38:171–174 [Google Scholar]
- 7.Byappanahalli MN, Shively DA, Nevers MB, Sadowsky MJ, Whitman RL. 2003. Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora (Chlorophyta). FEMS Microbiol. Ecol. 46:203–211 [DOI] [PubMed] [Google Scholar]
- 8.Harris MM. 1932. A bacteriological study of decomposing crabs and crab meat. Am. J. Epidemiol. 15:260–275 [Google Scholar]
- 9.Ostrolenk M, Kramer N, Cleverdon RC. 1947. Comparative studies of enterococci and Escherichia coli as indices of pollution. J. Bacteriol. 53:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shellenbarger GG, Athearn ND, Takekawa JY, Boehm AB. 2008. Fecal indicator bacteria and Salmonella in ponds managed as bird habitat, San Francisco Bay, California, USA. Water Res. 42:2921–2930 [DOI] [PubMed] [Google Scholar]
- 11.Stoeckel DM, Stelzer EA, Stogner RW, Mau DP. 2011. Semi-quantitative evaluation of fecal contamination potential by human and ruminant sources using multiple lines of evidence. Water Res. 45:3225–3244 [DOI] [PubMed] [Google Scholar]
- 12.Anderson KL, Whitlock JE, Harwood VJ. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71:3041–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinclair RG, Jones EL, Gerba CP. 2009. Viruses in recreational water-borne disease outbreaks: a review. J. Appl. Microbiol. 107:1769–1780 [DOI] [PubMed] [Google Scholar]
- 14.Soller JA, Schoen ME, Bartrand T, Ravenscroft JE, Ashbolt NJ. 2011. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. 44:4674–4691 [DOI] [PubMed] [Google Scholar]
- 15.Anderson IC, Rhodes MW, Kator HI. 1983. Seasonal variation in survival of Escherichia coli exposed in situ in membrane-diffusion chambers containing filtered and nonfiltered estuarine water. Appl. Environ. Microbiol. 45:1877–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies CM, Evison LM. 1991. Sunlight and the survival of enteric bacteria in natural waters. J. Appl. Bacteriol. 70:265–274 [DOI] [PubMed] [Google Scholar]
- 17.Fujioka RS, Hashimoto HH, Siwak EB, Young RH. 1981. Effect of sunlight on survival of indicator bacteria in seawater. Appl. Environ. Microbiol. 41:690–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon P, Billen G, Servais P. 2003. Mortality rates of autochthonous and fecal bacteria in natural aquatic ecosystems. Water Res. 37:4151–4158 [DOI] [PubMed] [Google Scholar]
- 19.Sinton LW, Hall CH, Lynch PA, Davies-Colley RJ. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujioka RS, Narikawa OT. 1982. Effect of sunlight on enumeration of indicator bacteria under field conditions. Appl. Environ. Microbiol. 44:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinton L, Hall C, Braithwaite R. 2007. Sunlight inactivation of Campylobacter jejuni and Salmonella enterica, compared with Escherichia coli, in seawater and river water. J. Water Health 5:357–365 [DOI] [PubMed] [Google Scholar]
- 22.Green HC, Shanks OC, Sivaganesan M, Haugland RA, Field KG. 2011. Differential decay of human faecal Bacteroides in marine and freshwater. Environ. Microbiol. 13:3235–3249 [DOI] [PubMed] [Google Scholar]
- 23.Okabe S, Shimazu Y. 2007. Persistence of host-specific Bacteroides-Prevotella 16S rRNA genetic markers in environmental waters: effects of temperature and salinity. Appl. Microbiol. Biotechnol. 76:935–944 [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Lara J, Menon P, Servais P, Billen G. 1991. Mortality of fecal bacteria in seawater. Appl. Environ. Microbiol. 57:885–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirlot S, Unrein F, Descy JP, Servais P. 2007. Fate of heterotrophic bacteria in Lake Tanganyika (East Africa). FEMS Microbiol. Ecol. 62:354–364 [DOI] [PubMed] [Google Scholar]
- 26.Servais P, Garcia-Armisen T, George I, Billen G. 2007. Fecal bacteria in the rivers of the Seine drainage network (France): sources, fate and modelling. Sci. Total Environ. 375:152–167 [DOI] [PubMed] [Google Scholar]
- 27.Wanjugi P, Harwood VJ. 2013. The influence of predation and competition on the survival of commensal and pathogenic fecal bacteria in aquatic habitats. Environ. Microbiol. 15:517–526 [DOI] [PubMed] [Google Scholar]
- 28.American Public Health Association 1999. Standard methods for the examination of water and wastewater. Standard method 9222D SM9222D. American Public Health Association, Washington, DC [Google Scholar]
- 29.United States Environmental Protection Agency 2002. Method 1600: enterococci in water by membrane filtration using membrane-enterococcus indoxyl-B-d-glucoside agar (mEI), EPA 821-R-02-022. United States Environmental Protection Agency, Washington, DC [Google Scholar]
- 30.United States Environmental Protection Agency 2002. Method 1603: Escherichia coli (E. coli) in water by membrane filtration using modified membrane-thermotolerant Escherichia coli agar (modified mTEC), EPA 821-R-02-023. United States Environmental Protection Agency, Washington, DC [Google Scholar]
- 31.Sinton LW, Braithwaite RR, Hall CH, Mackenzie ML. 2007. Survival of indicator and pathogenic bacteria in bovine feces on pasture. Appl. Environ. Microbiol. 73:7917–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau MM, Ingham SC. 2001. Survival of faecal indicator bacteria in bovine manure incorporated into soil. Lett. Appl. Microbiol. 33:131–136 [DOI] [PubMed] [Google Scholar]
- 33.Noble RT, Lee IM, Schiff KC. 2004. Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater. J. Appl. Microbiol. 96:464–472 [DOI] [PubMed] [Google Scholar]
- 34.Boehm AB, Yamahara KM, Love DC, Peterson BM, McNeill K, Nelson KL. 2009. Covariation and photoinactivation of traditional and novel indicator organisms and human viruses at a sewage-impacted marine beach. Environ. Sci. Technol. 43:8046–8052 [DOI] [PubMed] [Google Scholar]
- 35.Davies-Colley RJ, Bell RG, Donnison AM. 1994. Sunlight inactivation of enterococci and fecal coliforms in sewage effluent diluted in seawater. Appl. Environ. Microbiol. 60:2049–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]