Abstract
Salmonella is a zoonotic pathogen that poses a considerable public health and economic burden in the United States and worldwide. Resultant human diseases range from enterocolitis to bacteremia to sepsis and are acutely dependent on the particular serovar of Salmonella enterica subsp. enterica, which comprises over 99% of human-pathogenic S. enterica isolates. Point-of-care methods for detection and strain discrimination of Salmonella serovars would thus have considerable benefit to medical, veterinary, and field applications that safeguard public health and reduce industry-associated losses. Here we describe a single, disposable microfluidic chip that supports isothermal amplification and sequence-specific detection and discrimination of Salmonella serovars derived from whole blood of septic mice. The integrated microfluidic electrochemical DNA (IMED) chip consists of an amplification chamber that supports loop-mediated isothermal amplification (LAMP), a rapid, single-temperature amplification method as an alternative to PCR that offers advantages in terms of sensitivity, reaction speed, and amplicon yield. The amplification chamber is connected via a microchannel to a detection chamber containing a reagentless, multiplexed (here biplex) sensing array for sequence-specific electrochemical DNA (E-DNA) detection of the LAMP products. Validation of the IMED device was assessed by the detection and discrimination of S. enterica subsp. enterica serovars Typhimurium and Choleraesuis, the causative agents of enterocolitis and sepsis in humans, respectively. IMED chips conferred rapid (under 2 h) detection and discrimination of these strains at clinically relevant levels (<1,000 CFU/ml) from whole, unprocessed blood collected from septic animals. The IMED-based chip assay shows considerable promise as a rapid, inexpensive, and portable point-of-care diagnostic platform for the detection and strain-specific discrimination of microbial pathogens.
INTRODUCTION
Nontyphoidal Salmonella (NTS) is the greatest food-borne-disease burden in the United States, causing the most infections, hospitalizations, and deaths and 1.03 million illnesses annually (1, 2). The economic burden associated with the disease is dramatic, with the medical costs alone reaching $11.4 billion per year and substantial additional costs being incurred by the food industry (recalls, litigation, reduced consumer confidence) and by state, local, and federal public health agencies in response to NTS outbreaks (3). Globally, NTS is estimated to cause 93.8 million cases and 155,000 deaths each year (4), and NTS has emerged as the leading cause of bacteremia in sub-Saharan Africa, where its fatality rate reaches up to 25% (5, 6).
Salmonella enterica comprises six subspecies that are subdivided into more than 2,500 serovars (serological variants) on the basis of carbohydrate, lipopolysaccharide (LPS), and flagellar composition, with S. enterica subsp. enterica encompassing over 99% of human-pathogenic isolates (7–9). S. enterica infection can result in any of four distinct syndromes: enterocolitis/diarrhea, bacteremia, enteric (typhoid) fever, and chronic asymptomatic carriage (10, 11). Many serovars infect both humans and animals, whereby disease severity is a function of the serovar, strain virulence, and host susceptibility (10–13). The CDC has recently reported a plethora of Salmonella outbreaks in the United States (2011-2012) arising from the consumption of contaminated ground beef, turkey burgers, chicken livers, fish, cantaloupe, mangoes, papayas, pine nuts, alfalfa sprouts, dry dog food, and peanut butter or by exposure to any of a wide range of backyard animals (poultry, turtles, frogs, and hedgehogs [14]). The number of illnesses reported during each of these outbreaks may represent only a fraction of the total number of individuals affected, as it is estimated that for every confirmed case, there are as many as 30 that go unreported (1). Thus, an outbreak of 350 reported cases may, in fact, affect more than 10,000 people. Additionally, the false implication of food products can be devastating to a particular industry: tomatoes falsely implicated in the Salmonella jalapeno and Serrano pepper outbreak (2008) resulted in losses exceeding $200 million due to a dramatic reduction in tomato consumption (15).
The health and economic burden associated with Salmonella appears to be poised to worsen: the prolonged administration of antibiotics has resulted in the emergence of multidrug-resistant NTS strains that have disseminated worldwide. S. enterica subsp. enterica serovar Typhimurium DT104, for example, which has caused a heightened occurrence of food-borne disease outbreaks over the last 2 decades, is resistant to four of the five most commonly used antibiotics in veterinary medicine (tetracycline, β-lactams, aminoglycosides, and sulfonamides) (16, 17). These multidrug-resistant NTS strains are often more virulent, causing more hospitalizations and bacteremia (18, 19), and their maintenance in nature occurs at very low antibiotic concentrations that are commonly found in the environment, including groundwater (20). Also of concern is the recent isolation of hypervirulent Salmonella from natural microbial populations derived from livestock that are 100 times more virulent than other clinical isolates and are more capable of killing vaccinated animals (21). The NTS disease burden is thus predicted to be exacerbated, with the potential emergence of more potent multidrug-resistant strains that cause an increased health risk due to the lack of available antibiotics to treat infected patients (7, 22–24).
Salmonella control efforts remain challenging for a variety of reasons. Most livestock infections, for example, are subclinical (7, 25); disease outbreaks are sporadic and frequently caused by specific serotypes, although a diversity of serotypes are isolated from livestock production systems (7, 8, 14); and Salmonella survival/proliferation in the environment provides a continuous reservoir for livestock infection and a vehicle for cross-contamination from animal to human food products (26–29). The diversity of Salmonella serovars on farms and feedlots, the human disease manifestation dependence on the particular serovar, and the potential emergence of more potent multidrug-resistant strains highlight the medical need for rapid, reliable, and reproducible methods for strain detection and intraspecies strain discrimination.
A key challenge that prevents the rapid detection of microbial pathogens that cause invasive disease, such as Salmonella, is the often extremely low concentration of organisms in the relevant clinical (e.g., blood) or environmental (e.g., water) samples, which invariably requires amplification of either the bacteria themselves (via culturing) or their nucleic acids (via enzymatic methods) prior to detection (30). Conventional culturing methods are reliable but are laborious, time-consuming, and incompatible with field testing. Nucleic acid-based amplification, such as PCR, is commonly used for Salmonella detection and must be coupled to methods for the detection of amplification products via (i) the generic detection of the formation of double-stranded DNA (31), (ii) the detection of amplification products of specific lengths (32), or (iii) the sequence-specific detection of the amplified product (33, 34). These detection methods, however, are not without significant limitations: the generic detection of double-stranded DNA products can be complicated by spurious amplification, which produces double-stranded product; size-specific detection often requires cumbersome electrophoretic separation techniques that have proven difficult to fully miniaturize (35, 36); and sequence-specific detection has proven complex because traditional PCR amplification produces double-stranded DNA targets ill-suited for detection via hybridization (37). There thus remains a pressing need for convenient methods for coupling nucleic acid amplification with multiplexed, sequence-specific detection.
In response to the arguments presented above, we describe here the development of an integrated microfluidic electrochemical DNA (IMED) chip capable of the detection and intraspecies strain discrimination of closely related Salmonella serovars. Given the potentially wide applicability of such a platform, there are, of course, a myriad of different samples that we could have used to demonstrate its attributes. From among these we selected blood from a mouse model of septicemia because it is highly relevant to clinical diagnostics and because blood has historically proven a very challenging sample matrix (38, 39). Success with this matrix will thus inform the adaptability of the IMED chip to other sample substrates, including those relevant to food safety monitoring.
MATERIALS AND METHODS
Bacterial and animal strains.
Virulent S. Typhimurium reference strain ATCC 14028 (CDC 6516-60) and S. Choleraesuis strain χ3246, obtained from Roy Curtiss III, Center for Infectious Diseases and Vaccinology, Arizona State University, Tempe, AZ (40), were used in all studies. S. Typhimurium is a broad-host-range serovar typically associated with food-borne illness in humans (10, 11). S. Choleraesuis is a highly invasive host-adapted serovar that causes sepsis or extraintestinal focal infections in humans (41) and has recently been shown to generate hypervirulent strain variants during murine passage (21). Both S. Typhimurium and S. Choleraesuis cause a fatal bacteremia in mice (21, 40). Bacteria used for these studies were derived from stationary-phase cultures containing Luria-Bertani (LB) medium aerated at 37°C (42). Six- to 10-week old BALB/c mice were purchased from Charles River Laboratories. All mice were kept under specific-pathogen-free conditions at the Animal Resource Center, University of California, Santa Barbara.
Ethics statement.
All animal experimentation was conducted following the National Institutes of Health (NIH) guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after pertinent review and approval by the Institutional Animal Care and Use Committee at the University of California, Santa Barbara.
Salmonella infection and blood sampling.
Bacterial cells derived from overnight cultures were resuspended in 0.15 M NaCl and administered to BALB/c mice (103 CFU) via intraperitoneal (i.p.) injection (43). Blood (25 to 50 μl) from the tail vein of infected mice was sampled throughout the 5-day infection time course; additionally, blood from cardiac puncture (200 μl) was taken at day 5. Whole blood was collected into heparin-coated tubes (Microvette CB 300 LH; Sarstedt, Germany); 10 μl was used to determine the number of CFU by plating, and the remaining sample was used for IMED chip assays (3 μl per reaction). Whole-blood samples were stored at 4°C for up to 60 days prior to use and were added to the IMED chip assays without further purification/modification.
LAMP primer design and reaction conditions.
The loop-mediated isothermal amplification (LAMP) primers that we employed are specific for the target region in the recF genes in S. Typhimurium and S. Choleraesuis. The primers were designed with PrimerExplorer v4 software (http://primerexplorer.jp/e/), which selects certain primer sets on the basis of strict parameters, including melting temperature (Tm), resistance to the formation of secondary structure, G+C content, and the distance between primers along the target gene. The primer set that we developed (Table 1) consists of two outer (F3 and B3) and two inner (FIP and BIP) primers that amplify the recF gene in the vast majority of Salmonella serovars but produces products that differ significantly in their single-stranded regions and thus allow strain-specific discrimination. All primers were ordered from Integrated DNA Technologies (Coralville, IA) and used as received.
Table 1.
LAMP primers that amplify the Salmonella recF target region for strain discriminationa
| Primer | Sequence 5′–3′ | Gene position |
|---|---|---|
| FIP (F2 + F1c) | GATAAGCAGGGCGACAGCAA-CAGCTCCGCGATCTTGTG | 794–813 + 751–768 |
| BIP (B2 + B1c) | AGCCAATCGACGTCTCACGC-AGCAGGAAGCGTTTGTTCTT | 822–841 + 865–884 |
| B3 | CCTCCGGCGTAATCAACTG | 714–732 |
| F3 | GCCGCGTGATTCGTCATG | 885–902 |
The LAMP primer set used in these studies consists of two inner (FIP and BIP) and two outer (F3 and B3) primers that amplify the target region of the recF gene in the vast majority of Salmonella serovars. The products differ significantly in their single-stranded loop regions, allowing strain-specific discrimination (Fig. 2A and C). FIP comprises regions F2 and F1c (F2 + F1c) within the recF gene (Fig. 2A); F1c indicates the sequence complementary to the F1 region. Similar notation is used for the BIP primer.
DNA templates comprising either purified genomic DNA (from a stock of 1 ng/μl diluted in buffer or 200 copies/μl [QIAamp DNA minikit; Qiagen, Chatsworth, CA]) or bacterial cells (103 to 106 CFU/ml diluted in buffer or whole blood) were added to 25 μl (tube reactions) or 35 μl (IMED chip reactions) of a LAMP reaction mixture containing the following: 20 mM Tris buffer, 10 mM KCl, 2 mM MgSO4, 10 mM (NH4)2SO4, 0.10% Triton X-100, 0.8 M betaine, 1.4 mM (each) the four deoxynucleotide triphosphates, 16 units of Bst DNA polymerase (New England BioLabs, Inc., Beverly, MA), 1% (vol/vol) bovine serum albumin, 3.2 μM (each) the inner primers, and 0.4 μM (each) the outer primers. Control reactions performed in micro-Eppendorf tubes were run in a standard thermal cycler at 65°C. IMED chip experiments were run on a heat block at 65°C. LAMP products were analyzed using PAGE (4 to 20% TBE [Tris-borate-EDTA] gel; Invitrogen, Carlsbad, CA) and stained with SYBR Gold (Invitrogen, Carlsbad, CA).
Electrochemical DNA (E-DNA) probes and synthetic target DNA.
Two oligonucleotide probe sequences complementary to the Salmonella recF gene were ordered from Fidelity Systems, Inc. (Gaithersburg, MD). The 5′ end of these probes was modified with a flexible Letsinger trihexylthiol described previously (44), and the 3′ end of the molecule was modified with a methylene blue (MB) redox reporter (Table 2). To accurately calibrate the sensors for detection of LAMP products, synthetic oligonucleotides equivalent to the smallest expected LAMP products (here termed “barbells,” which describes the secondary structure that they adopt) were also obtained via commercial synthesis (Table 2). An equivalent barbell structure lacking the target sequence was employed as a negative control. These initial calibration tests with synthetic amplicons were performed at room temperature with the sensor in buffer alone to rule out nonspecific binding. All other tests were performed at 65°C (as described in the Fig. 1 legend).
Table 2.
Sequences for Salmonella serovar-specific E-DNA target regions, synthetic LAMP target amplicons, and E-DNA probes
| DNA and serovar | Sequencea (5′–3′) | Gene position |
|---|---|---|
| E-DNA target region | ||
| S. Typhimurium | CTTCGCTCTGTAATCGCCCGTG | 843–864 |
| S. Choleraesuis | CTTCGCCCTGCAGGCGCCCGTG | 843–864 |
| Synthetic LAMP target amplicons | ||
| S. Typhimurium barbell | GATAAGCAGGGCGACAGCAAGCTCCGCGATCTTGTGACCGTCGGTACCGTCGATACGGACCTTGCTGTCGCCCTGCTTATCTTTGGTTAAGCCAATCGACGTCTCACGCTCTTCGCTCTGTAATCGCCCGTGAAGAACAAACGCTTCCTGCT GCGTGAGACGTCGATTGGCT | |
| S. Choleraesuis barbell | GATAAGCAGGGCGACAGCAAGCTCCGCGATCTTGTGACCGTCGGTACCGTCGATACGGACCTTGCTGTCGCCCTGCTTATCTTTGGTTAAGCCAATCGACGTCTCACGCTCTTCGCCCTGCAGGCGCCCGTGAAGAACAAACGCTTCCTGCTGCGTGAGACGTCGATTGGCT | |
| Nonspecific barbell | AGCCAATCGACGTCTCACGCAGCAGGAAGCGTTTGTTCTTCACGGGCGATTACAGAGCGAAGAGCGTGAGACGTCGATTGGCTTAACCAAAGATAAGCAGGGCGACAGCAAGGTCCGTATCGACGGTACCGACGGTCACAAGATCGCGGAGTTGCTGTCGCCCTGCTTATC | |
| E-DNA probe | ||
| S. Typhimurium | HS-trihexylthiol-CACGGGCGATTACAGAGCGAAG-(CH2)7-NH-MB | |
| S. Choleraesuis | HS-trihexylthiol-CACGGGCGCCTGCAGGGCGAAG-(CH2)7-NH-MB | |
Strain differences are in bold, and binding regions are underlined. MB, methylene blue.
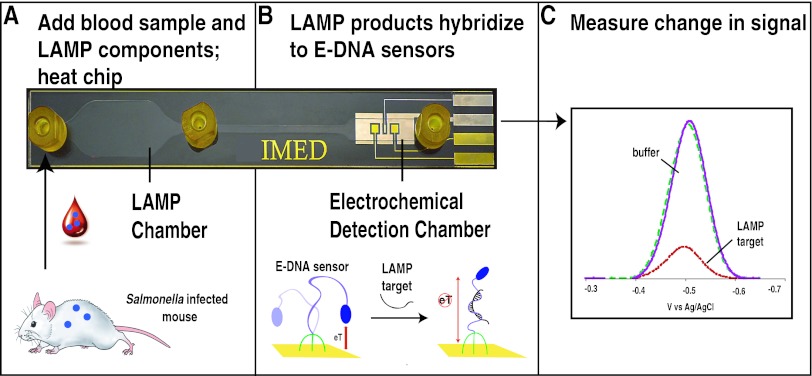
Fig 1.
Overview of the IMED chip. (A) The IMED chip consists of an amplification chamber that supports LAMP, a rapid amplification method that produces single-stranded products for hybridization-based detection. In the approach, bacteria from whole unprocessed blood of infected animals are added to the isothermal amplification chamber along with amplification reagents and heated at 65°C for 90 min. The products, which now include LAMP target amplicons, are then pushed into the electrochemical detection chamber via the injection of buffer. (B) The chamber contains a multiplexed electrode array (here biplex) supporting the simultaneous sequence-specific electrochemical detection of LAMP products derived from S. Typhimurium and S. Choleraesuis. The E-DNA probes used to sense the LAMP amplicons comprise a linear, redox-reporter-modified DNA molecule covalently attached to a gold surface. When the complementary LAMP target amplicons are absent, the flexibility of this single-stranded probe ensures that the end-located redox reporter collides freely with the electrode surface, producing a large current. (C) Conversely, upon hybridization to the target amplicon, the probe becomes rigid, restricting movement of the redox reporter and reducing the observed current. The potentials reported here and throughout this publication are relative to an Ag/AgCl reference electrode.
E-DNA sensor and IMED chip preparation.
The electrochemistry of E-DNA sensors has been extensively explored in the prior literature (45, 46). Polycrystalline gold disk rod electrodes (diameter, 2 mm) were employed for the initial characterization of the relevant E-DNA sensors. To prepare the electrodes, the exposed gold surfaces were manually hand polished by gently scrubbing against a felt cloth containing 1 μM diamond and 0.5 μM alumina slurries, followed by a brief sonication for 5 min. The electrodes were then electrochemically cleaned using a series of oxidation or reduction cycles in 0.5 M H2SO4, 0.01 M KCl or 0.1 M H2SO4, and 0.5 M H2SO4. The E-DNA probes were treated with 4 mM TCEP [Tris(2-carboxy-ethyl)phosphine hydrochloride] to reduce any disulfides present and then deposited on the freshly cleaned gold surface by incubation for 1 h at a concentration of 0.1 μM in 2× saline sodium citrate (SSC) buffer (300 mM NaCl plus 30 mM trisodium citrate, pH 7.0). The surface was then rinsed with distilled, deionized water and then subsequently passivated with 6-mercaptohexanol in 2× SSC buffer overnight. Prior to use in experiments, the electrodes were allowed to sit in 2× SSC buffer for at least 30 min at room temperature to equilibrate the sensor. Reagent-grade chemicals, including 6-mercapto-1-hexanol, sulfuric acid, and SSC buffer (all from Sigma-Aldrich, St. Louis, MO) were used without further purification.
Design and fabrication of the IMED chips followed protocols described previously (47). The microfabricated gold electrodes on the IMED chip do not require manual polishing steps postfabrication and are prepared using electrochemical cleaning. Since the IMED chips contain both gold pixel surfaces within a single enclosed chamber, differential labeling of the pixels requires a multistep procedure, which includes subsequent immobilization and electrochemical stripping steps (48). First, both pixels on the chip were immobilized with the S. Typhimurium-specific E-DNA probe and passivated with 6-mercaptohexanol in the same manner as the rod electrodes described above. Next, one pixel was selected and a low potential (−1.4 V versus Ag/AgCl) was applied and held for ∼90 s to electrochemically strip the gold surface of its monolayer. S. Choleraesuis-specific E-DNA probe was then injected into the chamber to immobilize onto the exposed gold pixel, followed by passivation with 6-mercaptohexanol, also as described above. Square-wave voltammetry (SWV) was performed in 6× SSC buffer between each step to verify that proper absorption/desorption of the monolayer was completed. The rod electrodes were differentially labeled by incubating each electrode in individual tubes containing either S. Typhimurium or S. Choleraesuis E-DNA probes.
E-DNA sensor and IMED chip measurements.
Electrochemical measurements on rod electrodes and IMED chips were performed on a CHI 660D potentiastat (CH Instruments, Austin, TX). Rod electrodes were measured with a platinum counter electrode and an Ag/AgCl (3 M NaCl) reference electrode. Both rod and IMED chip measurements were taken using SWV at 60 Hz and a 25-mV amplitude over a potential range of −0.05 to −0.50 V. The IMED chips, which can, in theory, be reused (49), are inexpensive to fabricate and thus were used only a single time to reduce the risk of cross contamination. Labeled gold rod electrodes may be used multiple times. A flush of deionized water for approximately 60 s removes the target DNA from the sensors and regenerates them for future use (49). For all experiments, each data point represents average readings from three independently fabricated sensors or chips. The means and standard deviations were calculated using Microsoft Excel and Kaleidagraph software.
RESULTS
IMED chip for detection and discrimination of Salmonella serovars.
S. enterica can cause any of a wide range of human diseases depending on the S. enterica subsp. enterica serovar (10, 11), exemplifying the pressing need for diagnostics capable of rapid detection and serovar discrimination. Toward this goal, we have developed an IMED microfluidic chip capable of performing amplification and sequence-specific, multiplexed detection of Salmonella on a single device. The IMED chip consists of an amplification chamber that supports LAMP, a single-temperature amplification method that produces single-stranded products amenable to hybridization-based detection (for a review, see references 50 to 52). The LAMP amplification chamber is connected via a microchannel that allows dilution and transfer of the complete LAMP products to the electrochemical detection chamber containing a multiplexed (here biplex) sensing array for sequence-specific E-DNA detection of the LAMP products (Fig. 1). Each E-DNA sensor array contains two sequence-specific DNA probes, each of which is specific for a given bacterial strain. Validation of the IMED device was carried out on S. enterica subsp. enterica serovars Typhimurium and Choleraesuis. These two serovars, the causative agents of enterocolitis and sepsis in humans, respectively, are quite closely related (homologous genes of Salmonella serovars have over 97% DNA sequence identity) and thus serve as a particularly challenging test of the specificity of our platform (41, 53–58; reviewed in reference 59).
LAMP of Salmonella-specific sequences.
A LAMP reaction of Salmonella-specific sequences requires the identification of DNA primers that recognize a region of the Salmonella genome that is sufficiently conserved—such that a single set of LAMP primers amplifies the region in a large subset of pathogenic Salmonella serovars—yet divergent enough that internal sequences within the amplicon can readily discriminate closely related serovars (60–62). To this end, we employed PrimerExplorer v4 software to design a primer set for the amplification of a region in the recF gene, a well-conserved gene encoding the RecF gap repair protein (63, 64). These LAMP primers consist of two outer (F3 and B3) and two inner (FIP and BIP) primers that should amplify the recF gene in Salmonella serovars, producing barbell-shaped DNA molecules possessing target-containing, single-stranded loops varying by 4 bases between S. Typhimurium and S. Choleraesuis (Fig. 2; Table 2). In terms of specificity, we designed these LAMP primers to amplify the recF gene, the sequence of which is >98% identical across the Salmonella serovars Typhimurium, Choleraesuis, Newport, Dublin, and Enteritidis (BLAST nucleotide sequence comparison). Given this, the primer set is expected to amplify a large fraction of all clinically relevant Salmonella serovars. However, the single-stranded regions in the recF gene produced in the resultant amplicons in this study were specifically designed to discriminate S. Typhimurium from S. Choleraesuis.
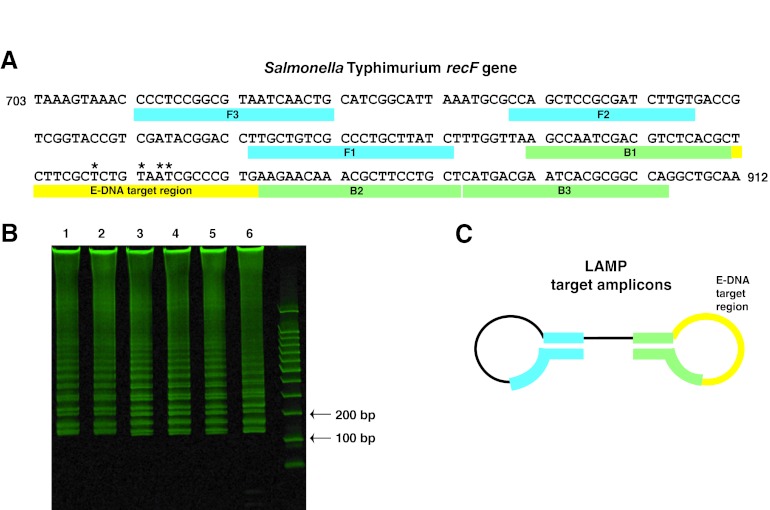
Fig 2.
Salmonella recF-specific primers produce LAMP target amplicons that contain an E-DNA target region. (A) A LAMP primer set was designed that amplified a region of the recF gene in Salmonella that contains a 22-base, single-stranded E-DNA target region (yellow), shown here for S. Typhimurium. *, bases that differ between the serovars. (B) A polyacrylamide gel loaded with triplicate LAMP reactions of S. Typhimurium (lanes 1 to 3) and S. Choleraesuis (lanes 4 to 6). The LAMP products derived from different serovars differ in sequence but are indistinguishable in terms of product lengths. (C) The lowest-molecular-size LAMP products that we detect are ∼150-base, barbell-like structures with a single-stranded loop region (in yellow) containing a 22-base, single-stranded sequence complementary to the E-DNA probes (Table 2).
Before we employed the approach on chip, we explored the LAMP protocol in micro-Eppendorf tubes using S. Typhimurium and S. Choleraesuis genomic DNA. Using the recF-specific primer set on both S. Typhimurium and S. Choleraesuis DNA, LAMP produced the characteristic ladder-like banding pattern (due to products of various sizes) on polyacrylamide gels indicative of positive amplification (Fig. 2B). Of note, although the amplicon products produced differ in sequence from serovar to serovar, they are identical in size, and thus, they cannot be distinguished by electrophoresis or other sized-based detection schemes. We confirmed the specificity of the LAMP reactions via control experiments either lacking template DNA or employing genomic DNA prepared from Shigella flexneri (ATCC 12022). As expected, neither of these negative controls resulted in a corresponding ladder pattern (data not shown).
E-DNA sensor characterization and detection of Salmonella-specific DNA sequences.
For the detection of LAMP products, we have employed serovar-specific linear probe E-DNA sensors (45, 49, 65). These sensors comprise a linear, single-stranded, redox-tagged DNA molecule covalently attached to a gold surface via a self-assembled monolayer (Fig. 1B) (45, 49, 65). Upon hybridization to a cDNA target (e.g., amplified sequences within the recF gene), the redox reporter (methylene blue) located on the distal end of the linear probe is held in a position fixed away from the electrode surface, reducing the observed redox current (Fig. 1C).
E-DNA sensors demonstrate a high level of specificity and selectivity, with mismatch discrimination down to 3 base pairs and detection capabilities in a wide array of clinical samples (e.g., blood, serum, soil, and foodstuffs [49, 66, 67]). In this study, the single-stranded regions produced in the LAMP amplicons, which are detected by their cognate E-DNA sensors, were specifically designed to discriminate S. Typhimurium and S. Choleraesuis, which, while closely related at the sequence level, nevertheless differ by 4 bases in the single-stranded recognition element present in our LAMP amplicons. Discrimination of additional Salmonella serovars is achievable on the IMED chip by modifying the E-DNA sensor sequence toward detection of additional targets.
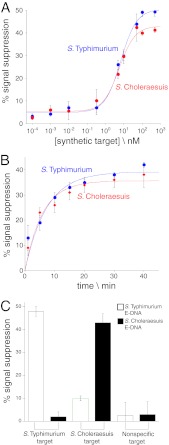
To characterize the sensors for use on the chip, the S. Typhimurium and S. Choleraesuis specific E-DNA sensors were first fabricated on gold disk rod electrodes and examined for sensitivity, kinetics, and cross-reactivity. To assess quantitatively the ability of E-DNA sensors to detect LAMP target amplicons, we employed synthetic DNA analogs of the lowest-molecular-size amplification products (Fig. 2C; Table 2). Synthetic amplicons were used to ensure that identical concentrations of single-stranded elements were present in all samples since the heterogeneous nature of LAMP products could otherwise lead to ambiguity. Titrating with these synthetic LAMP target amplicons, we found that our S. Typhimurium and S. Choleraesuis E-DNA sensors both recognize their cognate target amplicon at concentrations as low as 10 nM and saturate at ∼100 nM (Fig. 3A). No signal was observed when E-DNA sensors were tested with nonspecific barbell-shaped DNA (data not shown). To determine sensor response times, we incubated sensors with saturating concentrations (100 nM) of their cognate synthetic amplicon, finding that both sensors equilibrate with time constants of ∼6 min, even when they are challenged at room temperature to rule out nonspecific binding (Fig. 3B). No signal was observed when E-DNA sensors were tested with nonspecific barbell-shaped DNA (data not shown). Finally, we tested for cross-reactivity to rule out potential false positives arising from signal cross talk in our biplex devices. To do so, we tested each sensor against 100 nM cognate target (e.g., the S. Typhimurium E-DNA sensor with the S. Typhimurium target and the S. Choleraesuis E-DNA sensor with the S. Choleraesuis target) and noncognate target (the reverse). While both sensors exhibited high signal suppression (48% ± 2% and 43% ± 4%, respectively) in the presence of their appropriate target, the target suppression seen with the other sensor's target was significantly smaller (2% ± 2% and 10% ± 1%, respectively), with reported signal changes comparable to the nonspecific negative control value (Fig. 3C). This said, some cross-reactivity is seen when the S. Typhimurium sensor is challenged with the S. Choleraesuis target due to the nonzero affinity of this target-probe pair; the two serovars that we have used in our study are very closely related, and thus, there is significant complementarity even between the incorrect probe-target pair. No signal was observed when the E-DNA sensors were tested with synthetic barbell targets that possess a single-stranded loop region containing a sequence nonspecific (Table 2) to either sensor (Fig. 3C).
Fig 3.
Salmonella E-DNA sensors exhibit rapid and sensitive equilibration kinetics and can discriminate Salmonella serovars. IMED chips were fabricated to contain E-DNA sensors for the detection of both S. Typhimurium and S. Choleraesuis. (A) The analytical sensitivity of each E-DNA sensor was examined by challenging with synthetic LAMP target amplicons. (B) The equilibration kinetics of the sensors was characterized by injecting saturating (100 nM) concentrations of their cognate synthetic target amplicon. (C) The cross-reactivity of each sensor was examined by challenging it with saturating concentrations (100 nM) of its own target, the target of the other sensor, and a nonspecific DNA that neither sensor targets (Table 2). All electrochemical measurements in this figure and Fig. 4 and 5 were made using a CHI 660D potentiastat (CH Instruments, Austin, TX) (see Materials and Methods). The data points and error bars represent the means and standard deviations of measurements from three independently fabricated sensors/chips. The error bars are dominated by the sensor-to-sensor variability arising from the variation in the fabrication of individual sensors/chips.
IMED chip characterization of Salmonella serovars in rat blood.
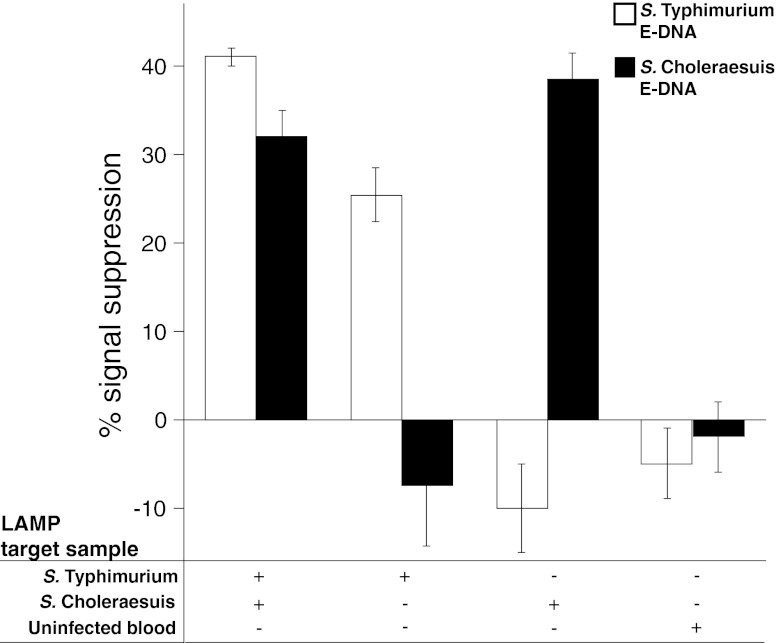
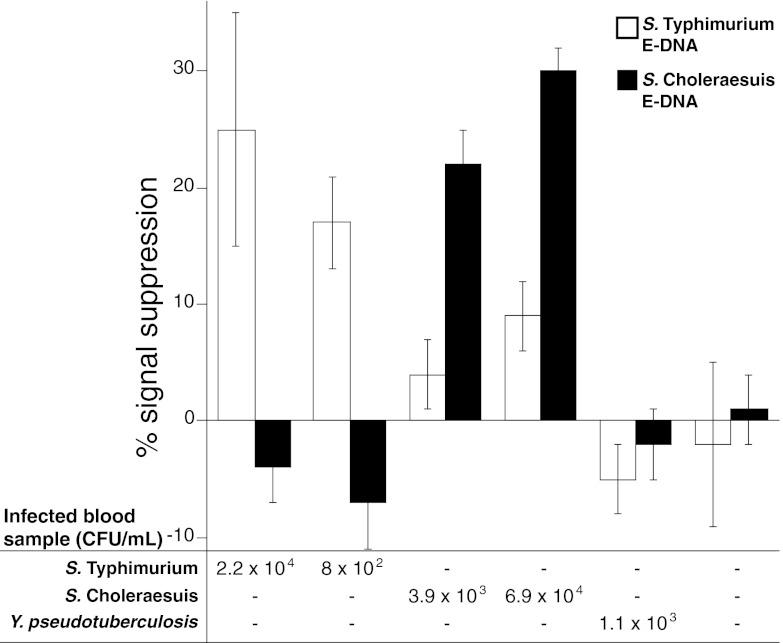
Before initiating the murine model of Salmonella for testing, we performed preliminary validation of the IMED device with LAMP on commercially available rat blood samples with the addition of ∼1,000 CFU/ml of either or both S. Typhimurium and S. Choleraesuis. Spiked blood samples (3 μl each) were added to the LAMP reaction components (35 μl total) and injected into the LAMP chamber on the chip. The chip was set on a heat block for 90 min, and the resulting products were pushed via buffer injection into the electrochemical detection chamber, where the E-DNA sensors reside for electrochemical measurement. IMED chips loaded with blood spiked with both S. Typhimurium and S. Choleraesuis generated a signal response for both E-DNA sensors, with responses of 41% ± 1% on the S. Typhimurium E-DNA sensor and 32% ± 3% on the S. Choleraesuis E-DNA sensor (Fig. 4). To discern the level of cross-reactivity between these two serovars, the IMED chips were loaded with blood spiked with either S. Typhimurium or S. Choleraesuis. The IMED chips responded only to the particular serovar loaded into the IMED chip (Fig. 4). No signal was observed when E-DNA sensors were tested in LAMP buffer with uninfected blood. Note that negative signals can arise when large amounts of nonspecific DNA are applied. Nontarget DNA can interact with the E-DNA sensors in a nonspecific manner near the electrode surface, as is the case when the S. Choleraesuis LAMP target is applied to an S. Typhimurium E-DNA sensor. These data indicate that the IMED chip-based assay is capable of detecting both Salmonella serovars at once with minimal cross-reactivity between S. Typhimurium and S. Choleraesuis, such that discrimination between the serovars is evidenced by distinct E-DNA sensor responses.
Fig 4.
IMED chip characterization of Salmonella serovars in commercially available rat blood. IMED chips containing E-DNA sensors for S. Typhimurium and S. Choleraesuis were loaded with LAMP reaction mixtures containing commercial rat blood spiked with either S. Typhimurium or S. Choleraesuis or not spiked with bacteria. The data points and error bars represent the means and standard deviations of measurements from three independently fabricated sensors/chips. Measurements of numbers of CFU/ml were determined by direct colony counting.
Clinically sensitive detection and identification of Salmonella serovars in whole, unprocessed blood from septic mice.
To demonstrate the clinical utility of the IMED chip, we tested the performance of the assay on the detection and discrimination of closely related Salmonella serovars in a murine model of typhoid fever, causing a fatal bacteremia resulting in bacteria in lymphoid tissues, blood, and visceral organs. BALB/c mice were infected via the intraperitoneal route of infection with a lethal dose (1,000 CFU, which is 100 times the 50% lethal dose) of either Salmonella reference strain ATCC 14028 or S. Choleraesuis strain χ3246 (21, 40). Blood (25 to 50 μl) was collected from the tail vein of mice at day 5 postinfection (late stage), 3 μl of which was added to the LAMP reaction components (total volume, 35 μl), and the mixture was injected into the amplification chamber of the IMED chip. An additional 10 μl was reserved to determine the number of CFU by direct colony counting upon plating (detection limit, ∼100 CFU/ml). Using these samples, we found that the IMED chip assay can detect and discriminate between S. Typhimurium and S. Choleraesuis serovars derived from whole, unprocessed blood, where the bacterial load ranged from 8 × 102 to 6.9 × 104 CFU/ml (Fig. 5). To assess the specificity of the IMED chip, we analyzed blood from uninfected mice and mice infected with Yersinia pseudotuberculosis, which also causes fatal bacteremia. The IMED chip assay detected little or no signal from blood samples from uninfected mice or from septic animals with Yersinia bacteremia at bacterial loads as high as 1.1 × 103 CFU/ml. These data indicate that the IMED chip assay is specific for Salmonella sequences in blood samples from septic mice.
Fig 5.
IMED chip-based detection and intraspecies strain discrimination of Salmonella serovars derived from whole blood of septic mice. IMED chips containing sensors for S. Typhimurium and S. Choleraesuis were loaded with LAMP reaction mixtures containing blood samples derived from septic mice infected with S. Typhimurium, S. Choleraesuis, or Y. pseudotuberculosis or from an uninfected mouse. The data points and error bars represent the means and standard deviations of measurements from three independently fabricated sensors/chips. Measurements of numbers of CFU/ml were determined by direct colony counting.
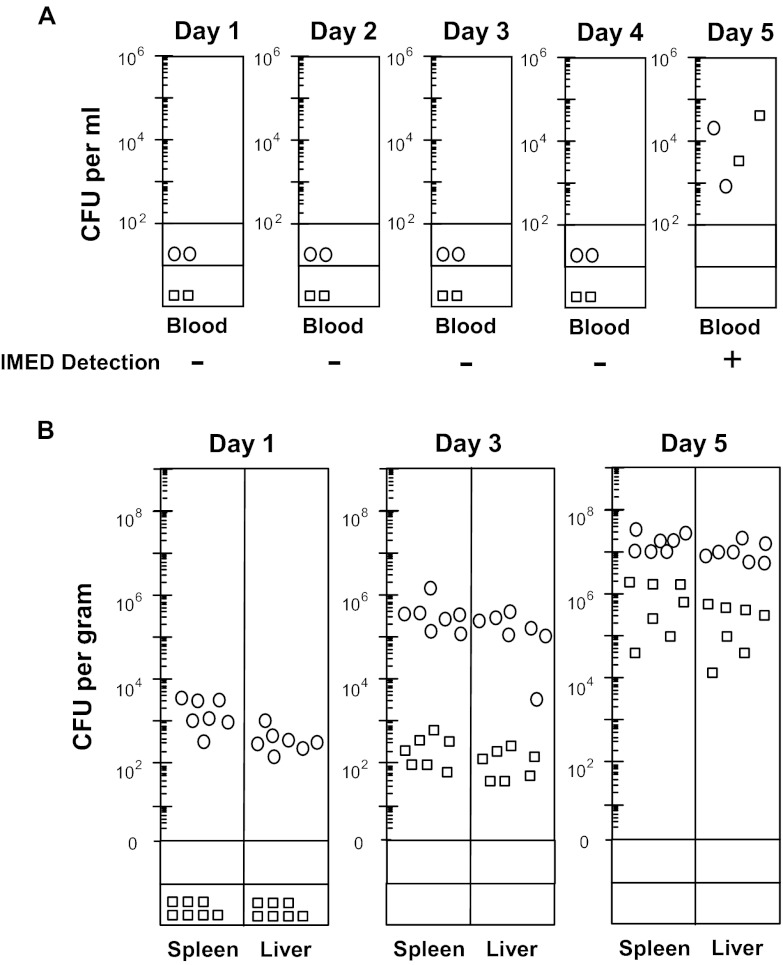
The sensitivity of the IMED device was compared to that of direct colony counting by using both detection methods on blood collected from the tail vein of i.p. infected mice throughout the 5-day infection time course. Salmonella bacteremia was not detected by either method until day 5 postinfection (late stage), when the bacterial load in the blood ranged from ∼103 to 105 CFU/ml (Fig. 6A). These data indicate that both methods are equally sensitive for detecting Salmonella bacteremia, where the bacterial load in the blood is extremely low until late stages of infection (limit of detection by plating, ∼100 CFU/ml). In contrast, colonization of the liver and spleen was more rapid and progressed to very high levels throughout the 5-day time course postinfection (Fig. 6B). Thus, although IMED can rapidly (within 2 h) detect Salmonella at clinically relevant blood concentrations of <1,000 CFU/ml, these findings exemplify the challenge of detecting invasive pathogens that are often present in the blood at extremely low concentrations until end-stage infection, even though mucosal and systemic tissues may be highly colonized at much earlier stages of infection. Moreover, the microbial infection kinetics of blood and host tissue sites may vary widely between and within microbial species (21), posing an additional challenge to the design and implementation of blood-based pathogen detection systems.
Fig 6.
Comparison of IMED device and direct colony counting sensitivity for detection of Salmonella serovars derived from whole blood of septic mice. BALB/c mice were infected i.p. (103 CFU) with S. Typhimurium ATCC 14028 (circles) or S. Choleraesuis χ3246 (boxes). (A) Blood collected from the tail vein throughout the 5-day infection time course was assayed for bacteria via the IMED device or by direct colony counting (detection limit, ∼100 CFU/ml). + and −, IMED signal detection and no detection, respectively. (B) Bacterial colonization of the liver and spleen (numbers of CFU) at days 1, 3, and 5 day postinfection was determined by direct colony count (detection limits, <40 CFU for spleen and <20 CFU for liver).
DISCUSSION
The FDA and USDA have made the development of improved methods for Salmonella monitoring from farm to fork a major priority to limit the size, frequency, and severity of food-borne outbreaks as well as to reduce the false implication of other food products to avoid catastrophic economic losses (15, 68–70). Improved monitoring of salmonellae will prevent contamination of food and water supplies at the outset and, in turn, significantly reduce pathogen exposure, transmission, animal disease, and the direct contamination of livestock-derived food products as well as the indirect contamination of fruit and vegetable food products via contaminated water. In response, we have established the utility of a microfluidic IMED chip to distinguish two closely related Salmonella serovars using blood samples derived from a murine model of septicemia. The extensive DNA similarity between Salmonella serovars (homologous genes having greater than 97% DNA sequence identity; reviewed in reference 59) serves as a demanding test of the specificity of our platform, and blood has proven to be a very challenging sample matrix. The IMED device facilitated rapid (<2 h) detection and intraspecies strain discrimination of S. Typhimurium and S. Choleraesuis directly from undiluted, unmodified whole-blood samples derived from infected animals at clinically relevant concentrations (<1,000 CFU/ml), pushing preparation requirements to an absolute minimum. The IMED platform is readily adaptable to diverse sample substrates in a number of potential commercial applications, including those relevant to clinical diagnostics (blood, urine, stool), food safety (stool, food matrices), and environmental/farm management (fecal pack, soil, surface water).
The IMED chip is an affordable assay—we estimate that current fabrication costs are about $15, with the potential for $1-per-chip costs with larger batch preparations—and can be configured for the simultaneous detection of multiple pathogens by changing the LAMP amplification primer set and the E-DNA probe sequences. This is evidenced by the clinical utility, specificity, and sensitivity of the LAMP reaction that has been established for the detection of pathogens, including Mycobacterium tuberculosis from sputum (71), Plasmodium falciparum (72) and Trypanosoma brucei (73) from blood, and dengue virus from serum (60). There have also been recent developments in the private sector, as Eiken Chemical Company is releasing two new clinical LAMP assays in the near future (74). The first is the Loop amp tuberculosis complex detection reagent kit for the detection of M. tuberculosis from untreated sputum samples. The second is a field-deployable test for human African trypanosomiasis, which is currently entering on-site field trials in Uganda and Congo with expected release later in 2013. Collectively, these LAMP studies provide a solid foundation for the development of the relatively nascent field of nucleic acid-based, point-of-care, or even field-portable pathogen detection.
The IMED diagnostic platform may have utility in hospitals as well as makeshift medical facilities for strain discrimination of a variety of pathogens. Streptococcus pneumoniae, for example, is a major cause of pneumonia, sepsis, and meningitis in the developed and developing world (75), and patient disease outcome is significantly improved if treatment occurs before the onset of the thrombohemorrhagic pathology of sepsis termed disseminated intravascular coagulation (DIC)—or “death is coming,” in physicians' vernacular. There are more than 90 pneumococcal capsular serotypes, each exhibiting a different prevalence in human populations and clinical disease outcome (i.e., pneumonia, septicemia, meningitis, infective complications of chronic obstructive pulmonary disease [76–78]). Improved methods for early detection and discrimination of S. pneumoniae capsular serotypes would thus be a major advance for clinicians and could be potentially achievable using the IMED device based on nucleic acid sequence differences between clinically relevant strains.
A key challenge that prevents the rapid detection of pathogens is the often extremely low concentration of pathogens in the blood. Clinically relevant HIV loads, for example, can be lower than 50 copies/ml in blood plasma (79, 80), and bacterial loads in the blood of septicemic patients are rarely above 1,000 CFU/ml (81). This is supported by our findings in a murine model of typhoid fever, in which blood CFU levels are below the detection limit of IMED or direct colony count methods until end-stage infection (limit of detection by plating, ∼100 CFU/ml). Patients may harbor a low pathogen load in the blood, and the capacity for early detection and monitoring the pathogen load in real time would significantly improve the utility of IMED. One limitation of the current IMED architecture is its rather small chamber size; LAMP amplification fails in whole blood at dilutions of more than ∼10%, and thus, the 35-μl volume of the LAMP chamber on our first-generation IMED chip precludes the detection of bacterial loads of less than ∼1 CFU/3.5 μl, or ∼350 CFU/ml. Given that this is purely a volumetric constraint, a severalfold improvement in the detection limit of the IMED chip should be easily realizable. Further, recent advances in IMED technology, termed microfluidic electrochemical quantitative loop-mediated isothermal amplification (MEQ-LAMP), have resulted in an integrated microfluidic platform for the rapid, sensitive, and quantitative detection of pathogenic DNA in real time, which should prove useful in the clinical setting (82).
Point-of-care pathogen detection methods would have significant utility in medical, veterinary, and field applications in cases wherein rapid, on-site diagnosis is not feasible (cases of acute illness, nonambulatory patients/animals, contaminated equipment), expensive, or time-consuming. Such methods would be particularly useful if compatible with (i) a wide range of clinical and environmental samples (e.g., blood, feces, urine, sputum, manure, feedlot effluent), (ii) varied surfaces (for testing the effectiveness of methods of disinfection of hospital equipment and food processors), and (iii) the discrimination of clinically relevant strain variants associated with an increased incidence and severity of disease. Implementation of the IMED device in risk-management strategies may promote human and animal health, reduce contamination of livestock-derived food products, and enhance food safety.
ACKNOWLEDGMENTS
This work was supported by the G. Harold and Leila Y. Mathers Foundation, the U.S. Army (W911NF-10-2-0111), the Agriculture and Food Research Initiative of the U.S. Department of Agriculture (USDA) (2008-01452) (to M.J.M.), and NIH (R01EB009764) (to H.T.S.). This work was also supported by the Institute for Collaborative Biotechnologies through grant W911NF-09-0001 from the U.S. Army Research Office.
The content of the information does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.
Footnotes
Published ahead of print 25 January 2013
REFERENCES
- 1. Gilliss D, Cronquist A, Cartter M, Tobin-D'Angelo M, Blythe D, Smith K, Lathrop S, Birkhead G, Cieslak P. 2011. Vital signs: incidence and trends of infection with pathogens transmitted commonly through food-foodborne diseases active surveillance network, 10 U. S. sites, 1996-2010. MMWR Morb. Mortal. Wkly. Rep. 60: 749– 755 [PubMed] [Google Scholar]
- 2. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17: 7– 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scharff RL. 2012. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 75: 123– 131 [DOI] [PubMed] [Google Scholar]
- 4. Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50: 882– 889 [DOI] [PubMed] [Google Scholar]
- 5. de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. 2012. Host-pathogen interaction in invasive salmonellosis. PLoS Pathog. 8: e1002933 doi:10.1371/journal.ppat.1002933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379: 2489– 2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2011. National Salmonella surveillance annual summary, 2008, p 1–5 US Department of Health and Human Services. Atlanta, GA [Google Scholar]
- 8. Centers for Disease Control and Prevention 2011. National Salmonella surveillance overview, p 1–12 US Department of Health and Human Services, Atlanta, GA: [Google Scholar]
- 9. Grimont PAD, Weill F-X. 2007. Antigenic formulae of the Salmonella serovars, 9th ed. WHO Collaborating Centre for Reference and Research on Salmonella, Paris, France: [Google Scholar]
- 10. Barrow PA, Jones NA, Thomson N. 2010. Salmonella, p 231–266 In Gyles C, Prescott J, Songer G, Thoen C. (ed), Pathogenesis of bacterial infections in animals, 4th ed. Wiley-Blackwell, Ames, IA [Google Scholar]
- 11. Coburn B, Grassl G, Finlay B. 2007. Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85: 112– 118 [DOI] [PubMed] [Google Scholar]
- 12. Tsolis RM, Adams LG, Ficht TA, Baumler AJ. 1999. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect. Immun. 67: 4879– 4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uzzau S, Brown DJ, Wallis T, Rubino S, Leori G, Bernard S, Casadesus J, Platt DJ, Olsen JE. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 125: 229– 255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention 2012. Reports of selected Salmonella outbreak investigations. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/salmonella/outbreaks.html [Google Scholar]
- 15. Maki DG. 2009. Coming to grips with foodborne infection—peanut butter, peppers, and nationwide Salmonella outbreaks. N. Engl. J. Med. 360: 949– 953 [DOI] [PubMed] [Google Scholar]
- 16. Arcangioli MA, Leroy-Setrin S, Martel JL, Chaslus-Dancla E. 1999. A new chloramphenicol and florfenicol resistance gene flanked by two integron structures in Salmonella typhimurium DT104. FEMS Microbiol. Lett. 174: 327– 332 [DOI] [PubMed] [Google Scholar]
- 17. Cloeckaert A, Schwarz S. 2001. Molecular characterization, spread and evolution of multidrug resistance in Salmonella enterica Typhimurium DT104. Vet. Res. 32: 301– 310 [DOI] [PubMed] [Google Scholar]
- 18. Glynn MK, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo FJ. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338: 1333– 1339 [DOI] [PubMed] [Google Scholar]
- 19. Varma JK, Molbak K, Barrett TJ, Beebe JL, Jones TF, Rabatsky-Ehr T, Smith KE, Vugia DJ, Chang HGH, Angulo FJ. 2005. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis. 191: 554– 561 [DOI] [PubMed] [Google Scholar]
- 20. Gullberg E, Cao S, Berg OG, Ilback C, Sandegren L, Hughes D, Andersson DI. 2011. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 7: e1002158 doi:10.1371/journal.ppat.1002158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heithoff DM, Shimp WR, House JK, Xie Y, Weimer BC, Sinsheimer RL, Mahan MJ. 2012. Intraspecies variation in the emergence of hyperinfectious bacterial strains in nature. PLoS Pathog. 8:e1002647 doi:10.1371/journal.ppat.1002647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brichta-Harhay DM, Arthur TM, Bosilevac JM, Kalchayanand N, Shackelford SD, Wheeler TL, Koohmaraie M. 2011. Diversity of multidrug-resistant Salmonella enterica strains associated with cattle at harvest in the United States. Appl. Environ. Microbiol. 77:1783– 1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hur J, Jawale C, Lee JH. 2012. Antimicrobial resistance of Salmonella isolated from food animals: a review. Food Res. Int. 45: 819– 830 [Google Scholar]
- 24. Parry CM, Threlfall E. 2008. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 21: 531– 538 [DOI] [PubMed] [Google Scholar]
- 25. Anderson RJ, House JK, Smith BP, Kinde H, Walker RL, Vande Steeg BJ, Breitmeyer RE. 2001. Epidemiologic and biological characteristics of salmonellosis in three dairy herds. J. Am. Vet. Med. Assoc. 219: 310– 322 [DOI] [PubMed] [Google Scholar]
- 26. Fossler C, Wells S, Kaneene J, Ruegg P, Warnick L, Bender J, Eberly L, Godden S, Halbert L. 2005. Herd-level factors associated with isolation of Salmonella in a multi-state study of conventional and organic farms. I. Salmonella shedding in cows. Prev. Vet. Med. 70: 257– 277 [DOI] [PubMed] [Google Scholar]
- 27. Fossler C, Wells S, Kaneene J, Ruegg P, Warnick L, Bender J, Eberly L, Godden S, Halbert L. 2005. Herd-level factors associated with isolation of Salmonella in a multi-state study of conventional and organic farms. II. Salmonella shedding in calves. Prev. Vet. Med. 70: 279– 291 [DOI] [PubMed] [Google Scholar]
- 28. Ruzante J, Lombard J, Wagner B, Fossler C, Karns J, Van Kessel J, Gardner I. 2010. Factors associated with Salmonella presence in environmental samples and bulk tank milk from US dairies. Zoonoses Public Health 57: e217– e225 doi:10.1111/j.1863-2378.2010.01333.x [DOI] [PubMed] [Google Scholar]
- 29. Smith B, Da Roden L, Thurmond M, Dilling G, Konrad H, Pelton J, Picanso J. 1994. Prevalence of salmonellae in cattle and in the environment on California dairies. J. Am. Vet. Med. Assoc. 205: 467– 471 [PubMed] [Google Scholar]
- 30. Bergeron MG. 2008. Revolutionizing the practice of medicine through rapid (< 1h) DNA-based diagnostics. Clin. Invest. Med. 31: E265– E271 [DOI] [PubMed] [Google Scholar]
- 31. Ihmels H, Faulhaber K, Vedaldi D, Dall'Acqua F, Viola G. 2005. Intercalation of organic dye molecules into double-stranded DNA. Part 2. The annelated quinolizinium ion as a structural motif in DNA intercalators. Photochem. Photobiol. 81: 1107– 1115 [DOI] [PubMed] [Google Scholar]
- 32. Kricka LJ. 2002. Stains, labels and detection strategies for nucleic acids assays. Ann. Clin. Biochem. 39: 114– 129 [DOI] [PubMed] [Google Scholar]
- 33. Call D, Borucki M, Loge F. 2003. Detection of bacterial pathogens in environmental samples using DNA microarrays. J. Microbiol. Methods 53: 235– 243 [DOI] [PubMed] [Google Scholar]
- 34. Keim P, Price LB, Klevytska AM, Smith KL, Schupp JM, Okinaka R, Jackson PJ, Hugh-Jones ME. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182: 2928– 2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen L, Manz A, Day PJR. 2007. Total nucleic acid analysis integrated on microfluidic devices. Lab. Chip 7: 1413– 1423 [DOI] [PubMed] [Google Scholar]
- 36. Viovy J. 2000. Electrophoresis of DNA and other polyelectrolytes: physical mechanisms. Rev. Mod. Phys. 72: 813– 872 [Google Scholar]
- 37. Patterson A, Caprio F, Valleé-Bélisle A, Moscone D, Plaxco KW, Palleschi G, Ricci F. 2010. Using triplex-forming oligonucleotide probes for the reagentless, electrochemical detection of double-stranded DNA. Anal. Chem. 82: 9109– 9115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomita N, Mori Y, Kanda H, Notomi T. 2008. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 3: 877– 882 [DOI] [PubMed] [Google Scholar]
- 39. Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. 2006. Microfluidic diagnostic technologies for global public health. Nature 442: 412– 418 [DOI] [PubMed] [Google Scholar]
- 40. Heithoff DM, Shimp WR, Lau PW, Badie G, Enioutina EY, Daynes RA, Byrne BA, House JK, Mahan MJ. 2008. Human Salmonella clinical isolates distinct from those of animal origin. Appl. Environ. Microbiol. 74: 1757– 1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiu C-H. 2005. The genome sequence of Salmonella enterica serovar Choleraesuis, a highly invasive and resistant zoonotic pathogen. Nucleic Acids Res. 33: 1690– 1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davis RW, Botstein D, Roth JR. 1980. A manual for genetic engineering: advanced bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [Google Scholar]
- 43. Heithoff DM, Sinsheimer RL, Low DA, Mahan MJ. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284: 967– 970 [DOI] [PubMed] [Google Scholar]
- 44. Phares N, White RJ, Plaxco KW. 2009. Improving the stability and sensing of electrochemical biosensors by employing trithiol-anchoring groups in a six-carbon self-assembled monolayer. Anal. Chem. 81: 1095– 1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan C, Plaxco KW, Heeger AJ. 2003. Electrochemical interrogation of conformational changes as a reagentless method for the sequence-specific detection of DNA. Proc. Natl. Acad. Sci. U. S. A. 100: 9134– 9137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. White RJ, Plaxco KW. 2010. Exploiting binding-induced changes in probe flexibility for the optimization of electrochemical biosensors. Anal. Chem. 82: 73– 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferguson BS, Buchsbaum SF, Swensen JS, Hsieh K, Lou X, Soh HT. 2009. Integrated microfluidic electrochemical DNA sensor. Anal. Chem. 81: 6503– 6508 [DOI] [PubMed] [Google Scholar]
- 48. Pavlovic E, Lai RY, Wu TT, Ferguson BS, Sun R, Plaxco KW, Soh HT. 2008. Microfluidic device architecture for electrochemical patterning and detection of multiple DNA sequences. Langmuir 24: 1102– 1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lubin AA, Lai RY, Baker BR, Heeger AJ, Plaxco KW. 2006. Sequence-specific, electronic detection of oligonucleotides in blood, soil, and foodstuffs with the reagentless, reusable E-DNA sensor. Anal. Chem. 78: 5671– 5677 [DOI] [PubMed] [Google Scholar]
- 50. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28: E63 doi:10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parida M, Sannarangaiah S, Dash PK, Rao PVL, Morita K. 2008. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 18:407– 421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mori Y, Notomi T. 2009. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 15: 62– 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deng W, Liou SR, Plunkett G, III, Mayhew GF, Rose DJ, Burland V, Kodoyianni V, Schwartz DC, Blattner FR. 2003. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J. Bacteriol. 185: 2330– 2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu WQ, Feng Y, Wang Y, Zou QH, Chen F, Guo JT, Peng YH, Jin Y, Li YG, Hu SN. 2009. Salmonella paratyphi C: genetic divergence from Salmonella choleraesuis and pathogenic convergence with Salmonella typhi. PLoS One 4: e4510 doi:10.1371/journal.pone.0004510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, Meyer R, Bieri T, Ozersky P, McLellan M. 2004. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 36:1268– 1274 [DOI] [PubMed] [Google Scholar]
- 56. McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413: 852– 856 [DOI] [PubMed] [Google Scholar]
- 57. Parkhill J, Dougan G, James K, Thomson N, Pickard D, Wain J, Churcher C, Mungall K, Bentley S, Holden M. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413: 848– 852 [DOI] [PubMed] [Google Scholar]
- 58. Sukhnanand S, Alcaine S, Warnick LD, Su WL, Hof J, Craver MPJ, McDonough P, Boor KJ, Wiedmann M. 2005. DNA sequence-based subtyping and evolutionary analysis of selected Salmonella enterica serotypes. J. Clin. Microbiol. 43: 3688– 3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Edwards RA, Olsen GJ, Maloy SR. 2002. Comparative genomics of closely related salmonellae. Trends Microbiol. 10: 94– 99 [DOI] [PubMed] [Google Scholar]
- 60. Parida M, Horioke K, Ishida H, Dash PK, Saxena P, Jana AM, Islam MA, Inoue S, Hosaka N, Morita K. 2005. Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J. Clin. Microbiol. 43: 2895– 2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li S, Fang M, Zhou B, Ni H, Shen Q, Zhang H, Han Y, Yin J, Chang W, Xu G, Cao G. 2011. Simultaneous detection and differentiation of dengue virus serotypes 1–4, Japanese encephalitis virus, and West Nile virus by a combined reverse-transcription loop-mediated isothermal amplification assay. Virol. J. 8: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han E-T, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T. 2007. Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J. Clin. Microbiol. 45:2521– 2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Makharashvili N, Mi T, Koroleva O, Korolev S. 2009. RecR-mediated modulation of RecF dimer specificity for single- and double-stranded DNA. J. Biol. Chem. 284: 1425– 1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sandler S, Chackerian B, Li J, Clark A. 1992. Sequence and complementation analysis of recF genes from Escherichia coli K-12, Salmonella typhimurium, Pseudomonas putida and Bacillus subtilis: evidence for a phosphate binding loop. Nucleic Acids Res. 20: 839– 845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xiao Y, Lai RY, Plaxco KW. 2007. Preparation of electrode-immobilized, redox-modified oligonucleotides for electrochemical DNA and aptamer-based sensing. Nat. Protoc. 2: 2875– 2880 [DOI] [PubMed] [Google Scholar]
- 66. Lubin AA, Vander Stoep Hunt B, White RJ, Plaxco KW. 2009. Effects of probe length, probe geometry, and redox-tag placement on the performance of the electrochemical E-DNA sensor. Anal. Chem. 81: 2150– 2158 [DOI] [PubMed] [Google Scholar]
- 67. Xiao Y, Qu X, Plaxco KW, Heeger AJ. 2007. Label-free electrochemical detection of DNA in blood serum via target-induced resolution of an electrode-bound DNA pseudoknot. J. Am. Chem. Soc. 129: 11896– 11897 [DOI] [PubMed] [Google Scholar]
- 68. Mahan MJ, Heithoff DM, House JK. 2012. Salmonella cross-protective vaccines: fast-forward to the next generation of food safety. Future Microbiol. 7: 805– 808 [DOI] [PubMed] [Google Scholar]
- 69. Mahan MJ, Kubicek-Sutherland JZ, Heithoff DM. 2013. Rise of the microbes. Virulence 4. http://dx.doi.org/10.4161/viru.23380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. US Food and Drug Administration 2012. FDA's strategy on antimicrobial resistance—questions and answers. US Department of Health and Human Services, Silver Spring, MD: [Google Scholar]
- 71.Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, Sanga E, Hoelscher M, Notomi T, Hase T. 2007. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J. Clin. Microbiol. 45: 1936– 1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Poon LLM, Wong BWY, Ma EHT, Chan KH, Chow LMC, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S. 2006. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin. Chem. 52: 303– 306 [DOI] [PubMed] [Google Scholar]
- 73. Kuboki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I. 2003. Loop-mediated isothermal amplification for detection of African trypanosomes. J. Clin. Microbiol. 41: 5517– 5524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eiken Chemical Co 2012. Press release. Eiken Chemical Co., Tokyo, Japan: http://www.eiken.co.jp/en/news/index.html [Google Scholar]
- 75.Tuomanen EI, Austrian R, Masure HR. 1995. Pathogenesis of pneumococcal infection. N. Engl. J. Med. 332: 1280– 1284 [DOI] [PubMed] [Google Scholar]
- 76. Weinberger DM, Trzciński K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. 2009. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 5: e1000476 doi:10.1371/journal.ppat.1000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, Lim WS. 2012. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 67: 540– 545 [DOI] [PubMed] [Google Scholar]
- 78. Brown JS. 2012. Pneumococcal capsular serotypes and lung infection. Thorax 67: 473– 474 [DOI] [PubMed] [Google Scholar]
- 79. Mylonakis E, Paliou M, Lally M, Flanigan TP, Rich JD. 2000. Laboratory testing for infection with the human immunodeficiency virus: established and novel approaches. Am. J. Med. 109: 568– 576 [DOI] [PubMed] [Google Scholar]
- 80. Barletta JM, Edelman DC, Constantine NT. 2004. Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am. J. Clin. Pathol. 122: 20– 27 [DOI] [PubMed] [Google Scholar]
- 81. Li Y, Karlin A, Loike J, Silverstein S. 2002. A critical concentration of neutrophils is required for effective bacterial killing in suspension. Proc. Natl. Acad. Sci. U. S. A. 99: 8289– 8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hsieh K, Patterson AS, Ferguson BS, Plaxco KW, Soh HT. 2012. Rapid, sensitive, and quantitative detection of pathogenic DNA at the point of care through microfluidic electrochemical quantitative loop-mediated isothermal amplification. Angew. Chem. 124: 4980– 4984 [DOI] [PMC free article] [PubMed] [Google Scholar]