Abstract
Purpose
Low-risk prostate cancer patients clinically eligible for active surveillance can also be managed surgically. We evaluated the pathologic outcomes for this cohort that was treated by radical prostatectomy and devised nomograms to predict patients at risk of upgrading and/or upstaging.
Materials and methods
Seven hundred and fifty patients treated by radical prostatectomy from Jan 2005 to the present fulfilled conventional active surveillance criteria and formed the study cohort. Preoperative data on standard clinicopathologic parameters were available. The radical prostatectomy specimens were graded and staged, and any upgrading to Gleason sum >6 or upstaging to ≥pT3 (‘worsening prognosis’) were noted. Multivariable logistic regression models were used to develop predictive nomograms.
Results
Of the 750 patients, 303 (40.4%) patients were either upgraded or upstaged. Multivariable analysis found that preoperative PSA, number of positive cores, and prostate volume were significantly predictive of worsening prognosis and formed the nomogram criteria.
Conclusions
Of patients deemed eligible for active surveillance based on conventional criteria, 40.4% have worse prognostic factors after radical prostatectomy. Current active surveillance criteria may be too relaxed, and the use of nomograms which we have devised, may aid in counseling primary prostate cancer patients considering active surveillance as their therapy of choice.
Keywords: Active surveillance, Nomograms, Prostate cancer
Introduction
Prostate cancer is the commonest nondermatologic cancer in Western men [1]. In recent years, increased public awareness and prostate-specific antigen (PSA) screening have led to more men being diagnosed earlier with prostate cancer that is deemed to be low risk disease, defined as PSA ≤10 ng/ml, biopsy Gleason score ≤6, and clinical stage ≤T2a [2]. For these patients, active surveillance (AS) with selective delayed intervention is becoming an increasingly popular alternative to upfront extirpative surgery or radiotherapy. It seeks to avoid the morbidity associated with overtreatment of indolent prostate cancer and delay treatment for disease until it becomes clinically significant. Current selection protocols into AS programs [3, 4] comprise variations of the criteria first proposed by Epstein et al. [5] in 1994 in which men with a PSA density ≤0.15 ng/ml/g, ≤Gleason grade 6, ≤2 positive biopsy cores, and no biopsy core with more than 50% involvement are eligible. These selection criteria have been reported to accurately predict for clinically insignificant disease with a positive predictive value of up to 95% and a negative predictive value of 66% [5].
However, recent studies have raised the concern of inappropriate treatment being recommended based on inaccurate reporting of the Gleason sum (GS) on initial needle biopsy report [6–8]. Hence, it appears that current AS criteria may include patients with higher Gleason sums than thought at clinical diagnosis. These patients may be at increased risk of worse prognosis should they be considered erroneously as suitable for AS strategies. Upstaging from biopsy to radical prostatectomy histology can also occur and again worsen the prognosis of potentially eligible AS candidates. Predicting upstaging from cT2a disease relies on the definition of upstaging: disease on final pathology encompassing more than half of one lobe or bilateral disease is pT2b/c, but this may simply be due to better sampling of the cancer and not due to clinically significantly increased tumor bulk beyond that predicted by clinical stage. Hence, in agreement with other investigators, we consider upstaging to ≥pT3 (disease no longer confined to the prostate) as our staging criterion of worsening prognosis. Herein, we report our experience in predicting upgrading to Gleason sum ≥7 and/or upstaging to ≥pT3 at final pathology in a cohort of AS-eligible men who underwent robotic-assisted radical prostatectomy by a single surgeon (AT) at a single institution.
Materials and methods
Patient selection
This is an institutional review board-approved, retrospective cohort study from a prospective database on patients undergoing robotic-assisted laparoscopic radical prostatectomy for prostate cancer. Data from 2,476 patients, from January 2005 to September 2010, were analyzed.Of these patients, 750 were classified as patients that fulfilled standard active surveillance (AS) criteria: PSA ≤10, clinical stage ≤2a, Gleason ≤6, ≤2 cores positive, and less than 50% cancer present in any one core. Preoperative data including PSA, biopsy Gleason score, number of cores taken, number of positive cores, maximal percentage of cancer on biopsy core, clinical stage, presence of HGPIN, and prostate volume were recorded.
Pathologic examination of prostate specimens
All biopsy and prostate specimens were reviewed at our institution by the surgical pathology department. Prostate specimens were serially sectioned from apex to base. Histologic examination reported standard diagnostic criteria including Gleason grading and pathologic stage. To ameliorate interobserver variability of Gleason sum and pathologic stage reporting, 182 consecutive radical prostatectomy specimens were externally reviewed by an experienced offsite genitourinary pathologist. After establishing a concordance rate of 89% between our in-house and offsite pathologists’ reports, external validation was discontinued for financial prudence.
Statistical analysis
Patients were defined into two groups: one cohort that remained low risk and suitable for AS, and a second group that was deemed “worsening prognosis” due to Gleason upgrading to ≥7 and/or upstaging to ≥pT3. Descriptive statistics (including mean, standard deviation, median, range, frequency, and percent) were calculated for the study cohort. Univariable analyses using t-tests, Wilcoxon rank-sum tests, and chi-square tests were performed to determine predictors of worsening prognosis from the list of preoperative variables. Multivariable logistic regression modeling was used to evaluate a priori defined variables (PSA, number of cores taken, number of positive cores, maximal percentage of cancer on biopsy core, clinical stage, presence of HGPIN, and prostate volume) for predicting upgrading, upstaging, and either upgrading or upstaging (worsening prognosis) (i.e., three separate models). Odds ratios (OR) and 95% confidence intervals (95% CI) were estimated from the univariable and multivariable models. The area under the curve (AUC) method was used to quantify the predictive accuracy (PA) of (1) each variable on univariable analysis and (2) the final multivariable models (via the concordance index [c-index] from the univariable and multivariable logistic regression models). All PA estimates were internally validated using 200 boostrap samples. Nomograms were created to predict upgrading, upstaging, and worsening prognosis, using variables from the multivariable models with associated P values less than 0.20 or large effect estimates (regardless of P value) [9]. Finally, calibration plots were fitted to evaluate the extent of over-or-under-estimation of the observed upgrading, upstaging, and worsening prognosis rates. All P values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SAS Version 9.2 (SAS Institute Inc., Cary, NC). The nomograms and calibration plots were constructed in R Version 2.10.1 (The R Foundation for Statistical Computing, 2009).
Results
Out of a cohort of 2,476 men undergoing robotic radical prostatectomy, 750 patients fulfilled all the following preoperative selection criteria for active surveillance: PSA <10 ng/dl; clinical stage ≤T2a, Gleason ≤6; ≤2 positive cores; and ≤50% cancer present in any one core. Review of these 750 radical prostatectomy specimens showed that 297 demonstrated Gleason upgrading on final pathology (39.6%), while 453 were not upgraded (60.4%). Of the 750 patients 29 (3.9%) patients were upstaged to at least pT3, and 303/750 (40.4%) were either upgraded or upstaged. Patients with Gleason upgrading at final pathology had significantly higher BMI (P = 0.004), higher preoperative PSA (P < 0.001), higher number of positive cores, higher maximum percentage of cancer in biopsy (P = 0.02), smaller prostates (P = 0.08), higher Gleason pathology score (P < 0.0001), higher percentage with pT3–4 disease (P < 0.0001), and higher positive margin rate (P < 0.0001) compared with patients who did not display GS upgrading (Table 1). Both cohorts had similar age, number of biopsy cores taken, HGPIN on biopsy, preoperative Gleason biopsy score, and clinical stage (Tables 2, 3).
Table 1.
Comparison of Gleason upgrading in potential active surveillance patients
| Variable | Group 1 (n = 750) All AS-eligible patients |
Group 1A (n = 453) No Gleason upgrading |
Group 1B (n = 297) Positive Gleason upgrading |
P value/X2 |
|---|---|---|---|---|
| Mean age | 59 ± 6.9 | 58.9 ± 7 | 59.4 ± 6.7 | 0.279 |
| Mean BMI | 27 ± 3.8 | 26.7 ± 3.8 | 27.5 ± 3.7 | 0.004 |
| Mean preop PSA | 4.6 ± 1.9 | 4.4 ± 1.9 | 5.0 ± 1.8 | <0.0001 |
| Total Bx cores | 0.89 | |||
| <18 | 87.9 | 87.7 | 88.1 | |
| ≥18 | 12.1 | 12.3 | 11.9 | |
| Total positive cores | <0.001 | |||
| 1 | 68.3 | 74.6 | 58.6 | |
| 2 | 31.7 | 25.4 | 41.4 | |
| Max % of cancer in biopsy | 0.02 | |||
| ≤5 | 50.9 | 54.5 | 45.5 | |
| >5 | 49.1 | 45.5 | 54.5 | |
| Clinical stage | 0.5 | |||
| T1 | 96.4 | 96 | 97 | |
| T2a | 3.6 | 4 | 3 | |
| HGPIN on Bx | 0.37 | |||
| No | 88.1 | 89.3 | 87.2 | |
| Yes | 11.9 | 10.7 | 12.8 | |
| Prostate volume | 54 ± 23.2 | 55.2 ± 24.1 | 52.1 ± 21.5 | 0.08 |
| Gleason pathology | <0.0001 | |||
| ≤6 | 60.4 | 100 | 0 | |
| 7 | 39.2 | 0 | 99 | |
| 8, 9, 10 | 0.4 | 0 | 1 | |
| pTNM | <0.0001 | |||
| T1–2 | 96.1 | 98.7 | 92.3 | |
| T3–4 | 3.9 | 1.3 | 7.7 | |
| Positive SM rate | 4.7 | 2.4 | 8.1 | <0.0001 |
Table 2.
Comparison of pathologic upstaging in potential active surveillance patients
| Variable | Group 1A (n = 721) No upstaging |
Group 1B (n = 29) Upstaging |
P value/X2 |
|---|---|---|---|
| Mean age | 59 ± 6.9 | 60.6 ± 7.0 | 0.208 |
| Mean BMI | 26.9 ± 3.8 | 28.7 ± 4.7 | 0.018 |
| Mean preop PSA | 4.6 ± 1.9 | 5.0 ± 1.9 | 0.261 |
| Total Bx cores | 0.582 | ||
| <18 | 87.8 | 84.4 | |
| ≥18 | 12.2 | 11.1 | |
| Total positive cores | 0.051 | ||
| 1 | 68.9 | 51.7 | |
| 2 | 31.1 | 48.3 | |
| Max % of cancer in biopsy | 0.502 | ||
| ≤5 | 51.2 | 44.8 | |
| >5 | 48.8 | 55.2 | |
| Clinical stage | 0.331 | ||
| T1 | 96.5 | 93.1 | |
| T2a | 3.5 | 6.9 | |
| HGPIN on Bx | 0.695 | ||
| No | 88.6 | 86.2 | |
| Yes | 11.4 | 13.8 | |
| Prostate volume | 54.2 ± 23.3 | 46.7 ± 16.7 | 0.025 |
| Gleason pathology | <0.0001 | ||
| ≤6 | 61.9 | 20.7 | |
| 7 | 37.8 | 75.9 | |
| 8, 9, 10 | 0.3 | 3.4 | |
| pTNM | <0.0001 | ||
| T1–2 | 100 | 0 | |
| T3–4 | 0 | 100 | |
| Positive SM rate | 3.7 | 27.6 | <0.0001 |
Table 3.
Comparison of either upgrading or upstaging in potential active surveillance patients
| Variable | Group 1A (n = 447) No Gleason upgrading/upstaging |
Group 1B (n = 303) Positive Gleason upgrading/upstaging |
P value/X2 |
|---|---|---|---|
| Mean age | 58.8 ± 6.9 | 59.4 ± 6.7 | 0.241 |
| Mean BMI | 26.7 ± 3.8 | 27.5 ± 3.8 | 0.003 |
| Mean preop PSA | 4.4 ± 1.9 | 4.9 ± 1.8 | <0.001 |
| Total Bx cores | 0.937 | ||
| <18 | 87.8 | 88 | |
| ≥18 | 12.2 | 12 | |
| Total positive cores | <0.0001 | ||
| 1 | 74.7 | 58.7 | |
| 2 | 25.3 | 41.3 | |
| Max % of cancer in biopsy | 0.015 | ||
| ≤5 | 54.6 | 45.5 | |
| >5 | 45.4 | 54.5 | |
| Clinical stage | 0.717 | ||
| T1 | 96.2 | 96.7 | |
| T2a | 3.8 | 3.3 | |
| HGPIN on Bx | 0.467 | ||
| No | 89.2 | 87.5 | |
| Yes | 10.8 | 12.5 | |
| Prostate volume | 55.1 ± 24.2 | 52.1 ± 21.4 | 0.078 |
| Gleason pathology | <0.0001 | ||
| ≤6 | 100 | 2 | |
| 7 | 0 | 97 | |
| 8, 9, 10 | 0 | 1 | |
| pTNM | <0.0001 | ||
| T1–2 | 100 | 90.4 | |
| T3–4 | 0 | 9.6 | |
| Positive SM rate | 2.2 | 8.3 | <0.0001 |
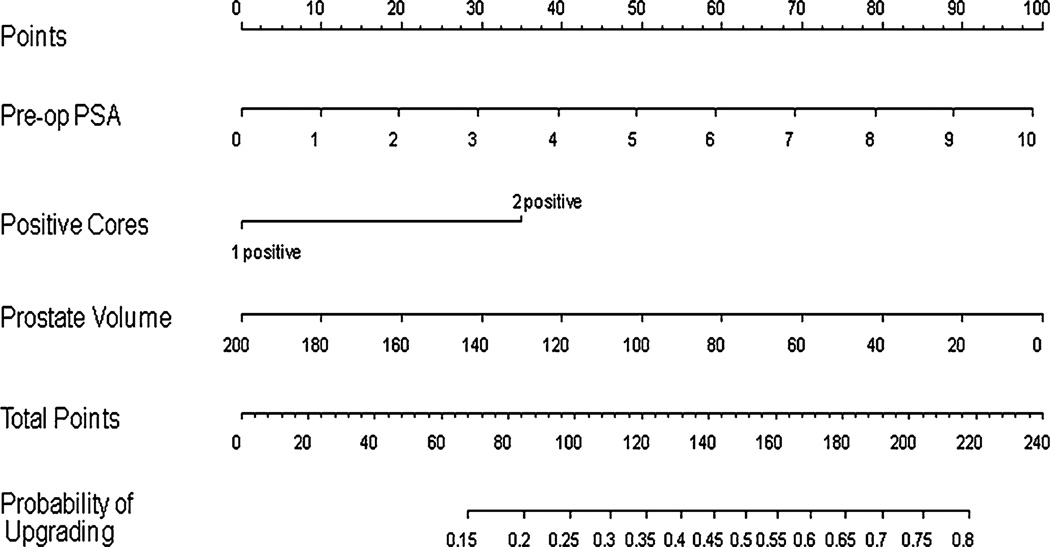
In an effort to determine variables that correlated with upgrading, upstaging, or either upgrading or upstaging (worsening prognosis), univariable and multivariable analyses were performed. Preoperative PSA, number of positive cores, and maximum percentage of cancer in biopsy cores were predictive of upgrading at P < 0.05 on univariable analysis with odds ratios of 1.17, 2.08, and 1.44, respectively (Table 4). Based on multivariable analysis, preoperative PSA, number of positive cores, and lower prostate volume were statistically significant contributors to upgrading at P < 0.20 with odds ratios of 1.24, 1.94, and 0.99, respectively (Table 5), and were entered into the upgrading nomogram. This nomogram (Fig. 1) was reasonably discriminatory at differentiating between patients deemed clinically eligible for AS based on current conventional criteria who remained eligible from those that would be upgraded were they to have undergone surgery (bootstrap corrected c-index 0.65). The nomogram was also well calibrated internally though did slightly underpredict the risk of upgrading in those at <30% and >50% actual risk (data not shown).
Table 4.
Univariable analysis for the prediction of Gleason upgrading in potential active surveillance patients
| Predictors of upgrading | Univariable analyses |
|||
|---|---|---|---|---|
| OR | 95% CI on OR | P value | PA; % | |
| Preop PSA (continuous) | 1.17 | 1.08, 1.27 | <0.0001 | 59.0 |
| Clinical stage (t2 vs. t1) | 0.76 | 0.34, 1.70 | 0.4990 | 50.5 |
| Biopsy cores (≥18 vs. <18) | 0.97 | 0.60, 1.55 | 0.8853 | 50.2 |
| Positive cores (2 vs. 1) | 2.08 | 1.52, 2.84 | <0.0001 | 58.0 |
| Max % Bx (>5% vs. ≤5%) | 1.44 | 1.07, 1.93 | 0.0152 | 54.5 |
| HGPIN Bx (yes vs. no) | 1.23 | 0.78, 1.93 | 0.3731 | 51.1 |
| Prostate volume (continuous) | 0.99 | 0.99, 1.00 | 0.0796 | 54.3 |
PA predictive accuracy (AUC from c-statistic)
Table 5.
Multivariable analysis for the prediction of Gleason upgrading in potential active surveillance patients
| Predictors of upgrading | P value | Adjusted OR | 95% CI for OR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Preop PSA | <0.0001 | 1.237 | 1.129 | 1.354 |
| Clinical stage | 0.374 | 0.675 | 0.284 | 1.606 |
| Biopsy cores | 0.864 | 1.044 | 0.639 | 1.704 |
| Positive cores | <0.0001 | 1.942 | 1.384 | 2.724 |
| Max % Bx | 0.344 | 1.168 | 0.847 | 1.611 |
| HGPIN Bx | 0.228 | 1.348 | 0.830 | 2.191 |
| Prostate volume | 0.011 | 0.990 | 0.982 | 0.998 |
PA = 64.5%; Preop PSA: Continuous in model; Clinical stage: t2 versus t1 (referent); Biopsy cores: ≥18 cores versus <18 cores (referent); Positive cores: 2 positive cores versus 1 positive core (referent); Max % Bx: >5% (median) versus ≤5% (referent); HGPIN Bx: yes versus no (referent); Prostate volume: Continuous in model
Fig. 1.
Nomogram for the prediction of Gleason upgrading in potential active surveillance patients. Nomogram instructions: To obtain the nomogram-predicted probability of upgrading, locate patient values on each axis. Draw a vertical line to the ‘Points’ axis to determine how many points are attributed for each variable value. Sum the points for all variables. Locate the sum on the ‘Total Points’ line to assess the individual probability of upgrading on the ‘Probability of Upgrading’ line
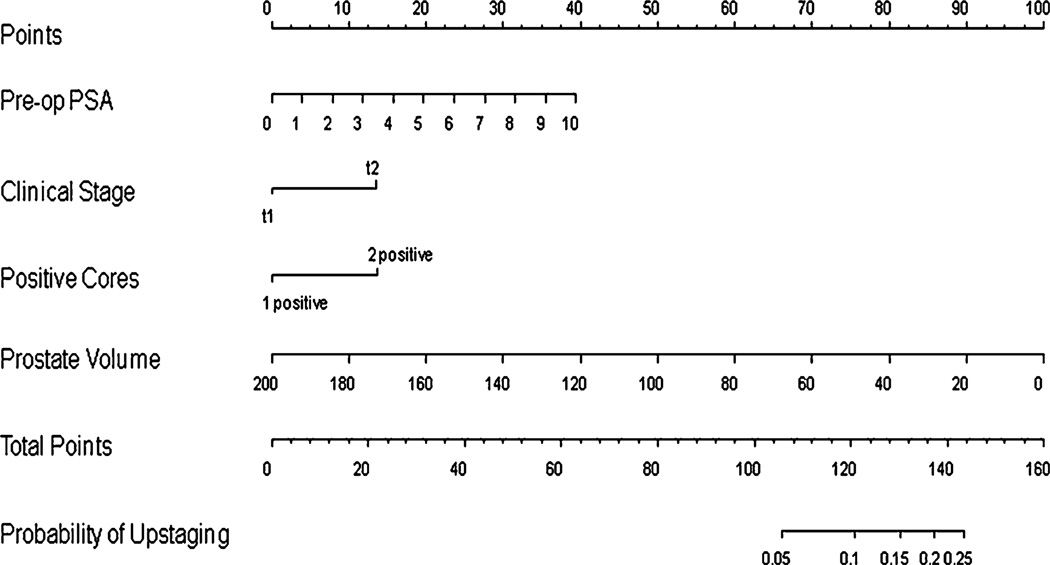
None of the variables were predictive of upstaging at P < 0.05 on univariable analysis (Table 6). However, based on a restricted multivariable analysis (i.e., due to the small number of outcome events only four variables could be explored in the model), preoperative PSA, number of positive cores, and lower prostate volume were statistically significant contributors to upstaging at P < 0.20 with odds ratios of 1.21, 1.95, and 0.98, respectively (Table 7), and were entered into the upstaging nomogram. Clinical stage was also entered into the upstaging nomogram as its effect estimate was large (OR 1.94) and the lack of significance was likely the result of the low number of outcome events (n = 29). This nomogram (Fig. 2) was also reasonably discriminatory at differentiating between patients deemed clinically eligible for AS based on current conventional criteria who remained eligible from those that would be upstaged were they to have undergone surgery (bootstrap corrected c-index 0.65). The nomogram was also well calibrated internally though the probability of upstaging was < 20% in our patients (data not shown).
Table 6.
Univariable analysis for the prediction of pathologic upstaging in potential active surveillance patients
| Predictors of upstaging | Univariable analyses |
|||
|---|---|---|---|---|
| OR | 95% CI on OR | P value | PA; % | |
| Preop PSA (continuous) | 1.12 | 0.92, 1.35 | 0.2611 | 55.6 |
| Clinical stage (t2 vs. t1) | 2.06 | 0.47, 9.16 | 0.3410 | 51.7 |
| Biopsy cores (≥18 vs. <18) | 0.90 | 0.27, 3.07 | 0.8698 | 50.5 |
| Positive cores (2 vs. 1) | 2.07 | 0.98, 4.36 | 0.0556 | 58.6 |
| Max % Bx (>5% vs. ≤5%) | 1.29 | 0.61, 2.72 | 0.5034 | 53.2 |
| HGPIN Bx (yes vs. no) | 1.24 | 0.42, 3.66 | 0.6951 | 51.2 |
| Prostate volume (continuous) | 0.98 | 0.96, 1.00 | 0.0799 | 59.5 |
PA predictive accuracy (AUC from c-statistic)
Table 7.
Restricted multivariable analysis for the prediction of pathologic upstaging in potential active surveillance patients
| Predictors of upstaging | P value | Adjusted OR | 95% C.I. for OR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Preop PSA | 0.060 | 1.212 | 0.992 | 1.481 |
| Clinical stage | 0.394 | 1.937 | 0.423 | 8.865 |
| Positive cores | 0.083 | 1.946 | 0.917 | 4.129 |
| Prostate volume | 0.046 | 0.976 | 0.953 | 1.000 |
PA = 65.2%; Preop PSA: Continuous in model; Clinical stage: t2 versus t1 (referent); Positive cores: 2 positive cores versus 1 positive core (referent); Prostate volume: Continuous in model
Fig. 2.
Nomogram for the prediction of pathologic upstaging in potential active surveillance patients. Nomogram instructions: To obtain the nomogram-predicted probability of upstaging, locate patient values on each axis. Draw a vertical line to the ‘Points’ axis to determine how many points are attributed for each variable value. Sum the points for all variables. Locate the sum on the ‘Total Points’ line to assess the individual probability of upstaging on the ‘Probability of Upstaging’ line
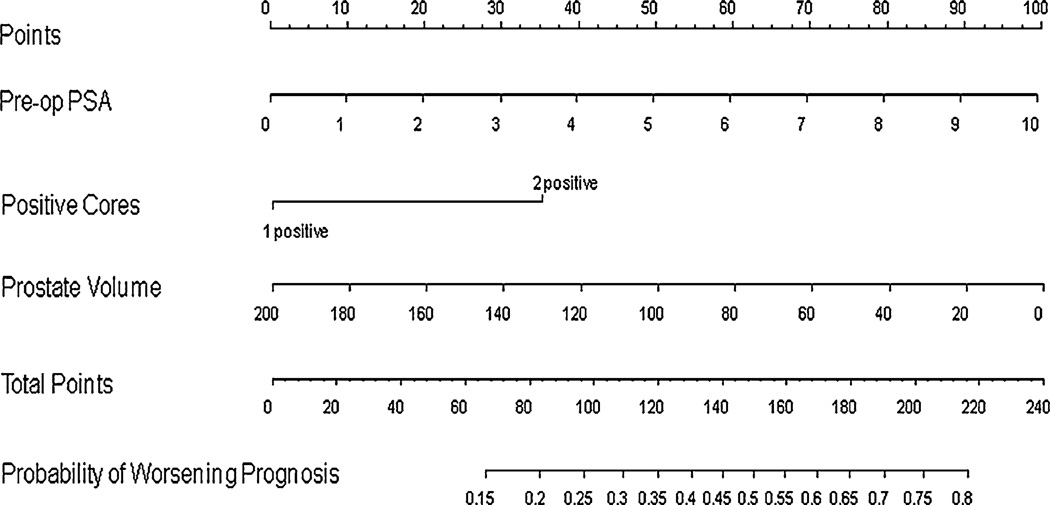
Preoperative PSA, number of positive cores, and maximum percentage of cancer in biopsy cores were predictive of either upgrading or upstaging (worsening prognosis) at P < 0.05 on univariable analysis with odds ratios of 1.17, 2.08, and 1.44, respectively (Table 8). Based on multivariable analysis, preoperative PSA, number of positive cores, and lower prostate volume were statistically significant contributors to worsening prognosis at P < 0.20 with odds ratio of 1.24, 1.93, and 0.99, respectively (Table 9), and were entered into the worsening prognosis nomogram. This nomogram (Fig. 3) was also reasonably discriminatory at differentiating between patients deemed clinically eligible for AS based on current conventional criteria who remained eligible from those that would be upgraded or upstaged were they to have undergone surgery (bootstrap corrected c-index 0.64). The nomogram was also well calibrated internally though did slightly underpredict the risk of worsening prognosis in those at <30% and >50% actual risk (data not shown).
Table 8.
Univariable analysis for the prediction of worsening prognosis in potential active surveillance patients
| Predictors of worsening prognosis | Univariable analyses |
|||
|---|---|---|---|---|
| OR | 95% CI on OR | P value | PA; % | |
| Preop PSA (continuous) | 1.17 | 1.08, 1.27 | <0.0001 | 58.9 |
| Clinical stage (t2 vs. t1) | 0.86 | 0.39, 1.91 | 0.7170 | 50.3 |
| Biopsy cores (≥18 vs. <18) | 0.98 | 0.62, 1.57 | 0.9369 | 50.1 |
| Positive cores (2 vs. 1) | 2.08 | 1.52, 2.84 | <0.0001 | 58.0 |
| Max % bx (>5% vs. ≤5%) | 1.44 | 1.07, 1.93 | 0.0152 | 54.5 |
| HGPIN Bx (yes vs. no) | 1.18 | 0.75, 1.86 | 0.4672 | 50.9 |
| Prostate volume (continuous) | 0.99 | 0.99, 1.00 | 0.0797 | 54.2 |
PA predictive accuracy (AUC from c-statistic)
Table 9.
Multivariable analysis for the prediction of worsening prognosis in potential active surveillance patients
| Predictors of worsening prognosis | P value | Adjusted OR | 95% C.I. for OR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Preop PSA | <0.0001 | 1.239 | 1.132 | 1.357 |
| Clinical stage | 0.575 | 0.785 | 0.338 | 1.825 |
| Biopsy cores | 0.800 | 1.065 | 0.654 | 1.735 |
| Positive cores | <0.0001 | 1.934 | 1.379 | 2.710 |
| Max % Bx | 0.343 | 1.168 | 0.848 | 1.609 |
| HGPIN Bx | 0.304 | 1.289 | 0.794 | 2.094 |
| Prostate volume | 0.010 | 0.990 | 0.982 | 0.998 |
PA = 64.4%; Preop PSA: Continuous in model; Clinical stage: t2 versus t1 (referent); Biopsy cores: ≥18 cores versus <18 cores (referent); Positive cores: 2 positive cores versus 1 positive core (referent); Max % Bx: >5% (median) versus ≤5% (referent); HGPIN Bx: yes versus no (referent); Prostate volume: Continuous in model
Fig. 3.
Nomogram for the prediction of worsening prognosis in potential active surveillance patients. Nomogram instructions: To obtain the nomogram-predicted probability of worsening prognosis, locate patient values on each axis. Draw a vertical line to the ‘Points’ axis to determine how many points are attributed for each variable value. Sum the points for all variables. Locate the sum on the ‘Total Points’ line to assess the individual probability of worsening prognosis on the ‘Probability of Worsening Prognosis’ line
Discussion
The proportion of men having low-risk prostate cancer at diagnosis has doubled in the past decade, largely as a result of screening, accounting for almost half of all men being diagnosed with prostate cancer in 2000–2001 in the CaPSURE registry [2]. The European Randomized Study of Screening for Prostate Cancer reported that although PSA-based screening reduced the rate of prostate cancer mortality by 20%, this was associated with a high risk of overdiagnosis [10]. Hence, AS with selective delayed intervention has become an increasingly attractive option for patients diagnosed with low-risk prostate cancer, who wish to avoid the myriad of complications from overtreatment for otherwise indolent disease. Emerging evidence from single- and multiinstitutional studies now suggest that AS is safe, is associated with a low risk of disease progression, and does not significantly compromise disease-specific outcomes [3, 11, 12]. However, underestimation of disease severity in AS-eligible men remains a primary concern, with the incidence of Gleason upgrading at final pathology varying from 18 to 50% in various series [6, 8, 13]. Also, the pT3 rate in such AS-eligible patients is 10–15% [14, 15]. Taken together, these findings suggest a significant percentage of men thought to be low risk and suitable for AS strategies may actually harbor more sinister disease.
Our analyses demonstrate that PSA, number of positive cores, and lower prostate volume are significant predictors of upgrading or upstaging in patients deemed eligible for AS by conventional criteria. Freedland et al. [16] and Briganti et al. [17] both demonstrated that smaller prostate glands were associated with more high-grade cancers, a higher likelihood of extracapsular extension, seminal vesicle invasion, and earlier biochemical progression after surgery. In 1999, D’Amico and colleagues first reported the significant association between prostate gland volume ≤75 cm3 and PSA >20 ng/dL with Gleason upgrading [6], which has been confirmed by other investigators [13, 18, 19]. However, Kulkarni et al. [20] failed to demonstrate an association between small prostate volumes and upgrading or upstaging, and the small effect estimate in our study of 0.99 suggests that prostate volume may not be very clinically significant. It may be that it is not prostate volume but rather more thorough preoperative needle sampling in a smaller gland that accounts for some studies showing worsening prognosis in men with small glands. Previous studies have indeed reported a lower likelihood of GS discordance with extended biopsy schemes [21, 22]. Turley et al. [23] recently reported that the association between smaller prostate volume and upgrading was significant only in men who had an extended-core biopsy (P < 0.001), but not in men with a sextant biopsy (P = 0.22). Other investigators, however, failed to confirm this finding or correlate number of cores taken with upstaging [7, 13]. In our study, the number of biopsy cores taken was not significant for predicting worsening prognosis in a multivariable model. However, number of positive cores (2 vs. 1) almost doubled the risk of worsening prognosis on multivariable analysis (OR 1.93).
Several studies have already documented that a higher baseline PSA as being associated with adverse pathologic features at radical prostatectomy [8, 22]. Baseline PSA density, which is a composite of PSA and prostate volume has been previously reported to be a significant predictor for adverse pathologic features and disease progression in men undergoing radical prostatectomy after AS [24]. In their series of men with prostate cancer managed with AS, Dall’Era et al. [25] reported that those with baseline PSA density >0.15 ng/ml/cm3 were five times more likely to receive active treatment than men with a lower PSA density. In their series of men undergoing robotic-assisted radical prostatectomies who had Gleason 6 microfocal prostate cancer on biopsy (who would be deemed to have low risk disease), Thong et al. [26] also reported that PSA density >0.20 ng/ml/cm3 was a significant predictor of both upgrading and upstaging. This is consistent with our study results showing that elevated PSA and lower prostate volume are significantly associated with upgrading and/or upstaging.
Interobserver variability has also been previously cited as a cause for upgrading or upstaging [27]. To ameliorate this possible confounder, 182 consecutive radical prostatectomy specimens were reviewed by an experienced external uropathologist after the specimens had been processed and reported by two dedicated in-house uropathologists according to our institutional protocols. An 89% concordance rate was achieved between both in-house and external pathologists, following which external validation was discontinued for financial prudence. Nonetheless, not being able to externally validate all of our radical prostatectomy specimens constitutes a limitation of this study.
Using the models of best fit from the multivariable analyses, we created nomograms to aid in the prediction of Gleason upgrading, pathologic upstaging, and either upgrading or upstaging (worsening prognosis). PSA density was not used in the nomogram derivation as this is a function of PSA and prostate volume, which are usually measured separately by most clinicians. These two variables were therefore separately used in the statistical modeling. Our nomograms should aid the practicing urologist, oncologist, and general practitioner who see patients with low-risk prostate cancer to adequately counsel them regarding their appropriateness for active surveillance. Rather than selecting arbitrary cut-offs, these nomograms enable the prediction of risk of worsening prognosis in clinically low risk patients deemed suitable for AS strategies. However, the models of best fit used in the derivation of the nomograms have concordance indexes (or area under the curve) of 0.64–0.65, making them only fairly discriminatory. In other words, there is a 64–65% chance that a pair of AS conventionally eligible individuals, one who is upgraded and/or upstaged on final histology and one who is not, will be correctly classified as such by the nomograms. The nomograms were also found to be well calibrated, especially in the middle range, meaning that the physician can give a reasonably accurate prediction of the probability that an AS conventionally eligible patient will be upgraded and/or upstaged were he to have surgery, assuming the patient was not deemed to be at the extremes of risk.
This work has a number of limitations. For one, the nomograms have not yet been validated by other surgical cohorts, and thus, the generalizability of their use remains in question. Other limitations of this study are its retrospective nature and inclusion of patients who did not have routine repeat restaging biopsy. In their cohort of 104 men who were eligible for AS but underwent immediate repeat biopsy, Berglund et al. [28] reported that 27% demonstrated GS upgrading or upstaging on repeat biopsy. These men with upgraded or upstaged disease were more likely to have higher pathologic stage and grade at radical prostatectomy than those who were not, suggesting that early repeat biopsy helps better select patients with low risk disease who would derive the most benefit from AS protocols [12]. Also, whether this upgrading and/or upstaging will result in differences in prostate cancer specific and/or overall survival will require longer follow-up of these patients, which is ongoing. Nevertheless, this study represents the largest study to date looking at predictors for upgrading and/or upstaging in AS-eligible men who chose to undergo radical prostatectomy.
Conclusions
Increasing evidence is emerging that contemporary selection criteria used for enrollment into active surveillance programs may be inadequate for keeping out those men with high risk of upgrading or upstaging at final pathology, with the attendant risks of delayed treatment, adverse pathologic features and biochemical progression. In our series, 40.4% of patients deemed eligible for AS by conventional criteria were upgraded and/or upstaged. Preoperative PSA, number of positive cores (2 vs. 1), and lower prostate volume are significant independent predictors for worsening prognosis. Until advances in proteomics, gene studies, and cancer biomarkers allow us to accurately distinguish aggressive from indolent prostate cancer, collaborative efforts using large multi-institutional databases to assess the true predictive value of these aforementioned predictors of worsening prognosis remain the best surrogate means of discriminating those men with true indolent disease who would derive the most benefit from active surveillance regimes. We suggest the use of nomograms, advocating caution in interpretation, to help clinicians and patients make decisions regarding enrollment into active surveillance protocols.
Acknowledgments
Dr. Prasanna Sooriakumaran is the ACMI Corp. Endourological Society fellow and also receives financial support from Prostate UK; Dr. Paul Christos is partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-RR024996); Dr. Ashutosh Tewari is the endowed Ronald P. Lynch Professor of Urologic-Oncology, the Director of the Prostate Cancer Institute, and Director of the Lefrak Center for Robotic Surgery, and has received grants in the past from Intuitive Surgical Inc. and the National Institute of Health. However, this work was not supported by such grants.
Contributor Information
Prasanna Sooriakumaran, Department of Urology, Lefrak Center of Robotic Surgery & Institute of Prostate Cancer, Weill Cornell Medical College, James Buchanan Brady Foundation, New York, NY 10065, USA.
Abhishek Srivastava, Department of Urology, Lefrak Center of Robotic Surgery & Institute of Prostate Cancer, Weill Cornell Medical College, James Buchanan Brady Foundation, New York, NY 10065, USA.
Paul Christos, Department of Statistics & Public Health, Weill Cornell Medical College, New York, NY, USA.
Sonal Grover, Department of Urology, Lefrak Center of Robotic Surgery & Institute of Prostate Cancer, Weill Cornell Medical College, James Buchanan Brady Foundation, New York, NY 10065, USA.
Maria Shevchuk, Department of Pathology, Weill Cornell Medical College, New York, NY, USA.
Ashutosh Tewari, Email: akt2002@med.cornell.edu, Department of Urology, Lefrak Center of Robotic Surgery & Institute of Prostate Cancer, Weill Cornell Medical College, James Buchanan Brady Foundation, New York, NY 10065, USA.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klotz L. Active surveillance for favorable risk prostate cancer: rationale, risks, and results. Urol Oncol. 2007;25(6):505–509. doi: 10.1016/j.urolonc.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Carter HB, Kettermann A, Warlick C, Metter EJ, Landis P, Walsh PC, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178(6):2359–2364. doi: 10.1016/j.juro.2007.08.039. discussion 2364–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368–374. [PubMed] [Google Scholar]
- 6.D’Amico AV, Renshaw AA, Arsenault L, Schultz D, Richie JP. Clinical predictors of upgrading to Gleason grade 4 or 5 disease at radical prostatectomy: potential implications for patient selection for radiation and androgen suppression therapy. Int J Radiat Oncol Biol Phys. 1999;45(4):841–846. doi: 10.1016/s0360-3016(99)00260-6. [DOI] [PubMed] [Google Scholar]
- 7.Fukagai T, Namiki T, Namiki H, Carlile RG, Shimada M, Yoshida H. Discrepancies between Gleason scores of needle biopsy and radical prostatectomy specimens. Pathol Int. 2001;51(5):364–370. doi: 10.1046/j.1440-1827.2001.01207.x. [DOI] [PubMed] [Google Scholar]
- 8.Pinthus JH, Witkos M, Fleshner NE, Sweet J, Evans A, Jewett MA, et al. Prostate cancers scored as Gleason-6 on prostate biopsy are frequently Gleason 7 tumors at radical prostatectomy: implication on outcome. J Urol. 2006;176(3):979–984. doi: 10.1016/j.juro.2006.04.102. discussion 984. [DOI] [PubMed] [Google Scholar]
- 9.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 11.Patel MI, DeConcini DT, Lopez-Corona E, Ohori M, Wheeler T, Scardino PT. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171(4):1520–1524. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 12.Eggener SE, Mueller A, Berglund RK, Ayyathurai R, Soloway C, Soloway MS, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2009;181(4):1635–1641. doi: 10.1016/j.juro.2008.11.109. discussion 1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong F, Jones JS, Stephenson AJ, Magi-Galluzzi C, Reuther AM, Klein EA. Prostate cancer volume at biopsy predicts clinically significant upgrading. J Urol. 2008;179(3):896–900. doi: 10.1016/j.juro.2007.10.060. discussion 900. [DOI] [PubMed] [Google Scholar]
- 14.Conti SL, Dall’era M, Fradet V, Cowan JE, Simko J, Carroll PR. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181(4):1628–1633. doi: 10.1016/j.juro.2008.11.107. discussion 1633–1624. [DOI] [PubMed] [Google Scholar]
- 15.Smaldone MC, Cowan JE, Carroll PR, Davies BJ. Eligibility for active surveillance and pathological outcomes for men undergoing radical prostatectomy in a large, community based cohort. J Urol. 2010;183(1):138–143. doi: 10.1016/j.juro.2009.08.152. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23(30):7546–7554. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 17.Briganti A, Chun FK, Suardi N, Gallina A, Walz J, Graefen M, et al. Prostate volume and adverse prostate cancer features: fact not artifact. Eur J Cancer. 2007;43(18):2669–2677. doi: 10.1016/j.ejca.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Turley RS, Hamilton RJ, Terris MK, Kane CJ, Aronson WJ, Presti JC, Jr, et al. Small transrectal ultrasound volume predicts clinically significant Gleason score upgrading after radical prostatectomy: results from the SEARCH database. J Urol. 2008;179(2):523–527. doi: 10.1016/j.juro.2007.09.078. discussion 527–528. [DOI] [PubMed] [Google Scholar]
- 19.Kassouf W, Nakanishi H, Ochiai A, Babaian KN, Troncoso P, Babaian RJ. Effect of prostate volume on tumor grade in patients undergoing radical prostatectomy in the era of extended prostatic biopsies. J Urol. 2007;178(1):111–114. doi: 10.1016/j.juro.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni GS, Lockwood G, Evans A, Toi A, Trachtenberg J, Jewett MA, et al. Clinical predictors of Gleason score upgrading: implications for patients considering watchful waiting, active surveillance, or brachytherapy. Cancer. 2007;109(12):2432–2438. doi: 10.1002/cncr.22712. [DOI] [PubMed] [Google Scholar]
- 21.San Francisco IF, DeWolf WC, Rosen S, Upton M, Olumi AF. Extended prostate needle biopsy improves concordance of Gleason grading between prostate needle biopsy and radical prostatectomy. J Urol. 2003;169(1):136–140. doi: 10.1016/S0022-5347(05)64053-0. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Kane CJ, Amling CL, Aronson WJ, Terris MK, Presti JC., Jr Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69(3):495–499. doi: 10.1016/j.urology.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turley RS, Terris MK, Kane CJ, Aronson WJ, Presti JC, Amling CL, et al. The association between prostate size and Gleason score upgrading depends on the number of biopsy cores obtained: results from the shared equal access regional cancer hospital database. BJU Int. 2008;102(9):1074–1079. doi: 10.1111/j.1464-410X.2008.08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkitaraman R, Norman A, Woode-Amissah R, Fisher C, Dearnaley D, Horwich A, et al. Predictors of histological disease progression in untreated, localized prostate cancer. J Urol. 2007;178(3 Pt 1):833–837. doi: 10.1016/j.juro.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 25.Dall’Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, Klotz LH, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112(8):1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 26.Thong AE, Shikanov S, Katz MH, Gofrit ON, Eggener S, Zagaja GP, et al. A single microfocus (5% or less) of Gleason 6 prostate cancer at biopsy—can we predict adverse pathological outcomes? J Urol. 2008;180(6):2436–2440. doi: 10.1016/j.juro.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Allsbrook WC, Jr, Mangold KA, Johnson MH, Lane RB, Lane CG, Amin MB, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol. 2001;32(1):74–80. doi: 10.1053/hupa.2001.21134. [DOI] [PubMed] [Google Scholar]
- 28.Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180(5):1964–1967. doi: 10.1016/j.juro.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]