Abstract
Guanidinopropionic acid (GPA) increases AMPK activity, mitochondrial function and biogenesis in muscle and improves physiological function, for example during aging. Mitochondrial dysfunction is a major contributor to the pathogenesis of Parkinson’s disease. Here we tested whether GPA prevents neurodegeneration of the nigrostriatal dopamine system in MPTP-treated mice. Mice were fed a diet of 1% GPA or normal chow for 4 weeks and then treated with either MPTP or saline. Indices of nigrostriatal function were examined by HPLC, immunohistochemistry, stereology, electron microscopy and mitochondrial respiration. MPTP intoxication decreased TH neurons in the SNpc of normal chow-fed mice, however GPA-fed mice remarkably exhibited no loss of TH neurons in the SNpc. MPTP caused a decrease in striatal dopamine of both normal chow- and GPA-fed mice, although this effect was significantly attenuated in GPA-fed mice. GPA-fed mice showed increased AMPK activity, mitochondrial respiration and mitochondrial number in nigrostriatal TH neurons, suggesting the neuroprotective effects of GPA involved AMPK-dependent increases in mitochondrial function and biogenesis. MPTP treatment produced a decrease in mitochondrial number and volume in normal chow-fed mice but not GPA-fed mice. Our results show the neuroprotective properties of GPA in a mouse model of Parkinson’s disease are partially mediated by AMPK and mitochondrial function. Mitochondrial dysfunction is a common problem in neurodegeneration and thus GPA may slow disease progression in other models of neurodegeneration.
Keywords: AMPK, ghrelin, dopamine, mitochondrial biogenesis, mitochondrial volume, nucleator, stereology
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder behind Alzheimer’s disease and cardinal features of PD include motor dysfunction such as rigidity, resting tremor, postural instability and bradykinesia. These debilitating symptoms manifest due to the massive loss of dopamine in the striatum, the nerve terminal region of dopamine neurons located in the substantia nigra pars compacta (SNpc). At present there are only a few known monogenic mutations accelerating the onset of PD, therefore most cases are considered sporadic and develop as a complex polygenic interaction with age and environment.
Mitochondrial dysfunction is well characterized as a major contributor to the onset of PD (Abou-Sleiman et al., 2006) and human PD patients show a mitochondrial complex 1 deficiency (Schapira et al., 1989). Indeed, the most common PD model in mice, MPTP, is a complex 1 inhibitor. Impaired mitochondrial mechanisms contribute to PD by decreasing ATP generating potential (Gispert et al., 2009), decreasing mitochondrial uncoupling (Andrews et al., 2005a; Andrews et al., 2005b; Conti et al., 2005), increasing oxidative stress and reactive oxygen species (Przedborski et al., 1992; St-Pierre et al., 2006), improper calcium handling (Gandhi et al., 2009; Marongiu et al., 2009), dysfunctional fission and fusion dynamics (Park et al., 2009), and reduced mitochondrial biogenesis (St-Pierre et al., 2006). In fact, many monogenic mutations that promote nigral degeneration and parkinsonism in humans, such as parkin, DJ1, PTEN-induced Kinase 1, all manifest mitochondrial pathologies (Clark et al., 2006; Palacino et al., 2004; Zhang et al., 2005). Thus, the most accepted mode of nigral degeneration, whether it is monogenic mutation or a polygenic interaction with age and environment, involves mitochondrial pathology.
Because of the importance of mitochondria in maintaining bioenergetic status and neuronal function in PD, factors positively regulating mitochondrial function are likely to slow disease progression. We recently established that the hormone ghrelin influences midbrain dopamine neuronal activity of both the ventral tegmental area (VTA) (Abizaid et al., 2006) and the SNpc (Andrews et al., 2009) and protects against MPTP-induced TH neurodegeneration by promoting mitochondrial respiration and biogenesis (Andrews et al., 2009).
Ghrelin is a hormone that is most well studied for its role in food intake and body weight regulation. Within the hypothalamus ghrelin initiates food intake by activating NPY neurons in the arcuate nucleus. Activation of the ghrelin receptor (GHSR) increases AMPK activity, mitochondrial biogenesis and respiration, and drives food intake. Furthermore, inhibition of AMPK prevents the ability of ghrelin to increase food intake. AMPK is an integrator of cell energy status and responses to metabolic stress by promoting pathways that favor energy production (fatty acid oxidation, glucose uptake) over energy (ATP) consumption. AMPK activation also promotes mitochondrial biogenesis and function in peripheral and neuronal tissues (Bergeron et al., 2001; Dasgupta and Milbrandt, 2007; Jager et al., 2007; Zong et al., 2002), and because of this, we hypothesized that ghrelin may mediate neuroprotection in the SNpc by increasing AMPK activity. However, the neuroprotective role of AMPK is controversial, as conflicting reports have been published (Culmsee et al., 2001; Kuramoto et al., 2007; Li et al., 2007; McCullough et al., 2005).
In this study, we used a dietary approach to chronically activate AMPK by feeding mice a normal chow diet containing 1% guanidinopropionic acid (GPA) before examining SNpc TH neurodegeneration in a mouse model of Parkinson’s disease. GPA is a creatine analogue that inhibits creatine kinase activity, reduces intracellular phosphate levels and thereby robustly increases AMPK activity (Bergeron et al., 2001; Reznick and Shulman, 2006). Further, GPA stimulates AMPK-dependent mitochondrial biogenesis (Zong et al., 2002) through increased PGC1 alpha in muscle tissue (Williams et al., 2009) and suggests a similar phenomenon may occur in TH SNpc neurons to promote neuroprotection. In this study, we hypothesized that GPA-fed mice would be resistant to MPTP-induced nigral cell degeneration due to increased AMPK activity and enhanced mitochondrial biogenesis and function.
Material and Methods
Animals
We used C57/B6 male mice at 10–12 weeks of age. Mice were placed on 1 of 2 different diets, either a normal chow diet (NCD) or a normal chow diet supplemented with 1% GPA (GPA; Research Diets). Mice were group housed and remained on the diets for 32 days. The Institutional Animal Care and Use Committee of Yale University have approved all procedures described below.
Metabolic profiling
Fat and lean body masses of mice on normal or GPA diet were measured by 1H-magnetic resonance spectroscopy (NMR; Bruker Biospin) after 32 days. Total body fat and muscle were calculated and expressed as percent increase at the end of the 32-day treatment relative to beginning weights. A comprehensive mouse metabolic monitoring system (CLAMS; Columbus Instruments) was used to measure VO2, VCO2, respiratory exchange ratio (RER), activity and heat. RER and heat were calculated from VO2 and VCO2 gas exchange data. The RER value depends on the primary energy source and is the ratio of VCO2/VO2. Carbohydrate oxidation only produces an RER of 1.0, whereas fatty acid oxidation only produces a RER of 0.7. Energy expenditure was calculated as follows: (3.815 + 1.232 × RER) × VO2. Activity was measured on x and z axes using infrared beams to count the number of beam breaks during the recording period.
AMPK activity assay
The brain was rapidly dissected and immediately freeze-clamped to prevent any degradation. Samples were homogenized in 1 ml lysis buffer (50mM Tris-HCl buffer, pH 7.5, 50mM NaF, 5mMNaPPi, 1mM EDTA, 1mM EGTA, 1mM dithiothreitrol, 1mM benzamidine, 1mM phenylmethanesulfonyl fluoride, 10% glycerol v/v, 1% triton X-100 v/v. Protein concentrations were determined following centrifugation of homogenates (20,000g 10 minutes 4C). Using 1mg of protein AMPK-α2 was immunoprecipitated with an AMPK-α2 antibody (Santa Cruz Biotechnology). Brain AMPK-α2 activity was determined following the incorporation of [32P]ATP into a synthetic peptide containing the AMARA sequence the following day.
Mitochondrial respiration
The striatum was rapidly dissected from mice on normal or GPA diet and homogenized in isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1% fatty acid-free BSA, 20 mM HEPES, 1 mM EGTA, pH adjusted to 7.2 with KOH). Mitochondria were isolated as described elsewhere (Andrews et al., 2008). Protein concentrations were determined with a BCA protein assay kit (Pierce, Rockford, IL). Mitochondrial respirations were assessed using a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, UK) at 37°C with pyruvate and malate (5 and 2.5 mM) as oxidative substrates in respiration buffer (215 mM mannitol, 75 mM sucrose, 0.1% fatty acid-free BSA, 20 mM HEPES, 2 mM MgCl, 2.5 mM KH2PO4, pH adjusted to 7.2 with KOH). ADP (150μM) was then added to test ADP dependent respiration rates as ADP is phosphorlyated to ATP. After the addition of oligomycin (1μM), which blocks ATP synthase activity, palmitate (100μM) was added to test fatty acid-induced mitochondrial respiration. Total uncoupled respiration was also measured after the addition of the photonophore FCCP (1μM) as an indicator of total respiratory capacity.
MPTP administration
To ensure a robust loss of TH neurons in the SNpc, mice were injected IP with 30 mg/kg of MPTP in saline on two consecutive days. Control animals were given saline. Animals remained on respective diets until sacrifice and were perfused 7 days after the first injection and processed for immunohistochemistry or HPLC for DA and metabolites.
Immunohistochemistry and stereology
Free floating sections were stained with TH (1:5000, Chemicon International) or pAMPK (1:1000, Cell Signaling) and visualized with DAB (TH) or fluorescence (pAMPK) using standard procedures for both light and electron microscopy as previously described (Andrews et al., 2005b). We then used design-based unbiased stereology methods to quantify TH immunoreactive cells in the SNpc. Cells were visualized by a Zeiss microscope and relayed via a MicroFibre digital camera (www.optronics.com) to a computer where they were counted by a blinded observer using the optical fractionator StereoInvestigator software (MicroBrightField, Williston, VT, USA). Systematic sampling of every fourth section was collected through the SNpc beginning at approximately bregma −2.06 mm and finishing at approximately −4.16 mm. Sections were cut at 50 μm to allow for a 20 μm optical dissector within each section after dehydration and mounting. The first sample section from the first 1–4 sections collected was chosen at random. TH cells were counted in grids randomly positioned by the software in the outlined counting area through all optical planes, thus creating a 3 dimensional counting area. Guard zones were set at 1μm on both surfaces of the section and the resulting optical dissector was approximately 20μm. The counting frame width and height was 55 μm producing an area of 3025 μm. With this counting frame area we discovered that we needed to sample approximately 150 sites throughout the entire SNpc and count approximately 120 neurons throughout the entire SNpc to give an acceptable coefficient of error (CE; using the Gunderson method) of 0.104 using the smoothness factor m=0. Our CE is the average across all the groups sampled (NCD, NCD MPTP, GPA, GPA MPTP). If considering the groups individually, only the NCD MPTP group had a CE over 0.1 (0.136), this is a direct reflection of the reduced TH number visible to counts. The smoothness factor defines the cell distribution of a structure that is sensitive to small changes in sampling. We have applied the more rigorous smoothness factor of m=0 to account for sudden changes in cell distribution across our region of interest. The coefficient of error provides a means to estimate sampling precision, which is independent of natural biological variance. As the value approaches 0, the uncertainty in the estimate precision reduces. A coefficient of error = 0.1 is deemed acceptable. Cells were only counted if they touched the inclusion border or did not touch the exclusion border of the sampling grid.
We also used an additional stereological probe, the nucleator method (Moller et al., 1990), to measure cell volume at the same time as we used the optical fractionator to estimate cell number. Thus, for every cell that was counted, the volume was also measured 7 days after MPTP injection. The nucleator uses orthogonal lines generated at the midpoint of the cell, in this case we used the centre of the nucleus as a common point. Using these lines, the boundary of the cell body can be identified. The distances between the central point and the lines intersecting the cell boundary are measured and the cell volume can be calculated from the average of the third powers of these measurements. To ensure unbiased sampling on neuronal volume, this method requires uniformly random sections or vertical uniformly random sections. In practical neuroscience it is difficult to meet these requirements as neuronal sections were cut in a coronal plane to allow for light and electron microscopy. Although this created a bias, as published elsewhere (Dorph-Petersen et al., 2004), we sampled from a large number of neurons in each treatment and believe we have a robust, albeit bias, estimate of dopamine neuronal cell volume in the SNpc. The CE of <0.01 reflects the robust nature of our sampling precision.
To estimate the percentage of cells expressing TH and pAMPK, we blindly analyzed between 146–385 neurons across in 15–18 sections in n=6 mice per group. Only cells with a clear observable nucleus were counted.
Mitochondria number and volume
Animals were perfused and their brains were processed for TH immunolabeling for electron microscopic examination. Ultra thin sections were cut on a Leica ultra microtome, collected on Formvar-coated single-slot grids and analyzed with a Tecnai 12 Biotwin (FEI Company) electron microscope. Mitochondria were counted blindly from randomly selected sections, and Scion Image was used to normalize cytoplasmic area so that mitochondrial number per cell is expressed in square micrometers.
In order to estimate mitochondrial volume, the same electron micrographs used to count mitochondrial number were imported into StereoInvestigator. We used the nucleator probe, as described above, to measure mitochondrial volume by placing the probe at the centre of the mitochondrial mass. The orthogonal lines generated were marked at the boundary of the mitochondrion. There is a sampling bias due to the fact that we could not use isotropic sections for volume measures as described above. Despite the bias, we believe we have generated a robust data set by sampling large populations of mitochondria in each group (normal diet n=469, normal diet MPTP n=411, 1% GPA n=497, 1% GPA MPTP n=626), this is reflected in our sampling precision CE values of <0.04. All analyses were conducted 7 days after MPTP injection in the same animals that were used for TH cell number and volume quantification.
Striatal DA and metabolite measurements
7 days after MPTP injection both striata were rapidly dissected on a chilled glass plate and frozen on dry ice. The samples were subsequently thawed in 0.4 ml of chilled 0.1 M perchloric acid and sonicated. Aliquots were taken for protein quantification using a spectrophotometric assay. Other aliquots were centrifuged, and DA levels were measured in supernatants by HPLC with electrochemical detection. Concentrations of DA and metabolites were expressed as ng/mg protein (mean ± SEM).
Results
Effect of GPA on body weight and composition
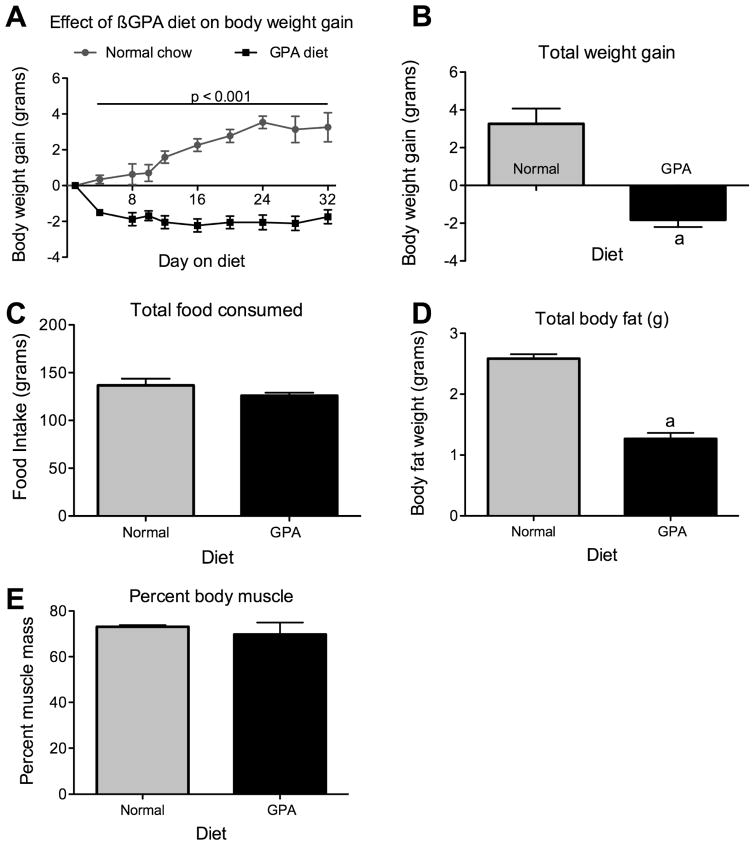
Mice were fed either a normal chow diet or 1% GPA chow diet for 4 weeks. Mice on the normal chow diet showed a slow but steady weight gain during the course of the experiment, however mice on the GPA diet lost 2 grams in body weight within the first week on the 1% GPA diet. Body weights in mice fed the 1% GPA diet remained constant for the remaining experimental period (Fig 1A, p<0.05). At the end of the experimental period, mice on the normal chow diet had gained 3.3±0.8 grams in body weight whereas the mice on a 1% GPA diet had lost 1.8±0.4 grams (Fig 1B, p<0.05). This loss of body weight was not caused by reduced food intake, as mice on GPA diet did not show a significant reduction in total food intake (NCD 136±7 vs. GPA 126±3 grams, n=15 and 20 respectively, p = 0.13, Fig 1C). The mice on GPA diet showed a significant decrease in percent body fat (NCD 10.6±0.4 vs. 5.3±0.6 percent, n = 4, p<0.05, Fig 1D) but not percent body muscle (NCD 73.1±0.7 vs. 69.75±5.2 percent, n = 4, p=0.27, Fig 1E) indicating that the loss of body weight is due to decreased body fat.
Figure 1. The effect of 1% GPA diet on body weight regulation.
A, Mice on a 1% GPA diet (n=19) exhibited a significantly reduce body weight relative to normal chow fed mice (n=15). Body weight loss stabilized after the 1st week on the diet and remained constant thereafter. B, Total body weight gain after 32 days on either normal chow or 1% GPA diet. C, Loss of body weight is not due to decreased food intake as no difference was observed over the diet period. D, Reduced body weight in GPA fed mice was due to a loss of body fat but not percent body muscle as measured by NMR, E. Data are presented as mean ± sem. a, significant with respect to normal chow fed mice (2-tailed t-test, p < 0.05).
Effect of GPA on metabolic parameters
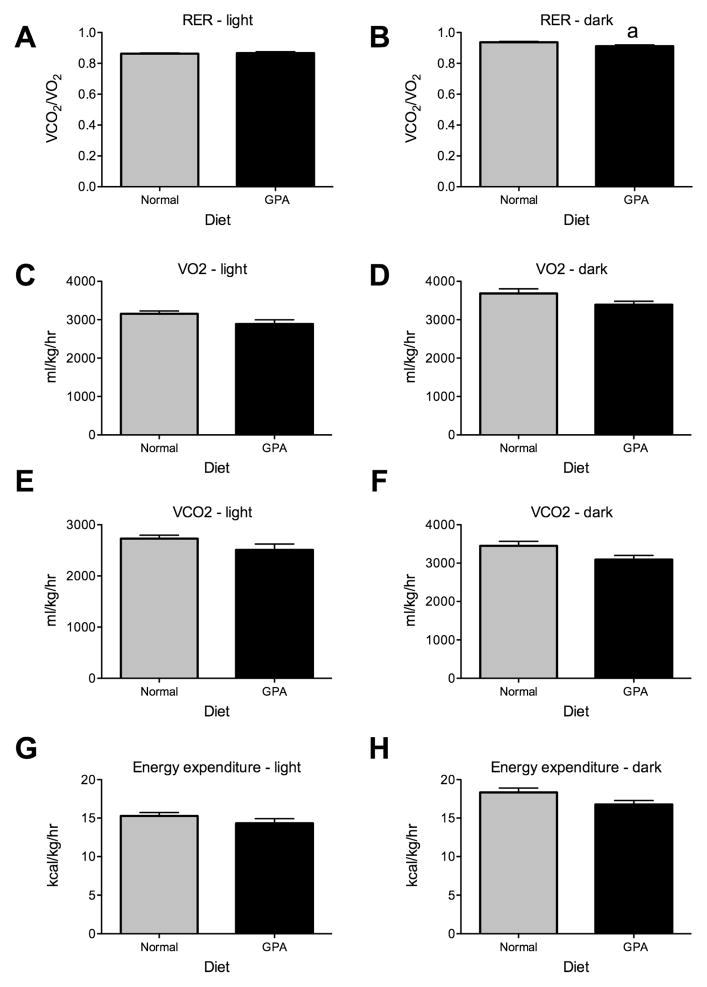
In order to determine whether GPA affects metabolic parameters and accounts for changes in body weight, we housed mice on normal chow or GPA diets in metabolic cages for 4 days. The analysis of results indicated GPA-fed mice exhibited significantly reduced RER during the dark phase (NCD 0.937±0.010 vs. GPA 0.911±0.016, n=4, p<0.05, Fig. 2B), but not during the light phase. The reduction in RER indicates these mice had greater rates of fatty acid oxidation relative to mice on normal chow diet. There was no difference in activity in mice fed a normal or GPA diet during either the light or dark phase indicating that the weight loss on the GPA diet was not a product of increased exercise (Fig. 2G&H). This was further supported by the lack of a significant difference in energy expenditure (Fig. 2I&J). Finally, we observed no significant differences in VO2 or VCO2 (Fig. 2C–F).
Figure 2. The effect of 1% GPA diet on metabolic parameters.
A&B, GPA decreased RER during the dark phase but not the light phase (n=4). C–F, No differences in VO2 or VCO2 were observed in either the light or dark phase. G&H, There were no significant differences in energy expenditure between mice or a normal chow or GPA diet, indicating that reduced body weight was solely due to increased fatty acid oxidation as measured by RER.
Data are presented as mean ± sem. a, significant with respect to normal chow fed mice (2-tailed t-test, p < 0.05).
GPA increases striatal AMPK and mitochondrial respiration
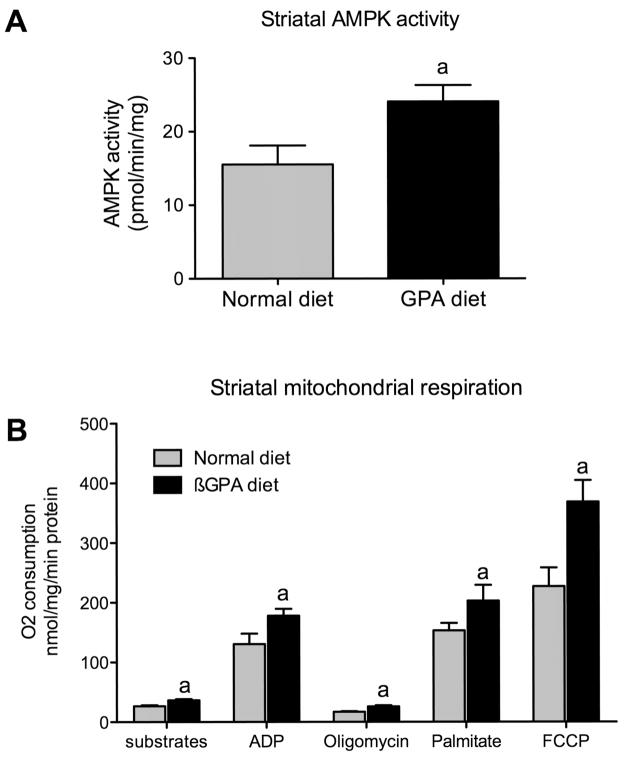
GPA is a creatine analog that decreases cellular phosphocreatine levels in all cell types including neuronal cells (Lunardi et al., 2006). In muscle, GPA increases AMPK activity by increasing the AMP/ATP ratio (Bergeron et al., 2001). In order to test whether GPA also increases AMPK activity in the striatum, we measured AMPK-α2 activity in GPA and NCD fed mice. We observed a significant increase in AMPK-α2 activity in GPA relative to NCD fed mice (NCD 15.5±2.5 vs. GPA 24.1±2.2 pmol/min/mg, n=8, p<0.05, Fig 3A). We previously demonstrated that AMPK activation with AICAR drives neuronal mitochondrial respiration (Andrews et al., 2008) and therefore we hypothesized that GPA-induced AMPK activity will increase mitochondrial respiration in the brain. We examined mitochondrial respiration in the striatum of GPA and NCD fed mice as the striatum is an important component of the nigrostriatal dopamine system and reduced striatal dopamine is a major hallmark of PD. We observed increased mitochondrial respiration in all states measured. Substrate driven mitochondrial respiration with pyruvate and malate was increased (NCD 26.7±1.5 vs. GPA 36.7±1.7 nmol oxygen/min/mg protein, n=6, p<0.05, Fig. 3B), as was ADP-dependent respiration (NCD 130.5±17.6 vs. GPA 178.1±11.3 nmol oxygen/min/mg protein, n=6, p<0.05) indicating enhanced capacity for phosphorylation to ATP. Oligomycin-induced respiration was also increased (NCD 17.6±1.7 vs. GPA 24.9±2.6 nmol oxygen/min/mg protein, n=6, p<0.05), suggesting that basal uncoupling was enhanced in GPA fed mice, an effect known to regulate nigrostriatal dopamine function and restrict MPTP-induced dopamine cell loss (Andrews et al., 2005b; Andrews et al., 2006). Fatty acid-induced respiration was increased (NCD 153.1±12.6 vs. GPA 203.1± 26.3 nmol oxygen/min/mg protein, n=6, p<0.05), indicating enhanced capacity to oxidize fatty acids. Finally, FCCP-induced respiration (NCD 215.4±33.3 vs. GPA 368.9±32.1 nmol oxygen/min/mg protein, n=6) was enhanced in GPA-fed mice, indicating these mice had elevated total respiratory capacity, suggesting that the GPA diet increased neuronal mitochondrial biogenesis as seen in muscle (Bergeron et al., 2001). These results collectively show that the GPA diet increased striatal AMPK activity and mitochondrial respiration.
Figure 3.
1% GPA diet increases striatal AMPK activity and mitochondrial respiration. A, Mice fed a 1% GPA diet for 32 days showed a significant increase in striatal AMPK- α2 activity (n=8, 2-tailed t-test, p < 0.05). B, GPA diet also increased mitochondrial respiration in all states examined. Substrates were pyruvate (5mM) and malate (2.5mM), followed by ADP (150μM), oligomycin (1μM), palmitate (100μM) and FCCP (1μM). a, Significant with respect to normal chow diet fed values (n=6, 2-tailed t-test, p < 0.05). Data are presented as mean ± sem.
GPA prevents MPTP-induced cell loss and attenuates striatal dopamine loss
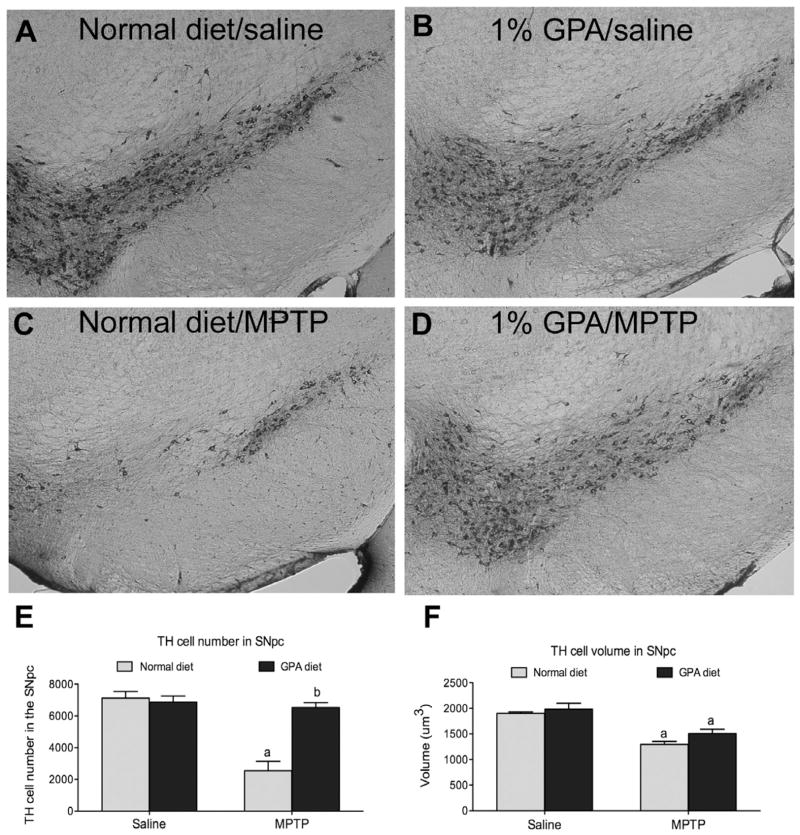
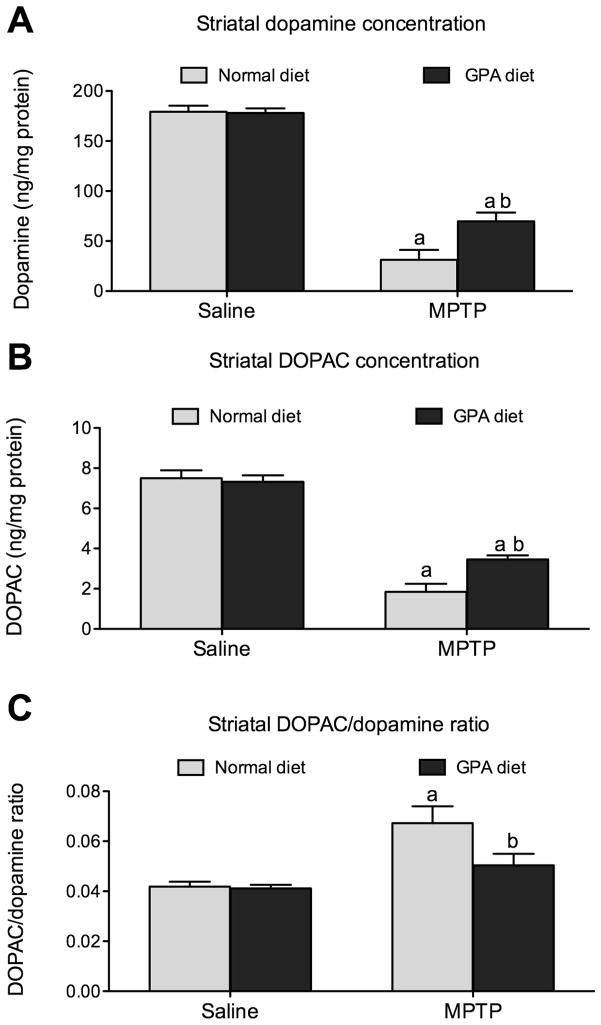
To test whether increased brain AMPK and mitochondrial respiration by GPA is neuroprotective in mouse models of PD, we administered MPTP to NCD- or GPA-fed mice. We used design-based stereology to estimate the number of TH neurons in the SNpc in an unbiased manner. TH cells were counted using the optical fractionator. In NCD-fed mice, MPTP resulted in a significant reduction in TH cell number in the SNpc (NCD 6798.6±557.9 vs. NCD MPTP 2117.7±643.5 cells, n=6, p<0.05, Fig 4E). Remarkably, mice on the GPA diet showed no cell loss when given MPTP compared to controls (NCD 6643.8±546.8 vs. GPA MPTP 6529.6±306.1, n=6, Fig 4E). The SNpc TH neurons send nerve terminals to the striatum thus we measured striatal dopamine, DOPAC and the DOPAC/dopamine as further characterization of the nigrostriatal pathway in NCD- and GPA-fed mice after MPTP administration. In NCD-fed mice, MPTP resulted in an 82% loss of dopamine in the striatum (NCD 177.2±6.0 vs. NCD MPTP 31.3±8.9, n=9 & n=5 respectively, p<0.05, Fig 5A). Despite showing no loss of TH neurons in the SNpc, MPTP administration to GPA fed mice still produced a 60% decrease in striatal dopamine (GPA 178.0±4.9 vs. GPA MPTP 69.81±7.79, n=13 & n=6 respectively, p<0.05, Fig 5A), suggesting retrograde degeneration from the nerve terminals back to the cell bodies as seen in human PD (Dauer and Przedborski, 2003). Although MPTP initiated significant dopamine loss in GPA fed mice, the amount of loss was significantly attenuated in GPA vs. NCD fed mice, further supporting the neuroprotective effect of GPA on the nigrostriatal dopamine system. GPA also attenuated the loss of the striatal DOPAC after MPTP compared to NCD fed mice (NCD MPTP 1.84±0.36 vs. GPA MPTP 4.0±0.6, p<0.05, Fig. 5B). Finally, we measured the DOPAC/dopamine ratio as an index of nigrostriatal dopamine neuronal activity and found that GPA significantly attenuates the increase in the DOPAC/dopamine ratio (NCD MPTP 0.07±0.01 vs. GPA MPTP 0.05 ±0.005, n=13 & n=6, p<0.05, Fig. 5C). In parallel with changes in striatal dopamine concentration we also observed similar difference in striatal TH immunoreactivity before and after MPTP treatment in both dietary groups (see supplementary information).
Figure 4. 1% GPA diet prevents TH cell loss in the SNpc.
A–D Representative figures of TH immunoreactivity in the SNpc (approximately Bregma -3.16). E, MPTP administration produced a significant decrease in SNpc TH neurons in normal chow diet fed mice, as measured using unbiased design-based stereology (optical fractionator probe). Strikingly, 1% GPA fed mice showed no significant loss of SNpc TH neurons after MPTP injection (n=6, 2-way ANOVA, p<0.05). F, TH cell volume was also measured using the nucleator as the stereological probe from all counted TH neurons. The mean TH cell volume was decreased in both normal chow fed and 1% GPA fed mice (n=319–738, ANOVA, p<0.05).
a, Significant with respect to saline injected controls, b, significant with respect to MPTP treated normal chow fed mice. Data are presented as mean ± sem.
Figure 5. 1% GPA attenuates striatal dopamine and DOPAC loss.
A, MPTP administration caused a significant loss of striatal dopamine from both normal chow and 1% GPA fed mice however the degree of loss was significantly attenuated in 1% GPA fed mice. B, Striatal DOPAC loss was significantly attenuated in mice on a 1% GPA diet. C, MPTP treated produced a significant increase in the DOPAC/dopamine ratio in normal chow fed but not 1% GPA fed mice. The increase in the DOPAC/dopamine ratio indicates greater stress placed on the remaining nerve terminals to maintain dopamine release.
a, Significant with respect to saline-injected controls, b, significant with respect to MPTP treated normal chow fed mice. Data are presented as mean ± sem (n=5–13, two-way ANOVA, p<0.05).
In order to closely examine TH cell morphology in both NCD and GPA fed diets after MPTP treatment we measured cell volume using the nucleator in the same sections as those used to determine TH cell number. Interestingly, both NCD-fed and GPA-fed MPTP-treated mice exhibited decreased cell volume compared to controls, despite no TH cell loss observed in GPA-fed mice (NCD 1902±27 vs. NCD MPTP 1291±60, n= 738 & n=319 respectively; (GPA 1984±117 vs. GPA MPTP 1508±82, n=619 & n=646 respectively, p<0.05, Fig. 4B). While the GPA diet did not prevent the reduction in cell volume, we believe it provided the necessary bioenergetic or metabolic status to mitigate TH cell loss in response to MPTP.
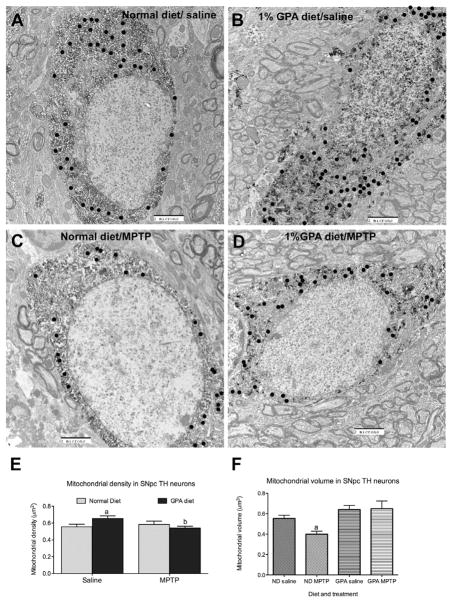
GPA prevents a decrease in mitochondrial number and volume
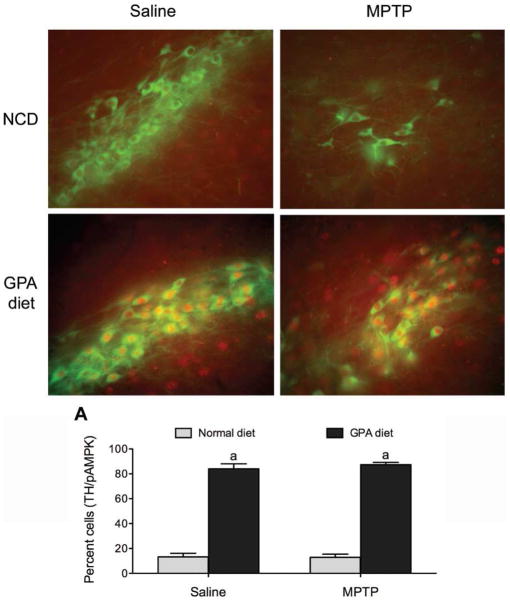
Because GPA increased mitochondrial respiration and is known to increased biogenesis in muscle, we hypothesized that attenuated loss in GPA-fed mice was due to enhanced mitochondrial biogenesis despite a reduction in cell volume. We found using electron micrographs that GPA significantly increased mitochondrial density in SNpc TH neurons compared to NCD mice 7 days after MPTP injection (NCD 0.56±0.03 vs. GPA 0.65±0.02 mitochondrial number/μm2, n=25 & 34 respectively, p<0.05, Fig 6E), suggesting that GPA promotes neuronal mitochondrial biogenesis, which helps to prevent TH cell loss after MPTP treatment. Because MPTP is a mitochondrial toxin that inhibits complex 1 activity, we reasoned that MPTP would decrease mitochondrial density. Surprisingly, we found MPTP reduced mitochondrial density only in TH neurons from GPA-fed mice and not NCD-fed mice (Fig. 6E). However, closer inspection of the data revealed that absolute mitochondrial number in NCD MPTP mice was decreased relative to GPA MPTP mice (NCD MPTP 33±3 vs. GPA MPTP 44±4 mitochondria counted/cell, n=19 and 31 respectively) and the lack of difference in mitochondrial density was due to parallel decreases in cell volume in NCD MPTP-treated mice. To further investigate the effect of GPA on mitochondrial morphology after MPTP intoxication, we measured mitochondrial volume in identified TH SNpc neurons in electron micrographs using the stereological probe, the nucleator. We observed that MPTP significantly reduces mitochondrial volume in NCD-fed mice (NCD 0.55±0.03 vs. NCD MPTP 0.39±0.03 μm3, n=469 & 411 respectively, p<0.05, Fig 6F), however MPTP did not produce a similar decrease in mitochondrial volume in SNpc TH neurons of GPA fed mice (GPA 0.64±0.04 vs. GPA MPTP 0.65±0.08 μm3, n=497 & 626 respectively, p<0.05, Fig 6F). These results suggest that GPA protects against MPTP-induced TH cell loss by increasing mitochondrial number and preventing the MPTP-induced loss in mitochondrial number and volume. We argue that GPA-induced AMPK activity may be a key cellular mechanism enhancing these mitochondrial mechanisms. In line with this idea, GPA causes a strong induction of pAMPK staining in TH neurons when compared to NCD-fed mice and this is still present after MPTP intoxication. Greater than 80% of SNpc TH neurons expressed pAMPK on a 1% GPA diet and this was not affected by MPTP injection (p<0.05, Fig 7), consistent with the idea that activation of AMPK by 1% GPA induces neuroprotective parameters to prevent degeneration. On the other hand, less than 20% of SNpc TH neurons express pAMPK on a normal chow diet. Our results argue that these neuroprotective parameters include increased mitochondrial function.
Figure 6. 1% GPA regulates mitochondrial number and volume within SNpc TH cells.
A–D, Representative electrons micrographs of SNpc TH neurons using in the estimation of mitochondrial number and volume (black closed circles indicate identified mitochondria). Darkened areas denote the cell positive for a DAB reaction. E, 1% GPA increased mitochondrial density in SNpc TH neurons without MPTP injection. After MPTP injection, mitochondrial density in SNpc TH cells from 1% GPA mice was significantly reduced to normal chow fed levels. The decrease in density was due to changes in total cell volume, when absolute mitochondrial number counted per cell was examined, normal chow fed MPTP-treated mice, relative to 1% GPA MPTP-treated mice, exhibited reduced mitochondrial number (n=25–34, two-way ANOVA, p<0.05).
F, Further stereological and ultra-structural analysis revealed decreased mitochondrial volume in SNpc TH neurons from normal chow fed mice treated with MPTP relative to saline controls. However, 1% GPA fed mice showed no decrease in mitochondrial volume after MPTP injection. (n=411–626, ANOVA, p<0.05). a, Significant with respect to saline-injected controls, b, significant with respect to saline-treated 1% GPA-fed mice. Data are presented as mean ± sem.
Figure 7. 1% GPA diet increases pAMPK in SNpc TH neurons.
Representative sections through the SNpc showing pAMPK (red fluorescence) staining in TH neurons (green fluorescence) from mice on a normal chow diet (NCD) or 1% GPA diet. A, Quantification of TH cell expressing pAMPK in the SNpc. a, Significant with respect to mice on a normal chow diet injected with saline. Data are presented as mean ± sem.
Discussion
In this study we show that orally administered GPA protects SNpc TH neurons and this may involve increased AMPK activity in SNpc TH neurons. We used design-based stereology to show that GPA regulates TH cell number, cell volume and mitochondrial number and morphology within SNpc TH neurons while decreasing degeneration. In particular, GPA prevented MPTP-induced decrease of TH cell number in the SNpc and partially retained dopamine levels in the striatum. We speculate that elevated AMPK activity confers the neuroprotective effects of GPA by regulating mitochondrial biogenesis and function. Robust pAMPK staining in TH neurons with and without MPTP indicates that GPA directly enhances AMPK function in SNpc TH neurons. The ability of GPA to activate AMPK in the brain is consistent with previous reports showing that GPA increases AMPK activity in peripheral tissues, such as the muscle (Bergeron et al., 2001; Chaturvedi et al., 2009; Reznick et al., 2007; Zong et al., 2002). Furthermore, GPA increased mitochondrial respiration in the striatum, which is consistent AMPK activation driving mitochondrial respiration (Canto et al., 2009). Collectively, these studies show that GPA promotes AMPK activity in the brain and also increases downstream consequences of AMPK activation such as mitochondrial respiration. It is noted that GPA has major effects of peripheral metabolism, especially muscle (Bergeron et al., 2001; Chaturvedi et al., 2009; Reznick et al., 2007; Zong et al., 2002). Indeed our results showed that GPA lowered body weight, independent of food intake changes. The reduced body weight, which is solely due to decreased body fat, is ascribed to elevated whole body fatty acid oxidation as measured by RER. We propose that GPA and GPA-induced AMPK activity drives fatty oxidation in the periphery and lowers body weight. This is supported by numerous papers, in which GPA and AMPK promote peripheral fatty acid oxidation (Bergeron et al., 2001; Canto et al., 2009; Chaturvedi et al., 2009; Jager et al., 2007; Lunardi et al., 2006; Williams et al., 2009).
Our recent study highlighted the neuroprotective role of ghrelin in the MPTP mouse model of PD (Andrews et al., 2009). In that study we observed that ghrelin increased mitochondrial respiration and biogenesis, which led to improved mitochondrial function, reduced free radical production and reduced TH SNpc cell death. These effects were completely dependent upon UCP2, due to the lack of effect seen in UCP2 knockout mice, supporting the previously established requirement for UCP2 in nigrostriatal function and neuroprotection (Andrews et al., 2005b; Andrews et al., 2006; Conti et al., 2005). Within the hypothalamus, ghrelin binds to its receptor, increases AMPK activity and downstream UCP2 activity to drive food intake (Andrews et al., 2008). This AMPK-UCP2 pathway is critical to enhance mitochondrial function, which fosters an optimal bioenergetic state and facilitates neuronal function. Although UCP2 was required for mitochondrial biogenesis, this does not discount other important regulators of mitochondrial biogenesis such as mitochondrial transcription factor A (Tfam). Tfam knockout in dopamine neurons causes progressive parkinsonism (Ekstrand et al., 2007) and UCP2 and Tfam are known to regulate mitochondrial biogenesis in muscle cells (Wu et al., 1999). Given that there are many ghrelin receptors present in the SNpc and that ghrelin stimulates mitochondrial function via an AMPK-UCP2 pathway, we tested whether a GPA diet, which is known to increase peripheral AMPK activity, (Bergeron et al., 2001; Reznick et al., 2007; Zong et al., 2002), would also promote brain AMPK activity and protect SNpc TH neurons by enhancing mitochondrial function.
Remarkably, GPA completely prevented the loss of TH cells in the SNpc after an MPTP dose that reduced significantly the number of TH cells in control animals. GPA administration also partially retained dopamine and DOPAC in nerve terminal regions. These data are consistent with the fact that MPTP-induced degeneration in the nigrostriatal pathway is initiated in nerve terminals and involves retrograde degeneration (Burke et al., 1992; Macaya et al., 1994; Sauer and Oertel, 1994) in a manner also seen in human PD patients (Dauer and Przedborski, 2003). Intriguingly, impaired energy metabolism in the striatum enhances dopamine cell loss in the SNpc (Sonsalla et al., 1997), thus GPA may prevent dopamine cell loss by enhancing energy metabolism in the striatum. In support of this concept, our current results show that GPA increases AMPK activity and mitochondrial respiration in the striatum.
In this study, we observed a decrease in TH cell volume after MPTP treatment in mice fed normal chow or GPA. Although there was a trend increase in GPA TH cell volume, no statistical difference in TH cell volume was observed between normal chow and GPA fed mice. Furthermore, TH cell volume results are largely consistent with published data, in which MPTP reduces TH cell volume as measured by the Cavalieri method (Aguirre et al., 1999; Ahmad et al., 2009). In these studies, average TH volume was ≈1000–1200 μm3 and in our study TH volume on average ranged from 1984 μm3 to 1291 μm3, we believe this is a more accurate reflection of true TH volume because of the large number of neurons sampled, the robust CE value and the use of the more valid probe, the nucleator (Moller et al., 1990). We argue that a decrease in TH cell volume is not the causative factor in degeneration because TH cell volume was equally reduced in both MPTP treated normal diet and GPA fed mice.
GPA significantly increased mitochondrial biogenesis in SNpc TH neurons consistent with reported effects in muscle tissue (Williams et al., 2009; Zong et al., 2002), restricts the decrease in absolute mitochondrial number and prevents the decrease in mitochondrial volume after MPTP treatment. To our knowledge, this is the first study to use stereology to analysis mitochondrial volume after MPTP treatment. There are reports in the literature of both increases and decreases mitochondrial size in models of Parkinson’s disease (Liang et al., 2007; Song et al., 2004; Stichel et al., 2007), however studies have only estimated mitochondrial size and no study has used unbiased stereology to investigate mitochondrial volume. Ours results are consistent with the idea that low mitochondrial mass predisposes to neurodegeneration (Liang et al., 2007) and supports the concept that mitochondrial dysfunction lies at the heart of Parkinson’s disease pathology (Abou-Sleiman et al., 2006; Hattingen et al., 2009). Moreover, recent studies show that monogenic mutations (parkin, DJ1, PTEN-induced Kinase 1) in human Parkinsonism, all manifest mitochondrial dysfunction (Clark et al., 2006; Palacino et al., 2004; Zhang et al., 2005).
Because GPA diet dramatically activates pAMPK in SNpc TH neurons, we argue that GPA–induced changes in mitochondrial number and volume are driven by increased AMPK activity within cells. In support of this GPA promotes AMPK activity and mitochondrial biogenesis in muscle tissue (Bergeron et al., 2001; Reznick and Shulman, 2006; Williams et al., 2009; Zong et al., 2002). AMPK also activates PGC1 alpha in muscle (Jager et al., 2007) and PGC1 alpha restricts MPTP-induced Parkinson’s disease by promoting mitochondrial anti-oxidative mechanisms (St-Pierre et al., 2006). PGC1 alpha is regarded as the master control of mitochondrial biogenesis (Lopez-Lluch et al., 2008) and GPA initiates mitochondrial biogenesis through PGC1 alpha in muscle (Williams et al., 2009). These studies, together with the results herein, build an attractive model whereby GPA robustly prevents MPTP-induced cell death by increasing mitochondrial respiration, number and volume through an AMPK-PGC1 alpha interaction in the SNpc. Recent genome wide expression data from SNpc dopamine neurons in human Parkinson’s disease patients show that PGC1 alpha is a potential therapeutic target for Parkinson’s disease (Zheng et al., 2010).
Our current study raises the possibility that neuronal AMPK activation is protective in models of neurodegeneration and brain disease, however there are conflicting reports in the literature. Two studies reported that pharmacological inhibition of AMPK and gene deletion of the alpha 2 catalytic subunit promoted neuroprotection after focal ischemia (Li et al., 2007; McCullough et al., 2005). However, these studies are yet to be independently verified and may be isolated in the literature given that resveratrol, an activator of neural AMPK (Dasgupta and Milbrandt, 2007), protects against models of stroke and cerebral ischemia (Dong et al., 2008; Raval et al., 2006; Tsai et al., 2007; Yousuf et al., 2009). Furthermore, resveratrol protects against neurodegeneration in models of PD (Bournival et al., 2009; Chao et al., 2008; Jin et al., 2008; Long et al., 2009; Lu et al., 2008) in line with our current study. We suspect that AMPK activation mediates the neuroprotective effects of resveratrol in PD models although this hypothesis is yet to be tested. Further evidence that supports a protective role for AMPK comes from studies, in which AMPK activation increases lifespan in mice and C. Elegans (Baur et al., 2006; Greer and Brunet, 2009; Greer et al., 2007).
In summary, we show for the first time that GPA restricts TH cell loss in the SNpc after MPTP treatment and may be an attractive therapeutic to prevent the development of Parkinson’s disease. The neuroprotective potential of GPA in other degenerative paradigms is yet to be investigated but seems likely to yield promising results. The actions of GPA on SNpc survivability are possibly mediated by an AMPK mechanism that promotes mitochondrial respiration, number and morphological stability. By maintaining these mitochondrial functions, SNpc TH neurons are robustly protected from MPTP-induced TH cell death. GPA also ameliorates hyperglycemia and restores insulin sensitivity (Meglasson et al., 1993) and active analogues have been developed to diabetes and obesity (Larsen et al., 2001; Vaillancourt et al., 2001). Recent epidemiological studies show that midlife diabetes and obesity can predispose to Parkinson’s disease later in life (Abbott et al., 2002; Bachmann and Trenkwalder, 2006; Hu et al., 2007; Hu et al., 2006). These results support the concept that energy metabolism and body weight regulation plays a critical role not only in peripheral disease states but also neurodegeneration (Andrews et al., 2009). Moreover, therapeutic agents that control glucose homeostasis and reduce obesity may also help to slow neurodegeneration.
Supplementary Material
Research highlights.
GPA prevents dopamine cell loss in substantia nigra
GPA prevents dopamine cell loss by maintaining mitochondrial biogenesis
GPA prevents dopamine cell loss by activating AMPK in substantia nigra dopamine neurons
Acknowledgments
This work was supported by a New Zealand Foundation for Research, Science and Technology fellowship, Monash Fellowship and NHMRC grant (NHMRC 546131) to ZBA, and NS 056181 to JDE.
Footnotes
Disclosure Statement
All authors have nothing to disclosure. There are no actual or potential conflicts of interest with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RD, et al. Midlife adiposity and the future risk of Parkinson’s disease. Neurology. 2002;59:1051–7. doi: 10.1212/wnl.59.7.1051. [DOI] [PubMed] [Google Scholar]
- Abizaid A, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Sleiman PM, et al. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–19. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Aguirre JA, et al. A stereological study on the neuroprotective actions of acute modafinil treatment on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigral lesions of the male black mouse. Neurosci Lett. 1999;275:215–8. doi: 10.1016/s0304-3940(99)00706-5. [DOI] [PubMed] [Google Scholar]
- Ahmad SO, et al. Effects of endurance exercise on ventral tegmental area neurons in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid-treated mice. Neurosci Lett. 2009;450:102–5. doi: 10.1016/j.neulet.2008.11.065. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, et al. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nature Reviews Neuroscience. 2005a;6:829–40. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057–65. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, et al. Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson’s disease.[erratum appears in J Neurosci. 2005 Feb 23;25(8):table of contents] Journal of Neuroscience. 2005b;25:184–91. doi: 10.1523/JNEUROSCI.4269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–51. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, et al. Uncoupling protein-2 promotes nigrostriatal dopamine neuronal function. European Journal of Neuroscience. 2006;24:32–6. doi: 10.1111/j.1460-9568.2006.04906.x. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Trenkwalder C. Body weight in patients with Parkinson’s disease. Mov Disord. 2006;21:1824–30. doi: 10.1002/mds.21068. [DOI] [PubMed] [Google Scholar]
- Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–6. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Bournival J, et al. Protective Effects of Resveratrol and Quercetin Against MPP(+) -Induced Oxidative Stress Act by Modulating Markers of Apoptotic Death in Dopaminergic Neurons. Cell Mol Neurobiol. 2009 doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, et al. Neonatal hypoxic-ischemic or excitotoxic striatal injury results in a decreased adult number of substantia nigra neurons. Neuroscience. 1992;50:559–69. doi: 10.1016/0306-4522(92)90447-a. [DOI] [PubMed] [Google Scholar]
- Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, et al. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45:1019–26. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum Mol Genet. 2009;18:3048–65. doi: 10.1093/hmg/ddp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–6. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Conti B, et al. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- Culmsee C, et al. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dong W, et al. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J Vasc Surg. 2008;48:709–14. doi: 10.1016/j.jvs.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, et al. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: volume, neuron number, and cell types. J Comp Neurol. 2004;472:449–62. doi: 10.1002/cne.20055. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–30. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–38. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispert S, et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–27. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C elegans. Curr Biol. 2007;17:1646–56. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingen E, et al. Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain. 2009 doi: 10.1093/brain/awp293. [DOI] [PubMed] [Google Scholar]
- Hu G, et al. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2007;30:842–7. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- Hu G, et al. Body mass index and the risk of Parkinson disease. Neurology. 2006;67:1955–9. doi: 10.1212/01.wnl.0000247052.18422.e5. [DOI] [PubMed] [Google Scholar]
- Jager S, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F, et al. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol. 2008;600:78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, et al. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–47. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen SD, et al. Synthesis and biological activity of analogues of the antidiabetic/antiobesity agent 3-guanidinopropionic acid: discovery of a novel aminoguanidinoacetic acid antidiabetic agent. J Med Chem. 2001;44:1217–30. doi: 10.1021/jm000095f. [DOI] [PubMed] [Google Scholar]
- Li J, et al. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–9. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CL, et al. Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson’s disease. Exp Neurol. 2007;203:370–80. doi: 10.1016/j.expneurol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Long J, et al. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson’s disease model. Rejuvenation Res. 2009;12:321–31. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, et al. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–9. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, et al. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem. 2008;56:6910–3. doi: 10.1021/jf8007212. [DOI] [PubMed] [Google Scholar]
- Lunardi G, et al. The creatine transporter mediates the uptake of creatine by brain tissue, but not the uptake of two creatine-derived compounds. Neuroscience. 2006;142:991–7. doi: 10.1016/j.neuroscience.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Macaya A, et al. Apoptosis in substantia nigra following developmental striatal excitotoxic injury. Proc Natl Acad Sci U S A. 1994;91:8117–21. doi: 10.1073/pnas.91.17.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu R, et al. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J Neurochem. 2009;108:1561–74. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, et al. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Meglasson MD, et al. Antihyperglycemic action of guanidinoalkanoic acids: 3-guanidinopropionic acid ameliorates hyperglycemia in diabetic KKAy and C57BL6Job/ob mice and increases glucose disappearance in rhesus monkeys. J Pharmacol Exp Ther. 1993;266:1454–62. [PubMed] [Google Scholar]
- Moller A, et al. Efficient estimation of cell volume and number using the nucleator and the disector. J Microsc. 1990;159:61–71. doi: 10.1111/j.1365-2818.1990.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–22. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Park J, et al. The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem Biophys Res Commun. 2009;378:518–23. doi: 10.1016/j.bbrc.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Przedborski S, et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992;12:1658–67. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, et al. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–7. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–9. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–6. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schapira AH, et al. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Song DD, et al. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol. 2004;186:158–72. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, et al. Inhibition of striatal energy metabolism produces cell loss in the ipsilateral substantia nigra. Brain Res. 1997;773:223–6. doi: 10.1016/s0006-8993(97)00941-4. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stichel CC, et al. Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum Mol Genet. 2007;16:2377–93. doi: 10.1093/hmg/ddm083. [DOI] [PubMed] [Google Scholar]
- Tsai SK, et al. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–53. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Vaillancourt VA, et al. Synthesis and biological activity of aminoguanidine and diaminoguanidine analogues of the antidiabetic/antiobesity agent 3-guanidinopropionic acid. J Med Chem. 2001;44:1231–48. doi: 10.1021/jm000094n. [DOI] [PubMed] [Google Scholar]
- Williams DB, et al. Muscle-specific differences in the response of mitochondrial proteins to beta-GPA feeding: an evaluation of potential mechanisms. Am J Physiol Endocrinol Metab. 2009;296:E1400–8. doi: 10.1152/ajpendo.90913.2008. [DOI] [PubMed] [Google Scholar]
- Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yousuf S, et al. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009;1250:242–53. doi: 10.1016/j.brainres.2008.10.068. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–73. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zheng B, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.