Abstract
Cell loss immediately adjacent to an intracerebral hemorrhage may be mediated in part by the toxicities of extracellular hemoglobin (Hb) and thrombin. However, at low concentrations, these proteins induce tolerance to hemin and iron that may limit further peri-hematomal injury as erythrocyte lysis progresses. The mechanisms mediating these preconditioning effects have not been completely defined, but increased expression of both heme oxygenase (HO)-1 and iron binding proteins likely contributes. In the present study, we hypothesized that iron chelator therapy would attenuate this protective response. Pretreatment of cortical glial cultures (> 90 % GFAP+) with 3 μM methemoglobin (metHb) or 5 units/ml thrombin for 24 h was nontoxic per se, and increased HO-1 and ferritin expression. When challenged with a toxic concentration of hemin, the increase in cellular redox-active iron was attenuated in preconditioned cultures and cell survival was increased. However, if cultures were pretreated with metHb or thrombin plus deferoxamine or 2,2′-bipyridyl, ferritin induction was prevented and cellular redox-active iron increased with hemin treatment. Preconditioning-mediated cytoprotection was consistently reduced by deferoxamine, while 2,2′-bipyridyl had a variable effect. Neither chelator altered HO-1 expression. A cytoprotective response was preserved when chelator therapy was limited to 11 hours of the 24 h preconditioning interval. These results suggest a potentially deleterious effect of continuous iron chelator therapy after ICH. Intermittent therapy may remove peri-hematomal iron without negating the benefits of exposure to low concentrations of Hb or thrombin.
Keywords: Heme, Intracerebral hemorrhage, Iron, Ischemia, Stroke, Subarachnoid hemorrhage
Introduction
A considerable body of experimental evidence suggests that release of thrombin and hemoglobin (Hb) from an intracerebral hematoma contributes to cell loss in adjacent tissue. Thrombin, via its interaction with protease-activated receptors, may increase perihematomal injury by activating matrix metalloproteinase-9, NADPH oxidase, and complement, upregulating NMDA receptors, and increasing neuronal cell cycle reentry [1–3]. Hb toxicity is mediated by release of its heme moieties after autoxidation to methemoglobin, resulting in hemin or iron-catalyzed free radical reactions [4]. The hypothesis that nonheme iron toxicity participates in the pathogenesis of intracerebral hemorrhage (ICH) is supported by observations that iron chelators are protective when administered to rodents or piglets as bolus injections [5–7], and is now being tested in clinical trials [8].
The protective effects of Hb and thrombin have been less-intensively investigated, but may be critical in limiting iron-mediated perihematomal injury as erythrocyte lysis progresses. Exposure to nontoxic concentrations of either protein induces robust tolerance to hemin or iron-mediated oxidative stress [9–11]. Although the mechanisms mediating these phenomena have not been defined, both thrombin and Hb increase expression of heme oxygenase (HO)-1 and iron binding proteins [9–12], thereby facilitating heme breakdown and iron sequestration.
The primary intracellular defense against redox-active iron is provided by ferritin, a 24-mer protein that can sequester and detoxify over 4000 iron atoms in its mineral core [13]. Cell ferritin synthesis is inhibited under physiologic conditions by the mRNA binding activities of iron regulatory protein (IRP)-1 and IRP-2. As cell iron levels increase, IRP’s are rapidly degraded and binding activity is decreased, enabling translation. We recently reported that both deferoxamine (DFO) and 2,2′-bipyridyl enhance iron export and inhibit its re-uptake in cultured neural cells [14], significantly decreasing nonheme iron. Since ferritin expression is a direct function of cell iron content, we hypothesized that chelators would prevent the increase in ferritin produced by Hb or thrombin preconditioning, and eliminate its subsequent protective effect. Given the potential relevance to iron chelator therapy for ICH, this hypothesis was tested in cultured glial cells, which are key drivers of preconditioning responses in the CNS [15].
Materials and Methods
Primary Glial Cultures
Cortical glial cultures (> 90 % GFAP +) were prepared from 2–3 day old C57BL/6 X 129/Sv mice that were bred in our animal facility, following a protocol that was approved by the local Institutional Animal Care and Use Committee and previously described in detail [16]. Approximately two-thirds of the culture medium was replaced at 5–6 days in vitro and then twice weekly, using growth medium containing minimal essential medium (MEM), 23 mM glucose, 2 mM glutamine, and 10 % equine serum (Hyclone, Logan, UT, USA).
Cytotoxicity Experiments
Confluent cultures were washed free of growth medium and were then exposed for 24 h to 3 μM bovine oxidized hemoglobin (methemoglobin, metHb) or 5 units/ml thrombin, alone or with 100 μM of either deferoxamine or 2,2′-bipyridyl (all reagents purchased from Sigma-Aldrich, St. Louis, MO). Preconditioning concentrations of metHb and thrombin were based on prior published studies [10, 17] and preliminary experiments that demonstrated efficacy in this cell culture model. Control cultures were incubated with experimental medium only, which consisted of Minimal Essential Medium (Gibco, Life Technologies, Grand Island, NY, USA) containing 10 mM glucose (MEM10). At the end of the preconditioning interval, cultures were washed with MEM10 (0.75 ml X 2) and were then treated with 30 μM hemin (Frontier Scientific, Logan, UT, USA) in MEM10, without any chelators. In all experiments, cell injury was estimated by inspecting cultures under phase contrast optics. Cell viability was then quantified using the lactate dehydrogenase (LDH) release assay, as previously described [18]. The low mean LDH activity in sister cultures subjected to medium exchanges only was subtracted from all values to obtain the LDH activity due to hemin toxicity, following the protocol of Koh and Choi [19]. Prior studies have demonstrated that cell vulnerability to hemin is consistent in cultures prepared from the same plating but varies somewhat from plating to plating, particularly if different serum samples are used. All direct comparisons were therefore made using sister cultures prepared with the same serum sample.
Immunoblotting
Cells were collected in 100 μl ice-cold lysis buffer (210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EDTA, 0.1 % sodium dodecyl sulfate, 0.1 % Triton X-100). After sonication and centrifugation, the protein concentration of the supernatant was quantified by the BCA method (Thermo Fisher Scientific, Inc., Rockford, IL, USA). Protein separation and transfer followed previously described protocols [20]. Membranes were incubated overnight at 4 °C with rabbit anti-HO-1 (1:5000, Enzo Life Sciences, Inc, Farmingdale, NY, USA), rabbit anti-horse spleen ferritin (1:250, Sigma-Aldrich, St. Louis, MO, USA), or rabbit anti-actin (1:1000, Sigma-Aldrich) as a gel-loading control, followed by HRP-conjugated secondary antibody (1:3500, Thermo Fisher) for 1 h at room temperature. Immunoreactive proteins were visualized using Super Signal West Femto Reagent (Thermo Fisher Scientific) and Kodak Gel Logic 2200.
Cell Labile Iron Assay
Changes in cytosolic redox active iron (the labile iron pool) were detected by quantifying calcein fluorescence quenching in hemin-treated cultures, as previously described [16, 21]. Cultures were pretreated with 3 μM metHb or 5 units/ml thrombin for 24 h, alone or with chelators, following the protocol described in the cytotoxicity experiments section. Since membrane integrity is essential for these experiments, hemin exposure was conducted in 3.3 % equine serum, which attenuates the toxicity of hemin, and was terminated by medium exchange at three hours. After treatment with 0.1 μM calcein-AM for 15 min, cultures were examined using an inverted microscope with epifluorescence attachment and an FITC filter. Digital 100X images in the center of each well were captured and analyzed using iVision–Mac™ software (BioVision Technologies, Exton, PA). Fluorescence of hemin-treated cultures was expressed as a percentage of that in sister cultures from the same plating subjected to medium exchanges only.
Data Analyses
All data were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni multiple comparisons post hoc test, using GraphPad InStat software (San Diego, CA).
Results
Iron Chelators Reduce Ferritin Induction by MetHb and Thrombin
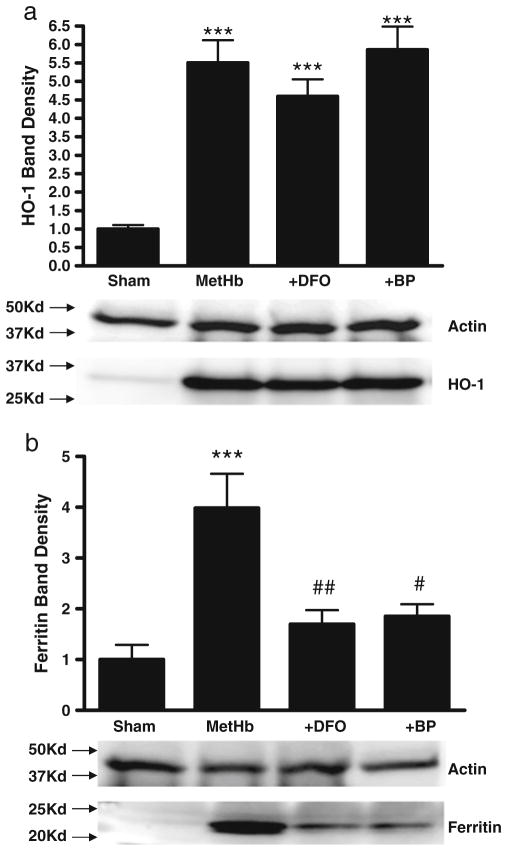
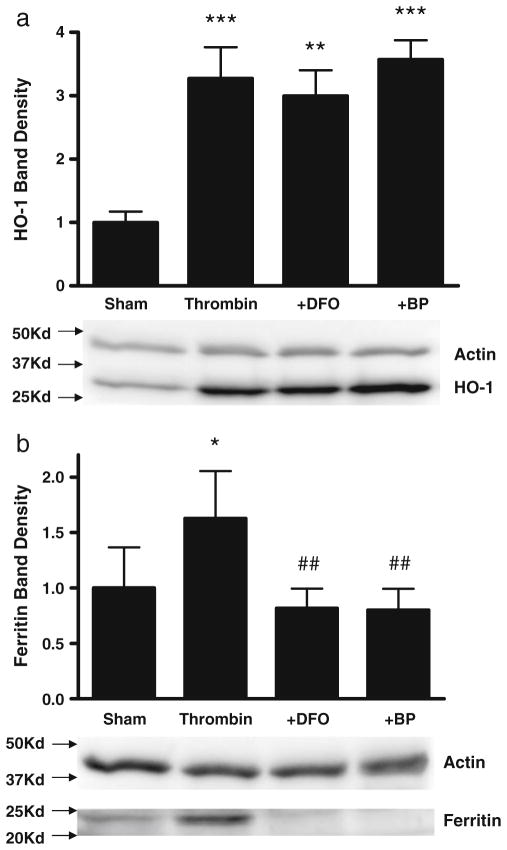
MetHb is the predominant extracellular Hb in an intracerebral hematoma [22], and was therefore used as a preconditioning stimulus rather than reduced Hb. Consistent with prior observations [10], no cell death was observed in glial cultures treated for 24 hours with 3 μM metHb [10]. Compared with control cultures subjected to medium exchanges only, HO-1 expression increased 5.5-fold after metHb treatment, while ferritin increased fourfold (Fig. 1). Concomitant treatment with either DFO and 2,2′-bipyridyl (100 μM) attenuated ferritin induction, but had no effect on HO-1. Thrombin (5 units/ml) also increased expression of HO-1 and ferritin, but less robustly than metHb (Fig. 2); the chelator effect was similar to that observed in metHb-treated cultures.
Fig. 1.
Iron chelators reduce ferritin induction by methemoglobin. Bars represent mean HO-1 (a) or ferritin (b) band densities in lysates of cultures (5/condition) treated with 3 μM methemoglobin (MetHb) for 24 hours, alone or with 100 μM of either deferoxamine (DFO) or 2,2′-bipyridyl (BP), expressed as a multiple of the mean value in control cultures subjected to sham medium changes only (= 1). Lane order of representative immuno-blots is same as bar order; actin is gel-loading control. ***P<0.001 v. sham controls, #P<0.05, ##P<0.01 v. metHb alone condition
Fig. 2.
Iron chelators prevent ferritin induction by thrombin. Cultures (5/condition) were treated with 5 units/ml thrombin for 24 hours alone or with 100 μM deferoxamine (DFO) or 2,2′-bipyridyl (BP). Bars represent mean HO-1 (a) or ferritin (b) band densities, scaled to the mean value in control cultures subjected to sham medium exchange only (= 1). Lane order of representative immunoblots is same as bar order; actin is gel-loading control. *P<0.05, **P<0.01, ***P<0.001 v. sham controls, ##P<0.01 v. thrombin alone condition
MetHb or Thrombin Preconditioning Reduces Cell Labile Iron after Hemin Treatment
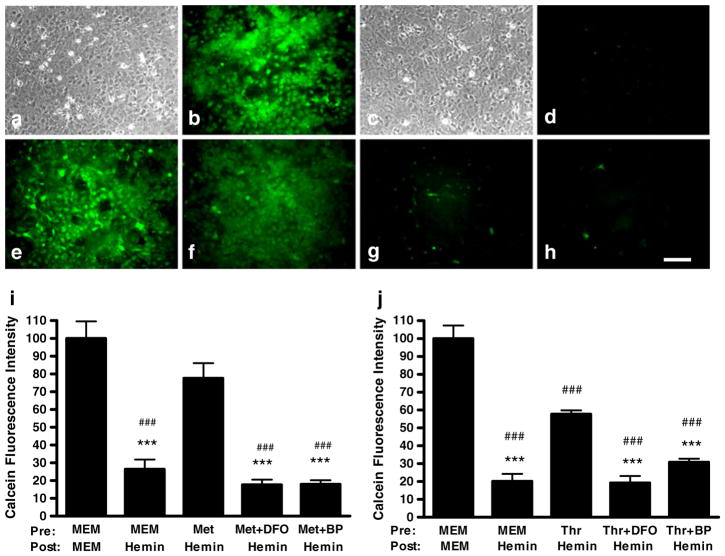
Since each ferritin molecule can sequester over 4000 ferric ions in it mineral core [13], we hypothesized that the increase in the labile iron pool that we have previously observed in these cultures after hemin treatment [16] would be reduced by metHb or thrombin preconditioning. Cytosolic labile iron, as detected by calcein fluorescence quenching [21], was significantly decreased in cultures pre-treated with 3 μM metHb followed by 30 μM hemin, compared with control cultures pretreated with MEM10 medium alone before hemin exposure (Fig. 3a–i). However, this effect was completely lost when the preconditioning stimulus was delivered with either DFO or 2,2′-bipyridyl (100 μM), followed by hemin treatment without chelators. Thrombin (5 units/ml) pre-treatment also attenuated the rise in labile iron after hemin treatment, and adding chelators to the preconditioning medium also negated its effect (Fig. 3j).
Fig. 3.
Methemoglobin and thrombin preconditioning reduce the cytosolic labile iron increase after hemin treatment; chelators reverse this effect. Phase contrast (a,c) and fluorescence (b, d-h) photomicrographs of cortical glial cultures stained with calcein AM after: a,b) control medium exchanges only; culture monolayer is intact with bright calcein fluorescence; c,d) sham pretreatment (MEM10) for 24 h followed by hemin 30 μM for 3 h, showing intact culture monolayer but calcein fluorescence quenching, indicating increased cytosolic labile iron; e) same culture as in c, d, after adding cell-permeable iron chelator 1,10-phenanthroline, which reverses calcein fluorescence quenching, demonstrating membrane integrity and ability to retain calcein; f) culture pretreated with 3 μM methemoglobin for 24 h, then 30 μM hemin for 3 hours; g,h) cultures pretreated with 3 μM methemoglobin plus 100 μM deferoxamine (g) or 100 μM 2,2′-bipyridyl (h) for 24 h, then hemin 30 μM for 3 hours; preconditioning effect is reduced. Scale bar = 100 μm. Bars represent mean calcein fluorescence intensity in cultures pretreated for 24 hours with MEM10 medium only, 3 μM methemoglobin (Met, i), or 5 units/ml thrombin (Thr, j), alone or with 100 μM deferoxamine (DFO) or 2,2′-bipyridyl (BP), then washed and treated with 30 μM hemin without chelators for 3 hours. Calcein fluorescence values are normalized to that in control cultures exposed to MEM10 medium only (= 100). ***P<0.001 v. fluorescence in cultures pretreated with methemoglobin or thrombin alone and then treated with hemin, ###P<0.001 v. that in control cultures treated with MEM10 medium only
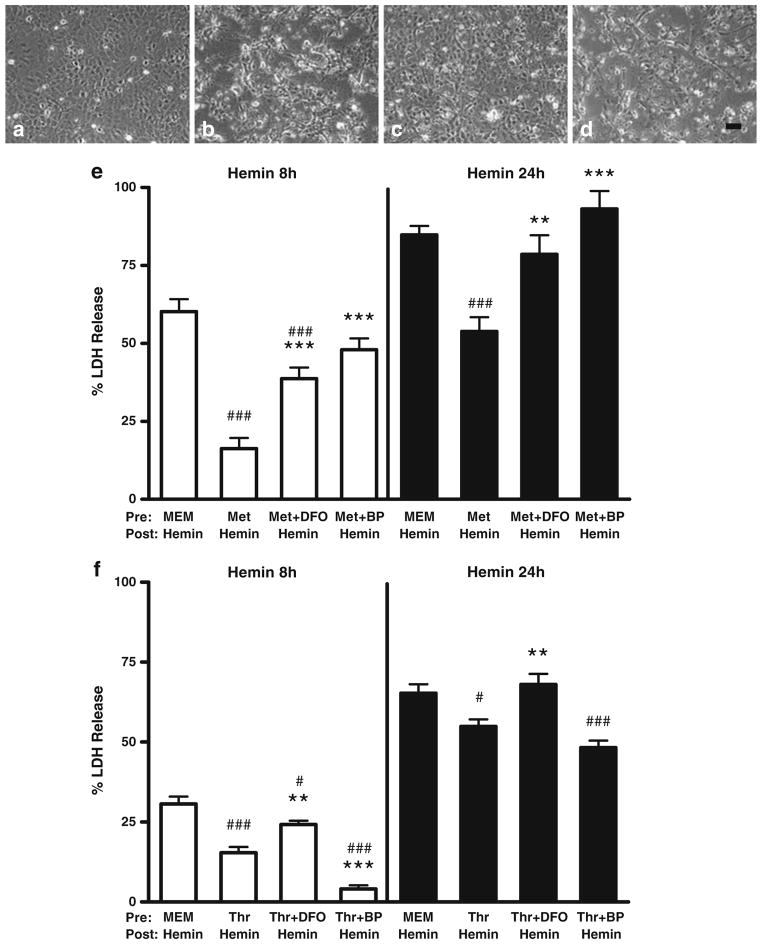
Protective Effect of Preconditioning is Reduced by Iron Chelators
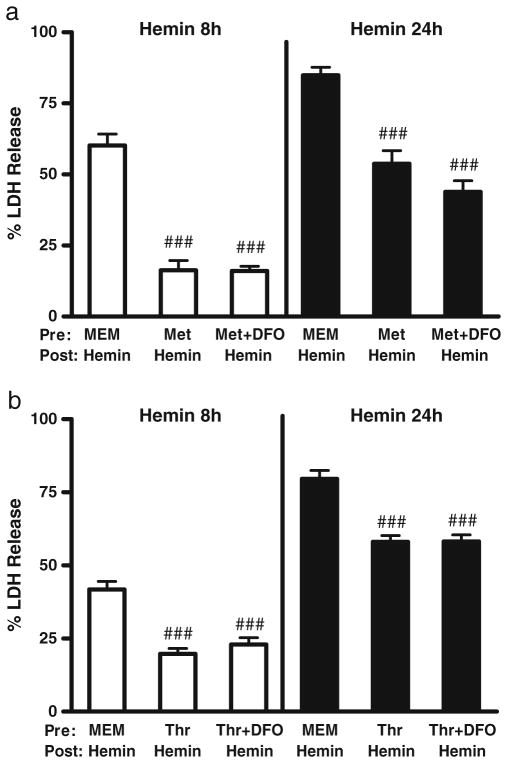
In control cultures pretreated with MEM10 medium only, exposure to 30 μM hemin for 24 hours consistently resulted in death, as measured by LDH release assay, of over 60 % of cells (Fig. 4). However, injury progression varied somewhat from culture to culture, resulting in cell loss at 8 hours ranging from 31±2 % to 60±4 %. Both metHb and thrombin preconditioning provided significant protection at both time points, although the effect was more robust with the former. When the preconditioning stimulus was delivered with 100 μM DFO, followed by washout and treatment with hemin alone, the protective benefit was consistently lost. 2,2′-bipyridyl (100 μM) had a more variable effect. In cultures pretreated with metHb, it negated preconditioning cytoprotection, consistent with DFO results. However, in thrombin preconditioning experiments, the presence of 2.2′-bipyridyl in the preconditioning medium provided additional protection to cultures treated with hemin for 8 hours, but not 24 hours.
Fig 4.
Protective effect of methemoglobin and thrombin preconditioning is attenuated by iron chelators. Phase contrast photomicrographs of glial cultures treated with: a) medium exchanges only; b-d) hemin 30 μM for 8 h, after pretreatment for 24 hours with MEM10 medium only (b), metHb 3 μM (c), or metHb 3 μM plus 2,2′-bipyridyl 100 μM (d). Scale bar=100 μm. Bars represent mean medium LDH in cultures pretreated for 24 h with 3 μM methemoglobin (Met, e) or 5 units/ml thrombin (Thr, f) alone or with 100 μM deferoxamine (DFO) or 100 μM 2,2′-bipyridyl (BP); other cultures were incubated with MEM10 medium only. After medium exchange, cultures were then treated with 30 μM hemin for 8 or 24 hours, without chelators. LDH values are scaled to the mean value in cultures from the same plating treated with 0.1 % Triton X-100 (= 100), which lyses all cells. The weak signal in control cultures subjected to medium exchange only was subtracted from all values to yield the LDH activity associated with neurotoxicity. **P<0.01, ***P<0.001 v. mean value in cultures pretreated with methemoglobin or thrombin alone, #P<0.05, ###P<0.001 v. value in cultures pretreated with MEM10 medium alone, n=14–18/condition
Ferritin is rapidly induced in glial cells [10], so we hypothesized that decreasing the duration of chelator therapy would preserve the cytoprotective effect of metHb and thrombin preconditioning. Additional cultures were therefore pretreated for 24 hours with either protein, but with DFO for only 11 hours. The latter interval was chosen since it approximates the longest duration of serum DFO levels in a 24 h period attained in a recent ICH clinical trial, which was estimated from the infusion interval of 8.3 hours and published pharmacokinetic data [8, 23]. This shorter duration of DFO treatment had no effect on the protection provided by either preconditioning stimulus (Fig. 5).
Fig. 5.
Decreasing the duration of chelator therapy preserves the cyto-protective effect of metHb and thrombin preconditioning. Cultures were pretreated for 24 h with 3 μM methemoglobin (Met, a) or 5 units/ml thrombin (Thr, b) as in Fig. 4, but 100 μM DFO was present only for the first 11 hours of the preconditioning interval. After medium exchange, cultures were then treated with 30 μM hemin for 8 or 24 hours, without DFO. Medium LDH values are scaled to the mean value in cultures treated with 0.1 % Triton X-100 (= 100), which lyses all cells. The weak signal in control cultures subjected to medium exchange only was subtracted from all values to yield the LDH activity associated with neurotoxicity. ###P<0.001 v. value in control cultures incubated in MEM10 medium alone, n=14–18/condition
Discussion
These results demonstrate that iron chelators antagonize the protective effect of metHb or thrombin preconditioning in glial cultures containing predominantly astrocytes. Both preconditioning stimuli increased expression of HO-1 and ferritin, which have previously been reported to protect astrocytes from hemin toxicity [10, 16, 24]. However, when the preconditioning medium also contained DFO or 2,2′-bipyridyl, ferritin induction was prevented, without change in HO-1 expression. This resulted in loss of iron homeostasis when cells were subsequently challenged with a toxic concentration of hemin in the absence of any chelators. Preconditioning with DFO also increased cell death due to hemin exposure, while lipophilic 2,2′-bipyridyl had a more variable effect. These observations suggest a potentially deleterious impact of iron chelators after CNS hemorrhage that should be further investigated in ICH models in vivo. To the extent that low concentrations of endogenous metHb and thrombin increase cellular resistance to hemin and iron in perihematomal tissue, this protective response may be weakened by continuous chelator therapy. These cells may then be more vulnerable to injury as hemin and iron levels increase, particularly if chelator therapy is discontinued prior to hematoma resolution. The present results also suggest that chelator therapy may not be compatible with preconditioning or postconditioning therapies involving the administration of exogenous thrombin or hemoproteins.
Considerable experimental evidence supports the hypothesis that DFO is beneficial after CNS hemorrhage [5, 6], although negative results have also been reported [25]. In these studies, DFO was administered by bolus intraperitoneal (i.p.) or intramuscular (i.m.) injection every 12 hours for 3–7 days, with therapy continuing until the animal was killed. When used clinically, DFO is always administered by slow intravenous (i.v.) or subcutaneous (s.c) infusion over several hours, due to its short half-life (0.3–0.56 h [23]) and early reports of severe hypotension after bolus injections to treat children with iron poisoning [26]. Although data regarding the pharmacokinetics of DFO when administered by bolus i.p. or i.m. injections are not available, it is very likely that the dosing regimens used in animal studies produce therapeutic levels intermittently and for less than half of a 24 h interval. In the present study, the beneficial effects of methemoglobin and thrombin were fully maintained when chelators were present for only 11 h of the 24 h preconditioning interval, suggesting that intermittent therapy may be preferable if a postconditioning stimulus is in fact beneficial. However, a hematoma will continuously produce iron, and the quantity produced may overwhelm any benefit of thrombin or methemoglobin postconditioning. It is therefore also possible that continuous chelator therapy is preferable. The latter treatment regimen has never been tested in an animal model, and should be further explored.
These results provide additional evidence that hemin toxicity in this culture system is mediated in part by chelatable iron. We have previously reported that heme oxygenase expression is a critical determinant of the vulnerability of glial cells to hemin [24, 27, 28]. In those studies, expression of HO-1 or HO-2 was modified by gene transfer or knockout, without any intervention to reduce ferritin upregulation in response to iron release. Cell death was observed to be an inverse function of HO expression and activity under those experimental conditions. In contrast, in the present study, addition of an iron chelator to the preconditioning medium had no impact whatsoever on HO-1 induction, but prevented ferritin upregulation and antagonized the cytoprotective effect of preconditioning in most experiments. Taken together, these observations demonstrate that HO activity is not sufficient per se to protect glial cells from hemin. Sufficient ferritin is also needed to sequester redox active iron that is released with hemin breakdown. These results are in agreement with observations by Balla et al. in aortic endothelial cells [9], and our prior studies using combined pretreatment with HO-1 and ferritin inducers in this model [10].
The beneficial effect of thrombin preconditioning on cell iron homeostasis during subsequent hemin exposure was antagonized by both DFO and 2,2′-bipyridyl. However, while DFO consistently had a negative impact on cell survival, as did 2,2′-bipyridyl in methemoglobin preconditioning experiments, pretreatment with 2,2′-bipyridyl plus thrombin was moderately more protective than thrombin alone. These results suggest that 2,2′-bipyridyl plus thrombin activates a protective signaling cascade that is not dependent on ferritin expression and chelatable iron levels, and that is not activated by 2,2′-bipyridyl plus metHb or by DFO plus either thrombin or metHb. The molecular basis for this cytoprotective effect is undefined and seems a worthy subject for future investigation. Compared with DFO, 2,2′-bipyridyl is more accessible to cells due to its lipophilicity [29, 30], and rapidly induces HIF-1α-dependent genes via prolyl hydroxylase inhibition [31]. Since thrombin preconditioning also increases expression of HIF-1α target genes [11], combined treatment may have an additive or synergistic effect. It is noteworthy that 2,2′-bipyridyl preconditioning was protective in a photothrombotic model of cortical ischemia via an undefined mechanism that was independent of iron chelation, reactive oxygen species formation and HO-1 expression [32]
The component of perihematomal injury that is mediated by heme is delayed until 3 days after hemorrhage in rodent models [33], which likely reflects the interval required for widespread erythrocyte lysis. It therefore may be highly amenable to postconditioning therapies with low concentrations of hemoglobin or thrombin. The present results suggest that any postconditioning benefit may be negated by concomitant treatment with DFO. If these observations are confirmed in vivo, then administration of thrombin or hemoglobin should be carefully timed to coincide with trough DFO levels.
The limitations of this study are acknowledged. The concentration of hemin (30 μM) was chosen due to its solubility in MEM10 and its previously-demonstrated toxicity in this culture system [10, 16, 18]. However, the use of alternative hemin concentrations and media may influence results in other models. The optimal hemin concentration and culture conditions must be empirically determined for each system. The highly controlled in vitro environment facilitates mechanistic investigation at the cellular and molecular level, but it cannot replicate many aspects of the complex and evolving cellular microenvironment within an intracerebral hematoma and surrounding tissue. Multiple factors that are absent in vitro may modify the cellular response to hemoglobin, hemin and thrombin, as well as the local availability of exogenous and endogenous iron chelators. While the present results are consistent with the hypothesis that iron chelators antagonize the protective effect of thrombin and methemoglobin preconditioning, further testing in appropriate in vivo models is warranted.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NS42273) to RFR.
References
- 1.Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38(5):1621–5. doi: 10.1161/STROKEAHA.106.478966. [DOI] [PubMed] [Google Scholar]
- 2.Park KW, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons: role of neuronal NADPH oxidase. J Neurosci Res. 2008;86(5):1053–63. doi: 10.1002/jnr.21571. [DOI] [PubMed] [Google Scholar]
- 3.Gong Y, Xi GH, Keep RF, Hoff JT, Hua Y. Complement inhibition attenuates brain edema and neurological deficits induced by thrombin. Acta Neurochir Suppl. 2005;95:389–92. doi: 10.1007/3-211-32318-x_79. [DOI] [PubMed] [Google Scholar]
- 4.Alyash A. Redox and radical reactions of hemoglobin solutions: toxicities and protective strategies. In: Winslow R, editor. Blood Substitutes. London: Academic Press; 2006. pp. 197–205. [Google Scholar]
- 5.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40(6):2241–3. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100(4):672–8. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 7.Wu H, Wu T, Li M, Wang J. Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiol Dis. 2012;45(1):388–94. doi: 10.1016/j.nbd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selim M, Yeatts S, Goldstein JN, Gomes J, Greenberg S, Morgenstern LB, et al. Safety and tolerability of deferoxamine mesylate in patients with acute intracerebral hemorrhage. Stroke. 2011;42(11):3067–74. doi: 10.1161/STROKEAHA.111.617589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, et al. Ferritin: a cytoprotective strategem of endothelium. J Biol Chem. 1992;267(25):18148–53. [PubMed] [Google Scholar]
- 10.Regan RF, Kumar N, Gao F, Guo YP. Ferritin induction protects cortical astrocytes from heme-mediated oxidative injury. Neuroscience. 2002;113:985–94. doi: 10.1016/s0306-4522(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 11.Hua Y, Keep RF, Hoff JT, Xi G. Thrombin preconditioning attenuates brain edema induced by erythrocytes and iron. J Cereb Blood Flow Metab. 2003;23(12):1448–54. doi: 10.1097/01.WCB.0000090621.86921.D5. [DOI] [PubMed] [Google Scholar]
- 12.Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30(6):1247–55. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- 13.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99(10):3505–16. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 14.Chen-Roetling J, Liu W, Regan RF. Iron accumulation and neurotoxicity in cortical cultures treated with holotransferrin. Free Radic Biol Med. 2011;51(11):1966–74. doi: 10.1016/j.freeradbiomed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gesuete R, Orsini F, Zanier ER, Albani D, Deli MA, Bazzoni G, et al. Glial cells drive preconditioning-induced blood-brain barrier protection. Stroke. 2011;42(5):1445–53. doi: 10.1161/STROKEAHA.110.603266. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Chen-Roetling J, Regan RF. Increasing expression of H- or L-ferritin protects cortical astrocytes from hemin toxicity. Free Radic Res. 2009;43(6):613–21. doi: 10.1080/10715760902942808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, et al. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22(4):404–10. doi: 10.1097/00004647-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Regan RF. Increasing expression of heme oxygenase-1 by proteasome inhibition protects astrocytes from heme-mediated oxidative injury. Curr Neurovasc Res. 2005;2(3):189–96. doi: 10.2174/1567202054368344. [DOI] [PubMed] [Google Scholar]
- 19.Koh JY, Choi DW. Vulnerability of cultured cortical neurons to damage by excitotoxins: differential susceptibility of neurons containing NADPH-diaphorase. J Neurosci. 1988;8 (6):2153–63. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen-Roetling J, Li Z, Chen M, Awe OO, Regan RF. Heme oxygenase activity and hemoglobin neurotoxicity are attenuated by inhibitors of the MEK/ERK pathway. Neuropharmacology. 2009;56(5):922–8. doi: 10.1016/j.neuropharm.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free Radic Biol Med. 2002;33(8):1037–46. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 22.Bradley WG., Jr MR appearance of hemorrhage in the brain. Radiology. 1993;189(1):15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 23.Kushner JP, Porter JP, Olivieri NF. Secondary iron overload. Hematology. 2001;2001(1):47–61. doi: 10.1182/asheducation-2001.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Benvenisti-Zarom L, Regan RF. Astrocyte-specific heme oxygenase-1 hyperexpression attenuates heme-mediated oxidative injury. Neurobiol Dis. 2007;26(3):688–95. doi: 10.1016/j.nbd.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warkentin LM, Auriat AM, Wowk S, Colbourne F. Failure of deferoxamine, an iron chelator, to improve outcome after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2010;1309:95–103. doi: 10.1016/j.brainres.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 26.Whitten CF, Gibson GW, Good MH, Goodwin JF, Brough AJ. Studies in acute iron poisoning. I. Desferrioxamine in the treatment of acute iron poisoning: clinical observations, experimental studies, and theoretical considerations. Pediatrics. 1965;36(3):322–35. [PubMed] [Google Scholar]
- 27.Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem Biophys Res Commun. 2004;318:88–94. doi: 10.1016/j.bbrc.2004.03.187. [DOI] [PubMed] [Google Scholar]
- 28.Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Cultured astrocytes from heme oxygenase-1 knockout mice are more vulnerable to heme-mediated oxidative injury. J Neurosci Res. 2005;82 (6):802–10. doi: 10.1002/jnr.20681. [DOI] [PubMed] [Google Scholar]
- 29.Cable H, Lloyd JB. Cellular uptake and release of two contrasting iron chelators. J Pharm Pharmacol. 1999;51(2):131–4. doi: 10.1211/0022357991772231. [DOI] [PubMed] [Google Scholar]
- 30.Nunez MT, Cole ES, Glass J. The reticulocyte plasma membrane pathway of iron uptake as determined by the mechanism of alpha, alpha′-dipyridyl inhibition. J Biol Chem. 1983;258 (2):1146–51. [PubMed] [Google Scholar]
- 31.Martens LK, Kirschner KM, Warnecke C, Scholz H. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator of the TrkB neurotrophin receptor gene. J Biol Chem. 2007;282(19):14379–88. doi: 10.1074/jbc.M609857200. [DOI] [PubMed] [Google Scholar]
- 32.Methy D, Bertrand N, Prigent-Tessier A, Mossiat C, Stanimirovic D, Beley A, et al. Beneficial effect of dipyridyl, a liposoluble iron chelator against focal cerebral ischemia: in vivo and in vitro evidence of protection of cerebral endothelial cells. Brain Res. 2008;1193:136–42. doi: 10.1016/j.brainres.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 33.Xi GH, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–6. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]