Abstract
Epithelial-to-mesenchymal transition (EMT) is a developmentally vital, molecularly complex cellular process by which epithelial cells lose apico–basal polarity and cell–cell contact, become motile, and acquire mesenchymal characteristics. Under pathophysiological conditions EMT has a central role in cancer progression and metastasis, and has been associated with fibrotic disorders. Microenvironmental changes such as alterations in oxygen levels and activation of hypoxic signaling through hypoxia-inducible factor (HIF) are emerging as important triggers and modulators of EMT. Recent insights into potential molecular mechanisms underlying oxygen-dependent regulation of this process and their relevance to disease are discussed.
Keywords: chronic kidney disease, EMT, extracellular matrix, fibrosis, hypoxia, hypoxia-indacible factors
Epithelial-to-mesenchymal transition (EMT) is a collective term for a series of phenotypic and molecular events during which epithelial cells lose apico–basal polarity and cell–cell contact, become motile and acquire mesenchymal characteristics. It is vital for normal morphogenesis and tissue remodeling during the early and late stages of embryonic development. The reverse event is called mesenchymal to epithelial transition and occurs physiologically when primary mesenchymal cells convert to secondary epithelial cells, for example, during nephron formation. Under pathophysiological conditions, EMT has been implicated in cancer progression and metastasis, wound healing and the development of fibrotic disorders, including pulmonary, hepatic and renal fibrosis (for reviews see Kalluri, Liu, Thiery, Thiery and Sleeman1–4). Phenotypically, EMT is characterized by the organized disassembly of epithelial cell–cell contacts such as adherens, tight and gap junctions, as well as desmosomes leading to cell–cell separations, which in conjunction with a dramatic actin cytoskeletal remodeling results in the transition to a spindle-shaped morphology coupled with increased migratory ability. On the molecular level EMT is associated with the loss of, or the redistribution of epithelial-specific junctional proteins such as ZO proteins and cadherins, and the de novo expression of mesenchymal markers, such as vimentin, α-smooth muscle actin (αSMA), fibroblast-specific protein 1 (FSP1/S100A4) and N-cadherin, and the expression of matrix metalloproteinases.1–3
Several molecular signaling pathways trigger EMT during embryonic development and/or under pathophysiological conditions and have context-dependent roles in the EMT process. These include signaling through (a) the activation of receptor tyrosine kinases by hepatocyte growth factor (HGF), fibroblast growth factor, and platelet-derived growth factor involving the Erk mitogen-activated protein kinase-signaling cascade, phosphatidylinositol-3′-kinase controlled pathways, and the Src-STAT-signaling pathway; (b) the activation of transforming growth factor (TGF)-β receptor serine-threonine kinases; and (c) the stimulation of the Notch and Wnt/β-catenin pathways leading to the activation of transcription factors CSL (C-promoter-binding factor 1/suppressor of hairless/LAG1) and lymphoid enhancer factor/T-cell factor (LEF/TCF), respectively. EMT-triggering pathways contribute to both gene regulation and cytoplasmic signaling, and functional interaction between pathways can result in signal amplification. Crucial nuclear targets are transcriptional regulators, which upon activation mediate the repression of epithelial proteins, such as E-cadherin and ZO-1, a central event in EMT. Epithelial repressors include the zinc-finger transcription factor snail homolog 1 (SNAI1, also known as Snail) and snail homolog 2 (SNAI2, also known as Slug), zinc-finger E-box binding homeobox (ZEB) 1 (ZFHX1A, also known as δEF1), ZEB2 (ZFXH1B, also known as SMAD-interacting protein 1 (SIP1)), twist homolog 1 (TWIST1) and TCF3 (E2A immunoglobulin enhancer-binding factors E12/E47).5,6
Recent reports have linked alterations in microenvironmental oxygen tension and the activation of hypoxia-inducible factor (HIF) signaling to EMT. Potential molecular mechanisms underlying oxygen-dependent regulation of EMT in development and disease, and their relevance to renal injury and the progression of chronic kidney disease (CKD) are discussed.
OXYGEN-SENSING MECHANISMS IN THE REGULATION OF EMT
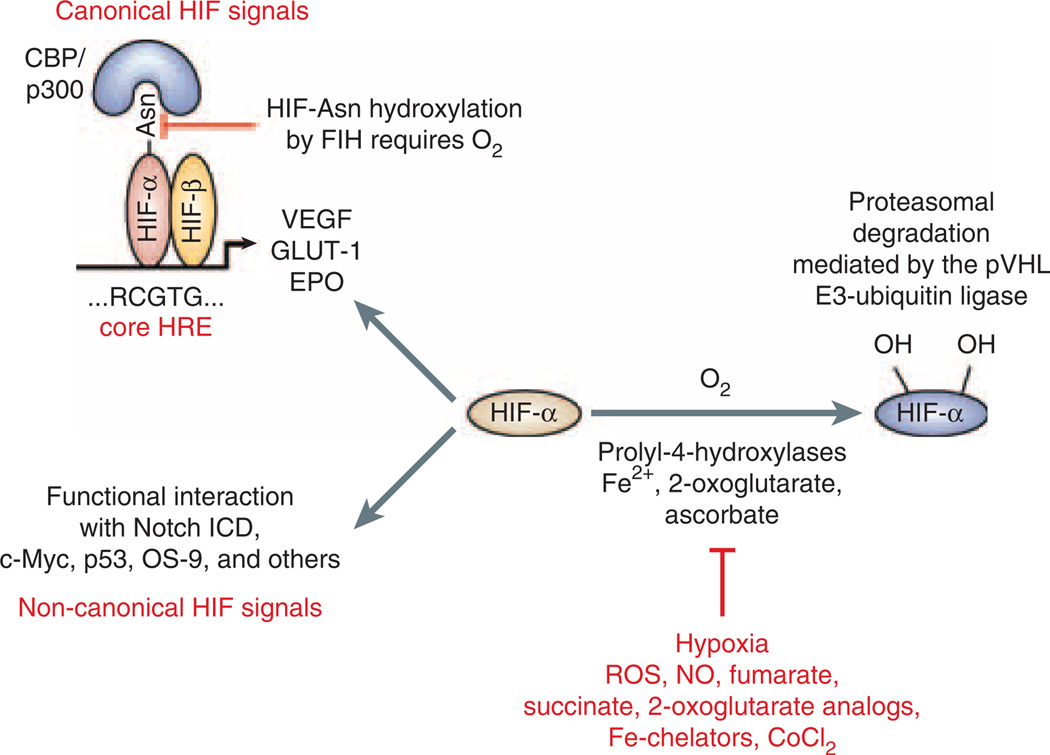
Alterations in intracellular PO2 have profound effects on cellular energy metabolism, proliferation, differentiation and cell type-specific functions and result in well-orchestrated molecular responses.7 Key intracellular oxygen sensors are 2-oxoglutarate-dependent dioxygenases (prolyl-4-hydroxylase domain (PHD) proteins), which target specific proline residues of the hypoxia-inducible factor α-subunit (HIF-α) for hydroxylation and require molecular oxygen, ferrous iron and ascorbate (Figure 1). HIF hydroxylation is a prerequisite for rapid proteasomal HIF inactivation under conditions of normal oxygen tension. Inhibition of HIF-α degradation in the presence of oxygen can result from the inherited or somatically acquired inability of cells to hydroxylate HIF-α, or from a defect in the ubiquitination of hydroxylated HIF-α and its targeting for proteasomal degradation, which is the case in hereditary leiomyomatosis and renal cancer syndrome, von Hippel-Lindau (VHL) disease or sporadic tumors associated with loss of VHL tumor suppressor function (for a review and relevant references see Haase8). Furthermore, normoxic stabilization of HIF-α can be achieved by pharmacological inhibition of PHDs with 2-oxoglutarate analogs,9 or can be mediated by nitric oxide and TNF-α, interleukin 1, angiotensin II, and a variety of growth factors including epidermal growth factor, insulin and insulin-like growth factors (for an overview and relevant references see Haase8). Nitric oxide, reactive oxygen species, v-Src and activated Ras have been shown to inhibit HIF prolyl-hydroxylation.10,11
Figure 1. Overview of HIF regulation.
Under normoxia hydroxylation of hypoxia-inducible factor (HIF)-α subunits by prolyl-4-hydroxylases is required for binding to the pVHL-E3-ubiquitin ligase complex, which poly-ubiquitinates HIF-α and targets it for proteasomal degradation. When prolyl-4-hydroxylation is inhibited HIFα is stabilized and translocates to the nucleus where it heterodimerizes with HIF-β. HIF-α/β heterodimers bind to the HIF consensus binding site RCGTG and regulate transcription of HIF target genes, for example, EPO, VEGF, and GLUT-1. Factor-inhibiting-HIF (FIH) is an asparagines hydroxylase that modulates cofactor recruitment to the HIF transcriptional complex through oxygen-dependent asparagines hydroxylation of the HIF-α carboxy-terminal transactivation domain. FIH also modulates the cross talk between Notch and HIF pathways through Notch ICD hydroxylation.34 Non-canonical HIF signaling occurs through functional interaction with other proteins, such as the Notch intracellular domain (for a more complete overview of HIF-α-interacting proteins see Wenger et al.12). Nitric oxide, reactive oxygen species, the Krebs cycle metabolites succinate and fumarate, cobalt chloride, and iron chelators such as desferrioxamine are known to inhibit HIF prolyl-4-hydroxylases in the presence of oxygen. Asn, asparagine; CoCl2, cobalt chloride; EPO, erythropoietin; Fe2+, ferrous iron; GLUT-1, glucose transporter 1; HRE, hypoxia response element; NO, nitric oxide; Notch ICD, Notch intracellular domain; VEGF, vascular endothelial growth factor; ROS, reactive oxygen species.
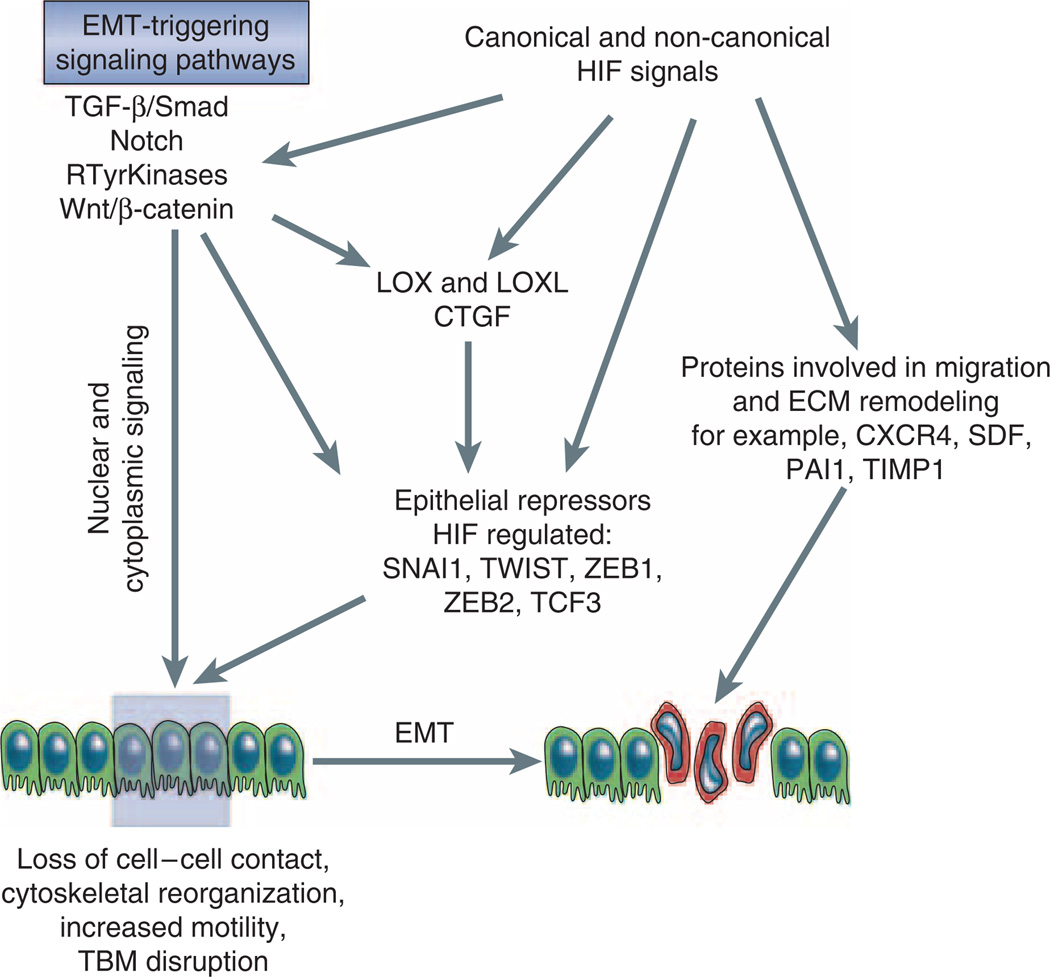
HIF transcription factors are members of the Per-ARNT-Sim family of heterodimeric basic helix–loop–helix (bHLH) transcription factors and consist of an oxygen-sensitive α-subunit and a constitutively expressed β-unit, also known as the aryl-hydrocarbon-receptor nuclear translocator (ARNT) or HIF-β.11,12 As transcription factors HIF heterodimers regulate the expression of gene products that have essential roles in the control of anaerobic energy metabolism, angiogenesis, cellular proliferation, apoptosis, differentiation, and other biological processes, which ultimately allows cells and tissues to adapt to and to survive an oxygen-deprived environment. Although a large number of HIF transcriptional targets are co-regulated by both HIF-1 and HIF-2, individual HIF homologs carry out distinct biological functions and regulate unique targets. This may be the result of tissue-specific interactions with other nuclear factors, differential interactions with transcriptional co-factors or a reflection of tissue and cell type-dependent differences in the ratios of individual HIF-α protein levels (for review see Ruas and Poellinger13). In addition to its role as a transcription factor, which mediates the canonical hypoxia response by binding to hypoxia response element (HRE) containing DNA sequences and transcriptional co-activator recruitment, HIF-α subunits also regulate cellular functions through molecular interactions with components of other signaling pathways in an HRE-independent manner. These include tumor suppressor protein p53, the c-Myc proto-oncogene and the Notch intracellular domain.12,14–17 More recent experimental data suggest that HIF has the ability to modulate the activity of several EMT-triggering signaling pathways, and that both the HRE-mediated HIF canonical response, as well as non-canonical HIF-signaling events are relevant to the EMT process (Figure 2).
Figure 2. HIF regulation of EMT.
Shown is a simplified overview of EMT-triggering signaling pathways and their effectors, which are modulated by HIF. CTGF, connective tissue growth factor; CXCR4, chemokine receptor 4; LOX, lysyl oxidase; LOXL, lysyl oxidase-like; PAI1, plasminogen activator inhibitor 1; TIMP-1, tissue-inhibitor of metalloproteinase 1; Rtyr kinases, receptor tyrosine kinases; SDF1, stromal cell–derived factor 1; SNAI1, snail homolog 1; TCF3, transcription factor 3 (E2A immunoglobulin enhancer-binding factors E12/E47); ZEB, zinc finger E-box binding homeobox.
HYPOXIA AND HIF MODULATION OF EMT-ASSOCIATED SIGNALING PATHWAYS
It has now become apparent that hypoxia and HIF activation have the potential of modulating the activity of major EMT-triggering pathways by either regulating the expression levels of EMT inducers and their receptors, by affecting the expression levels of pathway associated signaling molecules and downstream effectors, or by a signal enhancing functional interaction with EMT-associated nuclear factors.
Members of the TGF-β family of growth factors are major inducers of EMT during embryonic development, cancer progression and in the pathogenesis of fibrotic disorders.18 Experimental evidence suggests that hypoxia increases the expression levels of TGF-β,19–23 thus providing a potential mechanism by which hypoxia may modulate TGF-β triggered EMT. TGF-β binds to type I and type II receptor serine/threonine kinases and regulates EMT responses through complex mechanisms, which involve SMAD and non-SMAD signals. Receptor SMADs, SMAD2 and SMAD3, are activated following receptor trans-phosphorylation and form transcriptional complexes with SMAD4. These regulate the expression of gene products, which control cell proliferation, differentiation, apoptosis, and migration. Non-SMAD-mediated TGF-β signals activate the MAPK-signaling cascade, phosphatidylinositol-3′-kinase and regulate the activity of small Rho family GTPases thereby influencing motility (for a review on TGF-β signaling see Zavadil and Bottinger18). TGF-β targets include the zinc-finger repressors SNAI1 and SNAI2 the two-handed E-box-binding zinc-finger protein ZEB2/SIP1 and bHLH transcriptional repressors HEY (for further references see Nieto, Zavadil and Bottinger5,18). Although the molecular mechanisms underlying functional interactions between hypoxia and TGF-β signals are ill defined, close proximity of HIF and SMAD-binding DNA sequences in co-regulated target genes suggests that HIF and TGF-β may cooperate on a transcriptional level as was suggested for the regulation of VEGF expression.24 Furthermore, hypoxia increases SMAD3 mRNA levels and promotes the thrombospondin-dependent release of latent TGF-β2, thus activating TGF-β signaling,25 suggesting that hypoxia may affect the TGF-β signaling pathway at multiple regulatory levels. HIF and TGF-β also co-regulate gene products that have been shown to trigger EMT, or whose expression is increased as a result of the EMT process. For example, HIF and TGF-β both induce plasminogen activator inhibitor 1 (PAI1) and connective tissue growth factor (CTGF).26–28 CTGF, a member of the CCN (CTGF/cysteine rich 61/nephroblastoma overexpressed) family and mediator of TGF-β’s profibrotic actions, regulates multiple biological processes including cell adhesion and migration, extracellular matrix (ECM) production, angiogenesis, tumor growth and wound repair.29 It is rapidly induced by TGF-β in a SMAD-dependent manner and is a transcriptional target of HIF-1 in renal tubular epithelial cells.28 When added to cultured epithelial cells or overexpressed, CTGF triggers an EMT phenotype.30,31
Another important pathway that regulates EMT and is influenced by hypoxia is the Notch-signaling pathway.17,32–34 Notch controls differentiation, cell fate specification, proliferation and survival, and is important for stem cell maintenance. It promotes EMT during cardiac valve development and tumor progression through SNAI1 and SNAI2 induction,35 and is involved in TGF-β1/SMAD induced EMT through HEY1.36 Following ligand binding, the pathway is activated by proteolytic cleavage of the cytoplasmic tail of the Notch transmembrane receptor, liberating the Notch intracellular domain, which then translocates to the nucleus where it converts CSL to an activating transcription factor.37 HIF-1α interacts with the Notch intracellular domain functionally and increases its transcriptional activity. This functional interplay between HIF and Notch appears to be important for stem cell maintenance under hypoxia.17 In an oncogenic context hypoxia augments Notch signaling causing EMT changes as a result of increased SNAI1 expression.33 Interestingly, Notch also appears to potentiate HIF-dependent lysyl oxidase (LOX) expression, which in turn further increases SNAI1 protein stability thereby amplifying Notch signaling33 (see the following paragraph). The mechanisms by which HIF enhances Notch signals, however, appear to be cell-type dependent. In melanoma cells HIF-1 activation enhances Notch signals by increasing Notch1 mRNA levels.38 Enhanced Notch ligand expression has also been found in fibrotic kidney disease, indicating that the Notch pathway is potentially linked to the pathogenesis of CKD,39 the role of hypoxia and HIF activation in the context of this pathway and CKD progression, however, remains to be determined.
To add to the complexity of potential mechanisms underlying oxygen-regulated EMT, hypoxia and HIF have been shown to modulate the activity of HGF and Wnt,40,41 which both are ligands for two major EMT-triggering signaling pathways. Although hypoxia suppresses β-catenin-mediated transcriptional activity (HIF-1α competes with TCF4 for β-catenin binding), β-catenin enhances HIF-1-mediated transcription.41 With regard to HGF, HIF enhances HGF signaling through a HIF-dependent increase in c-met receptor tyrosine kinase expression in tumor cells.40 HGF is a major inducer of EMT during normal development and in cancer, and regulates cell motility by cell adhesion disassembly, ECM and basement membrane degradation and by altering the interactions between integrins and matrix components. In the context of chronic renal injury, however, HGF functions as an inhibitor of TGF-β triggered EMT and attenuates the development of renal fibrosis.2
The composition and integrity of the basement membrane is crucial for the maintenance of epithelial homeostasis, and its disruption is a key event in EMT associated with cancer metastasis and invasion, or fibrogenesis. In the kidney, transitioned tubular epithelial cells would migrate into the interstitium, produce ECM as myofibroblasts and thereby participate in the progression of renal fibrosis. These events are likely to be modulated by HIF activation through altered expression of proteins that regulate cell migration and ECM turnover. For instance, HIF has been shown to influence cell migration through the regulation of αvβ3 integrin cell surface expression42 and the induction of chemokine receptor CXCR4 and its ligand stromal cell–derived factor-1 (SDF-1/CXCL12), both of which have important functions in tumor metastasis and the homing of progenitor cells (for review see Chan and Giaccia43). Other relevant HIF-regulated proteins in this context include ECM modifiers PAI1, urokinase plasminogen activator receptor, TIMP1, matrix metalloproteinase (MMP)1 and MMP2,19,27,44,45 all of which contribute to the pathogenesis of renal fibrosis.
HIF REGULATION OF EMT-ASSOCIATED TRANSCRIPTIONAL REPRESSORS
A central event in EMT is the activation of epithelial repressors, which act as downstream effectors of EMT-triggering pathways. Epithelial repressors are transcription factors, which recruit specific chromatin-remodeling complexes and downregulate the expression of gene products that are important in the maintenance of the epithelial phenotype. These include cadherins, claudins, cytokeratins, mucins, plakophilin, occludin, and ZO proteins (for a recent review see Peinado et al.6). The loss of E-cadherin expression is consistently observed during EMT and is an important event during this process. E-cadherin is a key component of cell–cell adhesion junctions and is essential for the formation of epithelia during embryonic development and for the maintenance of adult epithelial homeostasis, and its loss is associated with tumor cell invasiveness.6 Some epithelial repressors can also directly or indirectly induce mesenchymal genes and gene products that are important in matrix remodeling as demonstrated by overexpression studies. So does activation of zinc-finger containing transcriptional repressor SNAI1, which indirectly increases MMP2 and MMP9 expression, as well as the expression of TIMP1 and PAI1.46–48
There is growing experimental evidence that HIF modulates EMT by regulating the expression and activity levels of major transcriptional repressors. Imai et al.49 observed that SNAI1 was increased by hypoxia in ovarian cancer cells, which correlated with increased HIF-α levels and decreased E-cadherin expression. HIF control of EMT-inducing epithelial repressors was subsequently demonstrated in normoxic renal cancer cells that were defective in the VHL tumor suppressor pVHL.50,51 pVHL is the substrate recognition component of the E3 ubiquitin ligase that targets hydroxylated HIF-α for ubiquitination and subsequent proteasomal degradation (Figure 1) and functional loss of pVHL results in constitutive activation of HIF signaling. In pVHL-deficient renal cancer cell lines Krishnamachary et al.50 reported that HIF-1 activation was associated with decreased E-cadherin expression, led to the loss of cell–cell adhesion and promoted EMT through the induction of transcriptional repressors TCF3, ZEB1/δEF1, and ZEB2/SIP1. Similarly, Evans et al.51 proposed that inactivation of pVHL resulted in E-cadherin suppression through HIF-dependent induction of SNAI1 and ZEB2/SIP1, whereas Esteban et al.52 suggested that HIF-2 may have a more potent suppressive effect on E-cadherin levels than HIF-1. SNAI1 and ZEB2/SIP1 are highly expressed in E-cadherin-deficient carcinoma cells where they promote tumor cell invasion and bind to E-boxes in the minimal E-cadherin promoter with similar suppressor effect.53–55
TWIST is an EMT-inducing bHLH transcription factor, which represses E-cadherin through E-boxes that are also targeted by SNAI1 and ZEB2/SIP1. During embryogenesis TWIST governs gastrulation and mesoderm specification, and when activated in tumor cells it promotes metastasis.56 TWIST is directly HIF regulated in Caenorhabditis elegans57 and in mammalian cells,57,58 and mediates hypoxia/HIF-induced EMT in breast cancer, nasopharyngeal cancer and lung tumor cells. TWIST-regulated migration and invasion of hypoxic tumor cells was non-redundant with co-existing SNAI1-regulated migration and invasion, that is, an additional reduction in migratory and invasive ability was achieved by knockdown of SNAI1 when TWIST was inhibited.58 In head and neck cancers HIF-1α, TWIST and SNAI1 expression correlated with metastasis and poor clinical prognosis.58 HIF-1 activation in primary renal epithelial cell culture enhanced the transition of epithelial cells towards a mesenchymal phenotype; however, increased transcription of the aforementioned epithelial repressors was not observed with exposure to short-term hypoxia, which may be a reflection of cell-type and context-dependent differences in hypoxic gene regulation, or the duration of hypoxia exposure.59
HIF-1 induction of LOX and lysyl oxidase-like (LOXL)-2 has been shown to promote migration in primary renal epithelial cells, human breast and cervical cancer cells, and to regulate E-cadherin expression in renal cell cancer.59–61 Although initially identified by their ability to cross-link collagen and elastin fibers, LOX and LOXL proteins carry out intracellular functions and display a range of biological activities that extend beyond extracellular cross-linkage. These include the regulation of ras- and NF-κB-signaling, possibly through the modification of DNA-histone and histone–histone interactions.62,63 A recent report by Peinado et al.64 indicated that LOXL2 and LOXL3 have the potential to regulate EMT by stabilizing and promoting the activity of transcriptional repressor SNAI1. Because LOX and LOXL2 are HIF regulated, LOXL-mediated control of SNAI1 activity may represent an additional molecular mechanism that underlies oxygen-dependent epithelial repression, which could be relevant to EMT in primary renal tubular epithelial cells and to the pathogenesis of renal fibrosis.59,65 Posttranslational regulation of SNAI1 was also shown in head and neck cancer cells, where both SNAI1 protein levels as well as its activity were induced by hypoxia through HIF-1, whereas SNAI1 mRNA levels did not change.58 Whether this involved LOX or LOXL remains to be investigated.
HYPOXIA, HIF AND OXYGEN-REGULATED EMT IN CHRONIC KIDNEY DISEASE
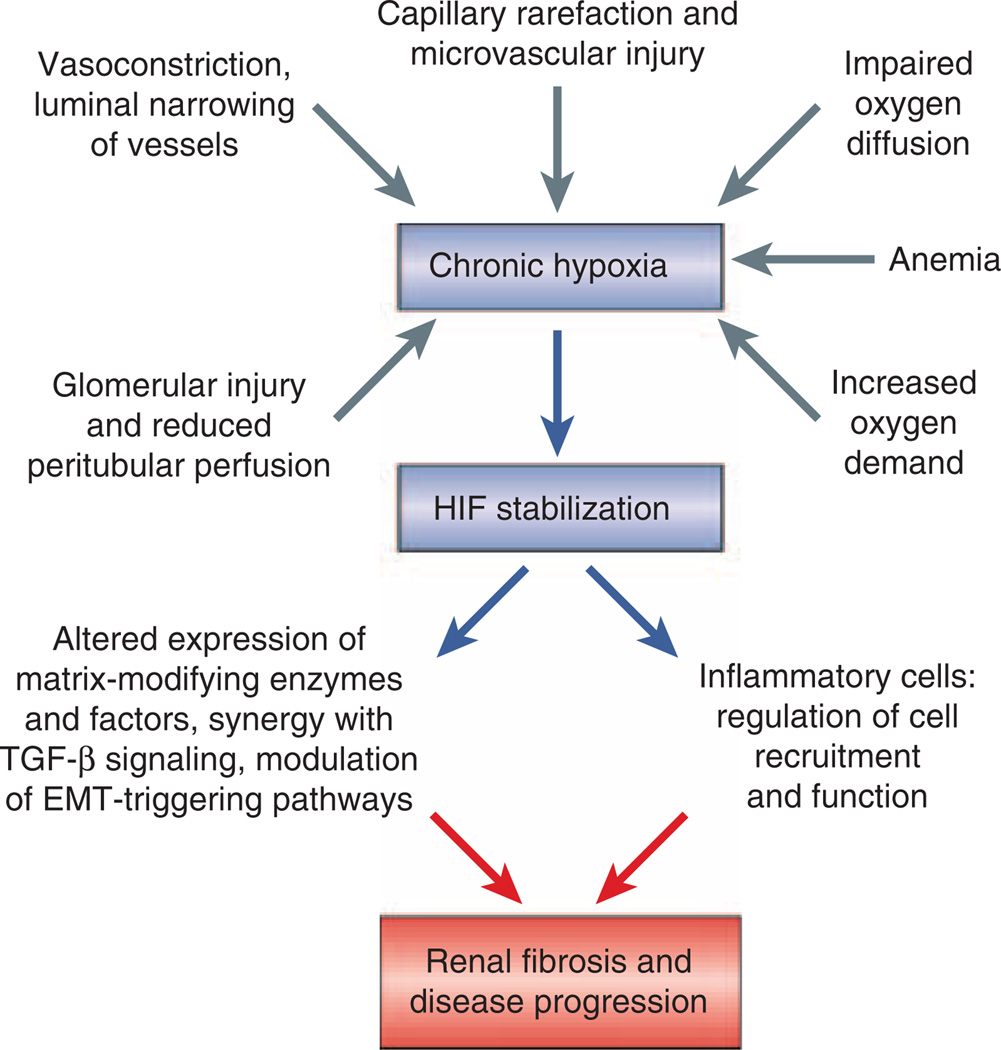
The molecular study of cancer, where hypoxia and HIF stabilization correlate with poor clinical outcome and resistance to therapy,43 has provided new and exciting mechanistic insights into oxygen-dependent regulation of EMT. Although it is unclear at the moment to which degree these findings are applicable to the pathogenesis of CKD, they are of great interest to experimental and clinical nephrologists and will certainly stimulate further investigations into the role of chronic hypoxia, HIF signaling and EMT in CKD progression. Notwithstanding the need for additional clinical and experimental studies, the presence of hypoxia (comparison to healthy controls) and HIF stabilization has been clearly demonstrated in chronic renal diseases of different etiologies (for example, diabetic nephropathy, IgA nephropathy, unilateral ureteral obstruction (UUO), 5/6 nephrectomy, anti-Thy1 glomerulonephritis and others) both in patients and in animal models employing blood oxygen level-dependent (BOLD) MRI, immunohistochemical, immunoblot, and PCR techniques, and by direct measurement of renal tissue oxygen levels using microelectrodes (for an overview of these studies see Haase, Eckardt et al., Heyman et al., Fine and Norman, Nangaku et al.8,66–69). Renal hypoxia associated with CKD is thought to result from a combination of structural and functional changes, which include capillary rarefaction, compromise of peritubular blood flow resulting from glomerular injury, vasoconstriction from altered levels of vasoactive factors and signaling molecules (for example, angiotensin II, endothelin, nitric oxide), luminal narrowing of atherosclerotic vessels, increased oxygen demand from hyperfiltration and tubular hypertrophy, limited oxygen diffusion as a consequence of ECM expansion, and renal anemia66–68 (Figure 3). In diabetic nephropathy for example, the clinical finding of increased HIF-1α expression in renal biopsy material59 is consistent with decreased renal oxygenation detected in experimental animal models by MRI.70,71 Moreover, the degree of HIF expression appears to correlate with severity of injury,59 suggesting that the reduction in renal tissue oxygen tension is more pronounced in advanced diabetic nephropathy than in early disease.
Figure 3. Potential profibrotic role of HIF activation in chronic kidney disease (CKD).
Shown is a simplified overview of functional and structural causes of hypoxia in CKD. As a result of hypoxia, activation of hypoxia-inducible factor (HIF) signaling in renal cells has the potential to promote renal fibrosis and to contribute to the progression of CKD as demonstrated experimentally in proximal tubule-specific HIF-1α knockout mice (unilateral uretal obstruction model) and in mice with increased tubular HIF-1α expression.59,72 HIF may exert its profibrotic function by modulating cellular and molecular events that have key roles in the pathogenesis of renal fibrosis. These include extracellular matrix production and processing through regulation of matrix-modifying factors and enzymes, such as CTGF, PAI1, TIMP1, and MMPs, the modulation of EMT-triggering pathways, and inflammation.
Recent experimental evidence indicates that hypoxia may accelerate CKD progression through its effects on renal cell survival, inflammatory cell recruitment, collagen gene expression, ECM turnover, and EMT8,67–69 (Figure 3). Our laboratory has identified tubular epithelial HIF-1α as a promoter of renal fibrosis in experimental UUO59 and demonstrated that genetic inactivation of HIF-1α in the proximal renal tubule led to a reduction in collagen accumulation, inflammatory cell infiltration and the number of FSP1-expressing interstitial cells.59 The notion of epithelial HIF-1α as a profibrotic factor is supported by overexpression studies in vivo.72 In addition, we and others have shown that hypoxia and HIF activation promotes EMT and cell motility of renal epithelial cells in vitro,59,73 which is likely to contribute to HIF’s fibrogenic role in vivo. EMT in the setting of CKD is considered a disease promoting mechanism by which renal tubular epithelial cells acquire a mesenchymal phenotype and migrate into the interstitium, where they together with resident cells produce ECM as myofibroblasts.2 Irrespective of their origin, the accumulation of myofibroblasts is closely correlated with the degree of interstitial damage and the risk of disease progression. The degree to which EMT contributes to the renal myofibroblast pool however, is debated. In an experimental model of UUO-induced fibrosis, which traced cells of epithelial origin by genetic means, Iwano et al.74 suggested that up to 36% of interstitial myofibroblasts are EMT-derived, approximately 15% from the bone marrow, whereas the rest is derived from resident fibroblast proliferation. Others have suggested that pericytes75 or endothelial cells76 can be major sources of ECM-producing renal interstitial myofibroblasts. Lending further support to the concept of disrupted epithelial homeostasis as a key event in renal fibrogenesis are studies in transgenic mice, which demonstrate that forced expression of transcriptional repressor SNAI1 induces renal fibrosis.65 Clinically, it would be important however to unequivocally determine the degree by which EMT contributes to tubulointerstitial fibrosis in patients with CKD.
Pharmacologic targeting of EMT-triggering signaling pathways such as the TGF-β pathway has been proposed as a therapeutic strategy to inhibit CKD progression. For example, HGF and bone morphogenic protein-7 (BMP-7), which both target TGF-β signaling, attenuate renal fibrosis in animal models (for review see Liu2). So does blockade of angiotensin II which has been shown to inhibit EMT in addition to its beneficial hemodynamic and other effects.2 Angiotensin II blockade may be especially of interest from the viewpoint of hypoxia-mediated renal injury, as it improves microvascular perfusion.77 It is unlikely that HIF itself may become a therapeutic target in this context, mainly because it regulates multiple biological processes, some of which have been shown to be cytoprotective or have beneficial effects on tissue perfusion (for example, HIF regulates the scavenging of reactive oxygen species, nitric oxide synthesis and promotes angiogenesis). Certain HIF-regulated proteins, however, irrespective of their role in EMT, may be suited as drug targets to retard CKD progression. Pharmacological inhibition of HIF-regulated lysyl oxidases, phenocopied the effects of genetic HIF-1α inactivation on cell motility in vitro and on fibrogenesis in vivo, suggesting that lysyl oxidases are important contributors to the pathogenesis of renal fibrosis. In keeping with this notion, increased LOXL2 expression was found in renal biopsy tissues from patients with CKD underscoring its relevance to the pathogenesis of CKD irrespective of etiology.59 Whether pharmacological inhibition of lysyl oxidases is feasible in clinical practice to slow the progression of CKD warrants further investigation. Notwithstanding the profibrogenic role of epithelial HIF in vivo and its function in the regulation of EMT-triggering pathways, systemic inhibition of HIF degradation (for example, with cobalt chloride or 2-oxoglurate analogs, which both inhibit HIF prolyl-hydroxylases) has been shown to be cytoprotective not only in acute ischemia, but also in certain chronic renal disease models (for an overview of studies see Haase, Heyman et al., Fine and Norman, Nangaku et al.8,67,69). It is unclear however, which cell type, biological process or HIF homolog confers cytoprotection or aids in the preservation of renal function. Cell type-specific genetic studies in mice, which target individual HIF homologs are needed to better understand the contributions of HIF transcription factors to chronic renal injury.
SUMMARY AND OUTLOOK
HIF has emerged as an important modulator of epithelial differentiation and cell fate, linking hypoxia to tumor metastasis and invasion, and to fibrogenesis. It interfaces with the EMT process at multiple regulatory levels, which include functional interactions and co-operation with well-established EMT triggering signaling pathways, the regulation of epithelial repressor activity, and the direct or indirect regulation of proteins that modify cell–matrix interactions and facilitate motility and invasion. Although experimental evidence for HIF regulation of EMT stems mainly from the study of tumor cells, the underlying molecular mechanisms that have been identified are relevant for normal embryonic development, wound healing and fibrogenesis, as decreases in tissue oxygen levels occur physiologically during embryogenesis, and are commonly associated with tissue injury and repair. Certainly, additional experimental and clinical studies are needed to (a) further understand the context-specific role of HIF signaling in developmental and disease-associated EMT, (b) to identify the relevant oxygen-sensitive signaling pathways, and most importantly (c) to address whether HIF-regulated signaling pathways can be targeted therapeutically to improve the outcome of malignant and non-malignant diseases that are commonly associated with EMT when tissue oxygenation cannot be improved with currently available pharmacological strategies.
ACKNOWLEDGMENTS
VHH is supported by the Krick-Brooks Chair in Nephrology and grants from the National Cancer Institute, and National Institute of Diabetes and Digestive and Kidney Diseases. By virtue of its nature, this minireview represents a limited and focused account of oxygen-dependent signaling events during EMT. The author apologizes to all colleagues whose original work was not cited. For an overview of original studies the reader is referred to review articles referenced in the main text.
Footnotes
DISCLOSURE
The author declared no competing interests.
REFERENCES
- 1.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 5.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 6.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 8.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivan M, Haberberger T, Gervasi DC, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 11.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 12.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 13.Ruas JL, Poellinger L. Hypoxia-dependent activation of HIF into a transcriptional regulator. Semin Cell Dev Biol. 2005;16:514–522. doi: 10.1016/j.semcdb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Ravi R, Mookerjee B, Bhujwalla ZM, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia- inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 15.Koshiji M, Kageyama Y, Pete EA, et al. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordan JD, Bertout JA, Hu CJ, et al. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 19.Orphanides C, Fine LG, Norman JT. Hypoxia stimulates proximal tubular cell matrix production via a TGF- beta1-independent mechanism. Kidney Int. 1997;52:637–647. doi: 10.1038/ki.1997.377. [DOI] [PubMed] [Google Scholar]
- 20.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 21.Scheid A, Wenger RH, Schaffer L, et al. Physiologically low oxygen concentrations in fetal skin regulate hypoxia-inducible factor 1 and transforming growth factor-beta3. FASEB J. 2002;16:411–413. doi: 10.1096/fj.01-0496fje. [DOI] [PubMed] [Google Scholar]
- 22.Schaffer L, Scheid A, Spielmann P, et al. Oxygen-regulated expression of TGF-beta 3, a growth factor involved in trophoblast differentiation. Placenta. 2003;24:941–950. doi: 10.1016/s0143-4004(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 23.Nishi H, Nakada T, Hokamura M, et al. Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast. Endocrinology. 2004;145:4113–4118. doi: 10.1210/en.2003-1639. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Elsner T, Botella LM, Velasco B, et al. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Akman HO, Smith EL, et al. Cellular response to hypoxia involves signaling via Smad proteins. Blood. 2003;101:2253–2260. doi: 10.1182/blood-2002-02-0629. [DOI] [PubMed] [Google Scholar]
- 26.Keeton MR, Curriden SA, van Zonneveld AJ, et al. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor beta. J Biol Chem. 1991;266:23048–23052. [PubMed] [Google Scholar]
- 27.Kietzmann T, Roth U, Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood. 1999;94:4177–4185. [PubMed] [Google Scholar]
- 28.Higgins DF, Biju MP, Akai Y, et al. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004;287:F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 29.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Meng X, Zhu Z, et al. Connective tissue growth factor regulates the key events in tubular epithelial to myofibroblast transition in vitro. Cell Biol Int. 2004;28:863–873. doi: 10.1016/j.cellbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Burns WC, Twigg SM, Forbes JM, et al. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J Am Soc Nephrol. 2006;17:2484–2494. doi: 10.1681/ASN.2006050525. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, De Marco MA, Graziani I, et al. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007;67:7954–7959. doi: 10.1158/0008-5472.CAN-07-1229. [DOI] [PubMed] [Google Scholar]
- 33.Sahlgren C, Gustafsson MV, Jin S, et al. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Linke S, Dias JM, et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc Natl Acad Sci USA. 2008;105:3368–3373. doi: 10.1073/pnas.0711591105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmerman LA, Grego-Bessa J, Raya A, et al. Notch promotes epithelialmesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zavadil J, Cermak L, Soto-Nieves N, et al. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadesch T. Notch signaling: the demise of elegant simplicity. Curr Opin Genet Dev. 2004;14:506–512. doi: 10.1016/j.gde.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Bedogni B, Warneke JA, Nickoloff BJ, et al. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008;118:3660–3670. doi: 10.1172/JCI36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrissey J, Guo G, Moridaira K, et al. Transforming growth factor-beta induces renal epithelial jagged-1 expression in fibrotic disease. J Am Soc Nephrol. 2002;13:1499–1508. doi: 10.1097/01.asn.0000017905.77985.4a. [DOI] [PubMed] [Google Scholar]
- 40.Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 41.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 42.Cowden Dahl KD, Robertson SE, Weaver VM, et al. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol Biol Cell. 2005;16:1901–1912. doi: 10.1091/mbc.E04-12-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007;26:333–339. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamachary B, Berg-Dixon S, Kelly B, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 45.Shyu KG, Hsu FL, Wang MJ, et al. Hypoxia-inducible factor 1alpha regulates lung adenocarcinoma cell invasion. Exp Cell Res. 2007;313:1181–1191. doi: 10.1016/j.yexcr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Bueno G, Cubillo E, Sarrio D, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 47.Jorda M, Olmeda D, Vinyals A, et al. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118:3371–3385. doi: 10.1242/jcs.02465. [DOI] [PubMed] [Google Scholar]
- 48.Taki M, Verschueren K, Yokoyama K, et al. Involvement of Ets-1 transcription factor in inducing matrix metalloproteinase-2 expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Int J Oncol. 2006;28:487–496. [PubMed] [Google Scholar]
- 49.Imai T, Horiuchi A, Wang C, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishnamachary B, Zagzag D, Nagasawa H, et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 51.Evans AJ, Russell RC, Roche O, et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol. 2007;27:157–169. doi: 10.1128/MCB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esteban MA, Tran MG, Harten SK, et al. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res. 2006;66:3567–3575. doi: 10.1158/0008-5472.CAN-05-2670. [DOI] [PubMed] [Google Scholar]
- 53.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 54.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 55.Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Gort EH, van Haaften G, Verlaan I, et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene. 2008;27:1501–1510. doi: 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- 58.Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 59.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 61.Schietke R, Wacker I, Herter T, et al. Hypoxic induction of lysyl oxidases leads to repression of E-cadherin – a possible link from the hypoxia-inducible factor to malignant transformation. Nephrol Dial Transpl. 2007;22(Suppl. 6):233. [Google Scholar]
- 62.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 64.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutet A, De Frutos CA, Maxwell PH, et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckardt KU, Bernhardt WM, Weidemann A, et al. Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl. 2005;68:S46–S51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- 67.Heyman SN, Khamaisi M, Rosen S, et al. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol. 2008;28:998–1006. doi: 10.1159/000146075. [DOI] [PubMed] [Google Scholar]
- 68.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 69.Nangaku M, Inagi R, Miyata T, et al. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol. 2008;110:e1–e7. doi: 10.1159/000148256. [DOI] [PubMed] [Google Scholar]
- 70.Edlund J, Hansell P, Fasching A, et al. Reduced oxygenation in diabetic rat kidneys measured by T2* weighted magnetic resonance micro-imaging. Adv Exp Med Biol. 2009;645:199–204. doi: 10.1007/978-0-387-85998-9_31. [DOI] [PubMed] [Google Scholar]
- 71.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging. 2003;17:104–113. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 72.Kimura K, Iwano M, Higgins DF, et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023–F1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manotham K, Tanaka T, Matsumoto M, et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004;65:871–880. doi: 10.1111/j.1523-1755.2004.00461.x. [DOI] [PubMed] [Google Scholar]
- 74.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norman JT, Stidwill R, Singer M, et al. Angiotensin II blockade augments renal cortical microvascular pO2 indicating a novel, potentially renoprotective action. Nephron Physiol. 2003;94:p39–p46. doi: 10.1159/000071289. [DOI] [PubMed] [Google Scholar]