Abstract
Cue-elicited craving or the intense desire to consume a substance following exposure to a conditioned drug cue is one of the primary behavioral symptoms of substance use disorders (SUDs). While the concept of cue-elicited craving is well characterized in alcohol and other substances of abuse, only recently has it been described in cannabis. A review of the extant literature has established that cue-elicited craving is a powerful reinforcer that contributes to drug-seeking for cannabis. Further, emergent research has begun to identify the neurobiological systems and neural mechanisms associated with this behavior. What research shows is that while theories of THC’s effects on the dopaminergic-reward system remain divergent, cannabis cues elicit neural activation in the brain’s reward network.
Keywords: marijuana, nucleus accumbens, ventral tegmental area, cue reactivity, fMRI
1. The urge to understand cue-elicited craving for cannabis
Craving is defined as the intense desire for a rewarding object or experience and is thought to be an important factor in the etiology of addiction (Robinson and Berridge, 1993, Wise, 1988). Cue exposure elicits craving by way of attribution of incentive salience to otherwise neutral stimuli after repeated pairing of cues with a rewarding stimuli (Kalivas and Volkow, 2005). Based on the incentive sensitization theory, addiction stems from drug-induced sensitization in dopaminergic reward structures, which attribute incentive-related salience to drug associated cues (for review see (Robinson and Berridge, 2008). In this way, after repeated coupling with the drug, the cue can trigger similar primary responses as the drug itself.
Cue exposure paradigms in nonhuman animals that utilize classical Pavlovian conditioning confirm that craving increases after drug-associated cue exposure (Fattore et al., 2010). Specifically, presentation of drug-associated cues (light or tone) successfully reinstates drug-seeking behavior in rats (lever pressing). It should be noted, however, that these findings in nonhuman animals have been inconsistent (see review by Solinas et al, 2007). For example, although conditioned place preference (CPP) has been reported in response to cannabinoids, the effects have largely been specific to low dosages. At high dosages, the effects not only diminish but have an opposite effect, such as the induction of conditioned place aversion (CPA) (see review by (Cooper and Haney, 2008)). Thus, determining an animal model of the effects of cannabis on reward-craving has been complex.
In humans, however, findings have been more consistent. The published human studies of cue-elicited craving for cannabis suggest that it is a reliable and valid phenomenon that is analogous to cue-elicited craving for other drugs of abuse (e.g. (Haughey et al., 2008) (Schacht et al., 2009) (Lundahl and Johanson, 2011)) (summarized in Table 1). Similar to other drugs of abuse, cannabis-related cues presented in a variety of sensory modalities elicit increases in self-reported craving. For example, auditorily-presented imagery scripts induced craving in cannabis smokers and the magnitude of this craving varied as a function of the amount of cannabis-related content presented in the script (Singleton et al., 2002). Specifically, it was found that after presentation of auditory scripts with low or high cannabis content, self-reported craving as measured by the marijuana craving questionnaire (MCQ) increased as a function of urge content. Auditory scripts paired with tactile cues such as a used cannabis pipe or bong have also been found to elicit subjective craving (Haughey, Marshall, 2008). In this large study of 105 daily cannabis users, the effects of exposure to cannabis cues on subjective craving and withdrawal were assessed before and after a 5-day abstinence. The results showed an increase in craving as a result of both abstinence and cue exposure. This suggests that exposure to cannabis cues increases subjective craving beyond the effects induced by abstinence alone. A follow-up study by Schacht et al. (2009) used a similar cue exposure paradigm with the addition of having participants smoke 1g of cannabis (via cigarette) immediately after the cue exposure (Schacht, Selling, 2009). Analysis of the MCQ indicated that craving increased significantly after cue exposure. Olfactory cues, such as a lit cannabis scent stick, paired with auditory and tactile cues have also been shown to increase self-reported craving for cannabis in regular cannabis users compared to controls (McRae-Clark et al., 2011). With virtual reality (VR) technology, craving in environments associated with cannabis use has also been examined. For example, Bordnick and colleagues (2009) presented participants with a VR paradigm that incorporated audio, visual and vibrotactile cues. The authors compared subjective craving during a scenario that included cannabis (i.e., a party where cannabis was present and being smoked, or a room with cannabis paraphernalia) with neutral scenarios (i.e., art gallery or nature). The results showed greater subjective craving during the cannabis cue VR environment compared to the neutral VR environments (Bordnick et al., 2009).
Table 1.
Human studies showing effect of cannabis cues on craving and reward pathway activation. Studies are arranged in order of publication year.
| Authors | Participants | Cannabis Cue | Response to Cannabis Cue |
|---|---|---|---|
| Singleton et al. 2002 |
|
|
|
| Field et al. 2006 |
|
|
|
| Haughey et al. 2008 |
|
|
|
| Wolfling, et al. 2008 |
|
|
|
| Bordnick et al. 2009 |
|
|
|
| Filbey et al. 2009 |
|
|
|
| Gray et al. 2009 |
|
|
|
| Schacht et al. 2009 |
|
|
|
| Nickerson et al. 2011 |
|
|
|
| McRae-Clark et al. 2011 |
|
|
|
“day of” abstinent = participants were asked not to consume cannabis during the day of the experiment. Length of abstinence was not controlled.
Physiologic measures of craving provide further support for the validity of cue-elicited craving for cannabis. For example, using cannabis-related visual cues, skin conductance and late positivity of visual event-related brain potentials in response to cues was greater in cannabis users relative to controls (Wolfling et al., 2008). Studies have also shown that there is increased attentional bias towards cannabis cues compared with neutral cues that is consistent with those seen with tobacco and alcohol. In a study where cannabis users were shown photos related to cannabis, cannabis users maintained their gaze on cannabis cues longer and had faster approach response times to cannabis-related stimuli than controls. The users also rated cannabis cues as more pleasant on a valence rating scale (Field et al., 2006). Similar response to cannabis cues has also been reported in the adolescent population. A preliminary investigation showed that in a group of 15 young, daily cannabis users (ages 16–21) increased skin conductance, heart rate and response on the MCQ was found after exposure to auditory, visual and tactile cannabis cues (Gray et al., 2008).
Taken together, the above findings suggest that cannabis cues, regardless of sensory modality, trigger subjective and physiologic craving for cannabis. A limitation of these studies is that the experiments did not utilize participants’ preferred or most frequently used modality (i.e., pipe, joint, bong, vaporizer, etc.) and instead presented a single cue type for all participants (Note: Haughey and Schacht studies asked participants to select from two cue options). Because of this, there is a possibility that the effects are diminished and perhaps greater effects would be observed if more personally relevant cues were used for each participant. In addition, how these effects are driven by each experiment’s required abstinence is also unknown. For instance, Haughey et al. required 5 days of abstinence prior to the cue exposure paradigm whereas Schacht et al. only required a period of 24 hours and Singleton et al had no abstinence requirement. Despite these disparate approaches, however, these studies observed increases in craving post cue-exposure regardless of length of abstinence and/or possible withdrawal. However, the effects of abstinence and/or withdrawal on craving were more pronounced in the secondary genotype analyses from the Schacht study. Specifically, Schacht et al did not replicate the difference in craving response between genotype groups that was previously reported by Haughey et al. (Schacht et al., 2009) (Haughey et al., 2008). Indeed, the authors attribute their discordant findings to the shorter abstinence period. Nevertheless, these behavioral studies describe a robust effect of cannabis cues on subjective craving that suggests that in cannabis users, conditioned cues have increased motivational salience and trigger craving. Because the putative pathway for motivational salience is the dopaminergic reward pathway, it can be inferred that cue-elicited craving mechanisms are also associated with the reward neurocircuitry (Hyman et al., 2006)).
2. Cannabis affects the reward neurocircuitry
As discussed above, in the absence of cannabis itself, cannabis-associated cues may trigger neural activation in the reward pathway via motivational salience. In this section, we briefly present the literature on how cannabis itself triggers events in the reward neurocircuitry. Historically, models of the rewarding effects of THC and synthetic cannabinoid agonists in nonhuman animals have been difficult to establish as mentioned earlier. A review by Solinas and colleagues (2007) highlights these difficulties, which have been attributed to various confounds including dose variations, experimental conditions, and priming effects. Thus, animal studies of self-administration, extinction, CPP and discrimination tasks with cannabinoids have been unreliable. However, despite these discrepancies, in humans and non-human primates the rewarding effects of cannabinoids are clearer (for review of evidence see (Solinas et al., 2007)).
There is growing evidence that the endocannabinoid system, particularly the ubiquitous cannabinoid 1 receptors (CB1), plays a key role in reward pathways (Cooper and Haney, 2008). First, cannabinoid-Δ9-tetrahydrocannabinol (THC) binds to CB1 receptors in the brain (Matsuda et al., 1990). Second, CB1 receptors are present in dopamine (DA) neurons in important regions in the reward neurocircuitry including the ventral tegmental area (VTA) and the nucleus accumbens (NAc) (Fattore et al., 2008). While it has been suggested that activation of CB1 receptors in the VTA results in DA release in the NAc (Yamamoto and Takada, 2000), the discovery of CB1 receptors in the NAc has incited great debate as to whether increased DA levels resulting from CB1 agonists are a result of direct receptor binding in the NAc or through VTA stimulation which activates NAc through feedback mechanisms involving multiple neurotransmitter systems. We briefly describe the existing evidence for both views below; however, extensive reviews of these differing models and supporting evidence for each have been discussed in the literature (see (Lupica et al., 2004) (Gardner, 2005)).
The direct influence of cannabinoids on NAc characterizes the release of DA as a result of CB1 receptor binding in the NAc. It has widely been shown that administration of CB1 agonists results in increased DA levels in the striatum, specifically in the NAc shell (Bossong et al., 2009) (Ng Cheong Ton et al., 1988) (Chen et al., 1990) (Tanda et al., 2000) (Malone and Taylor, 1999) (Fadda et al., 2006). Much of the evidence for stimulation of the NAc due to CB1 agonists comes from research involving GABA neurons in the NAc that possesses CB1 receptors. By attaching onto CB1 receptors on GABA neurons, GABA release is inhibited, which in turn, disinhibits DA release in the NAc (Hoffman and Lupica, 2001).
More evidence exists, however, for the indirect stimulation of DA neurons in the VTA through cannabinoid interaction with GABA and glutamate systems. It is well established that administration of CB1 agonists such as anandamide (Solinas et al., 2006) leads to increased firing of mesolimbic DA neurons, particularly in the dopamine rich VTA (French et al., 1997) (Gessa et al., 1998). This reaction is further supported by evidence of immunoreactivity for CB1 receptors and for synthesizing enzymes of DA within the VTA (Wenger et al., 2003). Analogous to the mechanisms resulting in NAc DA transmission, it is suggested that similar disinhibitory effects of cannabinoids on GABA neurons that terminate onto DA neurons underlie increased firing (Cheer et al., 2000). Cannabinoids can also increase DA firing by acting on the glutamatergic system in the VTA through depressed stimulation of excitation or DSE (Melis et al., 2004), which act to excite GABA neurons that, in turn, inhibit DA neurons (Johnson and North, 1992) (Robbe et al., 2002) (Marinelli et al., 2007). While the large body of evidence suggesting that cannabinoids’ indirect effect on reward systems originates from DA interaction within the VTA through glutamate/GABA systems, some research suggests that even more complex systems may come into play. For example, opiate antagonists attenuate DA firing only attenuated in the NAc (Chen, Paredes, 1990). This suggests that multiple pathways for DA neurotransmission other than from VTA to the NAc.
3. Do cannabis cues also affect the reward neurocircuitry?
As mentioned, cannabis cues lead to heightened subjective craving that are similar to response to the cannabis itself. Hence, similar mechanisms within the reward neurocircuitry are expected to underlie response to cannabis cues. Indeed, the human brain imaging literature suggests that the regions within the reward network underlie the development and experience of craving for cannabis (Filbey et al., 2009), which is similar to other drugs such as cocaine, heroin, methamphetamine, alcohol, and tobacco (for review see (Filbey et al., 2011) (Volkow et al., 2002) (Hommer, 1999)).
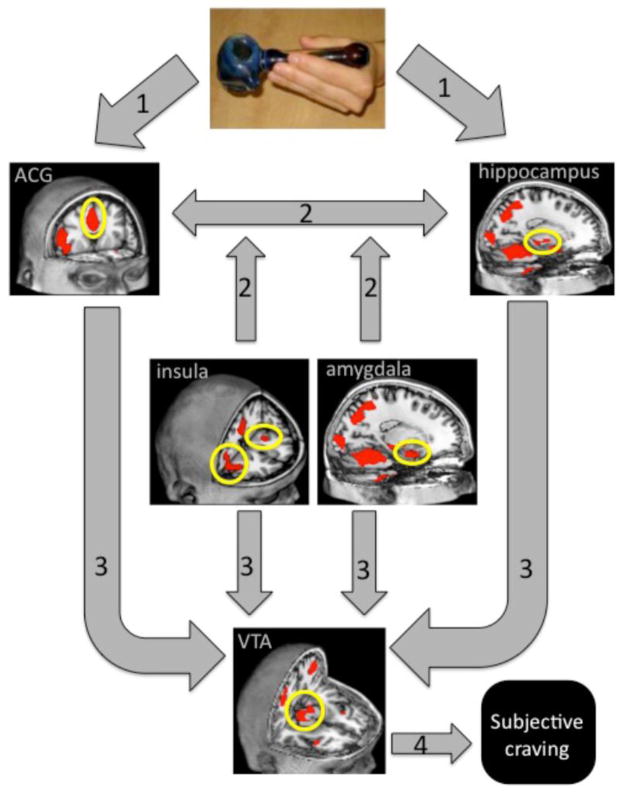
To date, we are only aware of one study that has looked at the neural mechanisms following exposure to cannabis cues. In this study, 38 72-hour abstinent regular cannabis users were exposed to tactile cannabis and neutral cues during an fMRI scan (Filbey, Schacht, 2009). The comparisons of the blood oxygenated level dependent (BOLD) response during exposure to the cannabis cue (used cannabis pipe) vs. exposure to the neutral cue (pencil) showed greater activation in several structures in the reward pathway. These areas include the VTA, anterior cingulate, insula, hippocampus, and amygdala, all of which pay a role in reward processing and incentive motivation. Specifically, the anterior cingulate has been implicated in decision-making processes surrounding reward and motivation. The insula has most recently been recognized for its role in interoceptive processes in response to cues. The amygdala plays a role in interpreting the emotional content of the cue, while the hippocampus is responsible for object recognition and memory processes related to the cues. The model presented in Figure 1 integrates these findings with theories put forth in the literature on processes related to addiction (see (Koob and Volkow, 2010)). In this model of cannabis cue-elicited craving, the neurobiological response to cannabis cues (i.e., pipe) triggers a cascade of events that underlies aspects of reward processing and attribution of salience. The model suggests multiple paths of convergence into the VTA that underlie assessment of emotional context, memory integration, evaluation of valence, as well as internal representations of the stimuli.
Figure 1. A proposed model of cannabis cue-elicited craving.
Based on the existing neuroimaging findings (Filbey, Schacht, 2009), it is proposed that in response to a conditioned stimulus (i.e., pipe), sensory information is (1) processed for motivation salience in prefrontal areas (e.g., anterior cingulate gyrus), as well as for emotional content in the amygdala. (2) Signal between this mesocorticolimbic pathway is modulated by projections from the insula that mediates interoceptive processes as well as projections from the hippocampus that underlies memory for contextual content. (3) These pathways all converge on the ventral tegmental area, through which the cascade of dopamine neurotransmission emerges and (4) leads to subjective feelings of craving.
Interestingly, in the fMRI study of cue-elicited craving response for cannabis, there was no significant activation found in the orbitofrontal cortex (OFC) or NAc, key areas in the reward neurocircuitry for processing rewards, during response to cues. This may be due to limitations in the experimental design noted in the behavioral studies described earlier. Specifically, it may be that these effects are diminished because the participants’ preferred cue type was not used. Thus, OFC and NAc activation may be stronger if cues that participants have personal relevance for were used. Moreover, because the NAc is a relatively small area, the applied statistical thresholds based on cluster-thresholding may have been too stringent for the NAc signal to survive. Thus, a larger sample size may be required if the same level of thresholding will be used. While the main effects of cannabis cues in reward areas enable the determination of how the brain responds to cues, it is equally important to determine how this brain response translates to behavior symptoms related to cannabis use. Regarding the latter, findings from the Filbey study found positive correlations between the OFC and NAc response with problems related to cannabis use. They found that greater BOLD activation in these two regions was associated with greater number of items on a cannabis problem scale (Figure 2).
Figure 2.
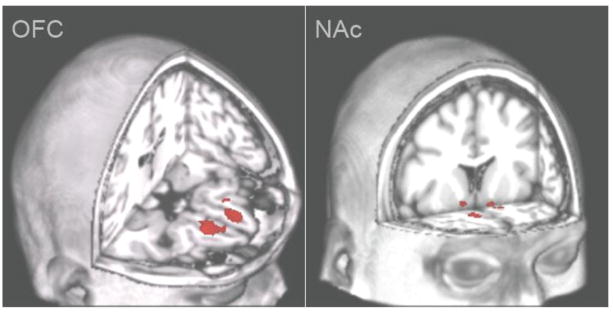
Magnitude of neural response in the orbitofrontal cortex (OFC) and nucleus accumbens (NAc) is related to marijuana related problems (Filbey, Schacht, 2009). Right side of image represents left side brain activation.
These findings are also strengthened by the report that cannabinoid receptor genes modulate this response. Filbey and colleagues (2010) reported that the cannabinoid receptor type 1 (CNR1) and fatty acid amide hydrolase (FAAH) each moderated neural response to cues in reward areas of the brain, and that there is an additive effect between the two single nucleotide polymorphisms (SNPs), such that the greater the number of risk alleles, the greater the neural response in reward areas (Filbey et al., 2010). Specifically, tests showed that those with a CNR1 G allele had significantly greater activity in the expected reward areas of the brain such as the OFC, inferior frontal gyrus (IFG), insula, and caudate during the cannabis cue compared to the those homozygous for the CNR1 A allele. Additionally, those with the FAAH C/C genotype had greater activation in the OFC, IFG, insula, NAc, compared to those who carried the FAAH A allele. In addition, correlation analyses of number of risk alleles and BOLD response to cues showed that the greater number of risk alleles (i.e., CNR1 G, FAAH C) the greater the BOLD response to cues in the OFC and striatum. This suggests a neuroregulatory function of these cannabinoid receptor alleles that cumulatively lead to altered reward neurocircuitry function, and, thus, greater susceptibility to the rewarding effects of cannabis and related cues (Figure 3).
Figure 3.
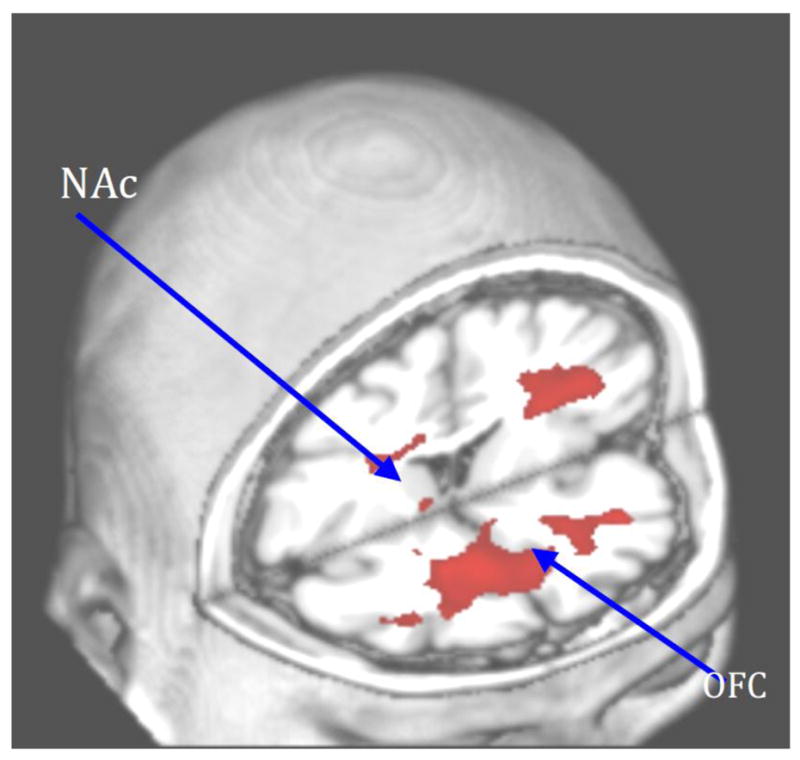
Genes that regulate endocannabinoid function modulate neural activity in orbitofrontal cortex (OFC) and nucleus accumbens (NAc) in response to cannabis cue-elicited craving (Filbey, Schacht, 2010). Right side of image represents left side brain activation.
These neuroimaging studies evidence neural response to cannabis cues similar to those of other addictive stimuli (e.g., alcohol, tobacco), however, replication of these findings still awaits. Interpretation of these findings must take some caveats into consideration. As discussed earlier, it is difficult to ascertain what the measured craving response, either self-reported or neurobiological is attributed to. Specifically, it may be possible that these findings are informing on the effects of withdrawal alone (e.g., 3-day abstinence in Filbey study). Thus, it would be important to determine whether similar response would be observed in an ad libitum sample. In other words, there may be differences in neural response to cues that could be due to the reinforcing properties of the cues, such as positive reinforcement in ad libitum states and negative reinforcement during withdrawal states. Future studies are needed to disentangle the nature of craving processes and how they may evolve during the course of drug use. In sum, while more work lies ahead in this field, the neural mechanisms evidenced in these cue-exposure experiments are analogous to those activated by the drug itself. Replication of these early studies is necessary to establish the involvement of the reward network during cannabis cues. In addition, the role of each region and how they modulate each other within the reward neurocircuitry would be important to elucidate.
4. Summary and Conclusions
To date, the literature on the mechanisms that underlie cue-elicited craving for cannabis is sparse. Behaviorally, cannabis cue-elicited craving is well characterized with studies showing that regardless of sensory modality, cues trigger both subjective and physiologic craving responses in adult and adolescent cannabis users. Although the exact mechanisms (direct vs. indirect) remain undetermined, the notion that cannabis itself increases DA neurotransmission within the reward neurocircuitry is well established. It is likely that no single pathway underlies DA neurotransmission following cannabis. Rather, multiple paths of DA enhancement with some direct and some indirect influences are responsible. The single published study on the neural mechanisms of cannabis cue-elicited craving is concordant with the existing literature that cue-elicited craving (1) is a valid phenomenon, and (2) is associated with neural activation in the reward neurocircuitry.
Future work is needed to fill the paucity in the literature on the mechanisms for cue-elicited craving. Specifically, replication of the single published study is of utmost importance. In addition to confirming the brain regions elicited by cue-elicited craving, determination of the intra-network (i.e., OFC, NAc, VTA) functional connectivity and, also, directional modulation (e.g., does VTA lead to NAc activation?) would be informative in understanding the relationship between cues and reward system response. Lastly, since behavioral and neurobiological studies have suggested that genetic factors modulate craving for cannabis, it would also be important to examine if and how environmental factors also affect craving, and how these factors interact.
List of abbreviations
- BOLD
Blood Oxygenated Level Dependent
- CB1
Cannabinoid 1 Receptors
- CNR1
Cannabinoid Receptor Type 1
- CPA
Conditioned Place Aversion
- CPP
Conditioned Place Preference
- DA
Dopamine
- DSE
Depressed Stimulation of Excitation
- FAAH
Fatty Acid Amide Hydrolase
- IFG
Inferior Frontal Gyrus
- MCQ
Marijuana Craving Questionnaire
- NAc
Nucleus Accumbens
- OFC
Orbitofrontal Cortex
- SNPs
Single Nucleotide Polymorphisms
- SUDs
Substance Use Disorders
- THC
Tetrahydrocannabinol
- VR
Virtual Reality
- VTA
Ventral Tegmental Area
Footnotes
Authors Contribution
FMF and SJD drafted the manuscript. All authors critically reviewed the manuscript content and approved the final submitted version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bordnick PS, Copp HL, Traylor A, Graap KM, Carter BL, Walton A, et al. Reactivity to cannabis cues in virtual reality environments. J Psychoactive Drugs. 2009;41:105–12. doi: 10.1080/02791072.2009.10399903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–66. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–62. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addiction biology. 2008;13:188–95. doi: 10.1111/j.1369-1600.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, et al. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17:1629–32. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Spano MS, Pistis M, Fratta W. Neurobiological mechanisms of cannabinoid addiction. Mol Cell Endocrinol. 2008;286:S97–S107. doi: 10.1016/j.mce.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol. 2010;160:724–35. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Hutchison KE. A Neuroimaging Approach to the Study of Craving. In: Adinoff A, Stein E, editors. Neuroimaging in Addiction. London: Wiley-Blackwell; 2011. pp. 133–56. [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–21. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35:967–75. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–52. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Upadhyaya HP. Cue reactivity in young marijuana smokers: a preliminary investigation. Psychol Addict Behav. 2008;22:582–6. doi: 10.1037/a0012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103:1678–86. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Functional imaging of craving. Alcohol Res Health. 1999;23:187–96. [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–68. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl LH, Johanson CE. Cue-induced craving for marijuana in cannabis-dependent adults. Exp Clin Psychopharmacol. 2011;19:224–30. doi: 10.1037/a0023030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–34. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol. 1999;128:21–6. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Florenzano F, Fezza F, Viscomi MT, van der Stelt M, et al. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology. 2007;32:298–308. doi: 10.1038/sj.npp.1301118. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, et al. Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, et al. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: an exploratory analysis. Psychopharmacology (Berl) 2009;203:511–7. doi: 10.1007/s00213-008-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton EG, Trotman AJ, Zavahir M, Taylor RC, Heishman SJ. Determination of the reliability and validity of the Marijuana Craving Questionnaire using imagery scripts. Exp Clin Psychopharmacol. 2002;10:47–53. doi: 10.1037//1064-1297.10.1.47. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–19. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–4. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S. Neuromorphological background of cannabis addiction. Brain Res Bull. 2003;61:125–8. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988;97:118–32. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008;27:976–83. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Takada K. Role of cannabinoid receptor in the brain as it relates to drug reward. Jpn J Pharmacol. 2000;84:229–36. doi: 10.1254/jjp.84.229. [DOI] [PubMed] [Google Scholar]