Abstract
Objective
Determine if incident AIDS-defining Kaposi sarcoma (KS) or Pneumocystis jiroveci pneumonia (PJP) is associated with combination antiretroviral therapy (cART) initiation.
Design
Compare risk for KS and PJP by time on cART and CD4 reconstitution.
Methods
In the FHDH-ANRS CO4 cohort (N=66,369), KS (N=1811) and PJP (N=1718) incidence rates were computed by demographic and HIV strata. Crude and adjusted relative risk (RR) with 95% confidence intervals (CI) following cART initiation were calculated by Poisson regression with untreated patients during 1996–2009 as reference. CD4 counts were compared by Wilcoxon rank sum tests.
Results
KS risk was very high during months 1–3 on cART (N=160, RRCrude 3.94, CI 3.26–4.76), which was incompletely attenuated by adjustment for demographics and contemporaneous CD4 count (RRAdj 1.25, CI 1.02–1.53). Corresponding PJP risk was minimally elevated (N=84, RRCrude 1.80, CI 1.42–2.30) and markedly reduced with adjustment on the same variables and PJP prophylaxis (RRAdj 0.52, CI 0.41–0.67). HIV load had no added effect. Median CD4 cell count at cART initiation was much lower in patients with incident KS (82/mm3) or PJP (61/mm3) within 3 months compared with those without (>250/mm3). Notably, median CD4 change was +44 cells/month with incident KS within 3 months of cART initiation versus 0 cells/month with incident PJP (P=0.0003).
Conclusions
Failure of CD4 reconstitution during months 1–3 on cART fully accounted for incident PJP. In contrast, there were 1.6 additional KS cases per 1000 person-years during months 1–3 on cART, suggesting that immune reconstitution may contribute to the risk for AIDS-defining KS.
INTRODUCTION
Immune reconstitution inflammatory syndrome (IRIS) is a heterogeneous, clinically defined aggregation of opportunistic infections and other conditions that paradoxically worsen or first appear with initiation of combination antiretroviral therapy (cART) for human immunodeficiency virus (HIV) infection.[1–3] Paradoxical progression of Kaposi sarcoma (KS) following cART initiation occurs [4–8] and was reported as a common manifestation of IRIS among patients with an AIDS-defining clinical condition (ADC) in Seattle [9]. KS, a malignancy of lymphatic endothelial cells that is often highly aggressive in people with HIV infection and severe immunodeficiency, is caused by dysregulated infection with the KS-associated herpesvirus, also known as human herpesvirus 8 (KSHV/HHV8) [10, 11].
First diagnosis of KS has been included in the IRIS spectrum [3, 6, 12]. In the HIV Outpatient Study, nine (2.4%) participants had a first diagnosis of KS within 7–180 days of starting cART, a lower cumulative incidence than observed for four other ADC (range 2.7% - 17.3%) [13]. Similar rates for KS and other ADC following cART initiation were reported from South Africa [14].
Prior ADC and very low CD4 count are risk factors for IRIS, KS, and probably both [2, 13]. However, linking immune reconstitution to an incident condition is difficult because CD4 count is dynamic and there is no diagnostic assay for IRIS [15]. Falling CD4 count prior to cART initiation was the major predictor of incident cancer in the CASCADE collaboration [16]. Nonetheless, adjusted for pre-cART change in CD4 count, a 2.3-fold elevated risk of incident cancer within 3 months of cART initiation was also seen, suggesting a genuine effect of IRIS [16]. As CASCADE includes only people with a known date of infection who are in care, it is not representative of what happens for most patients, whose HIV infection is diagnosed at later stages.
IRIS generally occurs within weeks to months after cART initiation, although the time at highest risk is variable and unsettled. During the first 5 months on cART in the Swiss HIV Cohort Study, KS risk fell 76%, pointing to a rapid effect [17]. Thus, as for other IRIS conditions [18], KS incidence might be postulated to reflect the steep decline in HIV viremia during the first 2–4 weeks, or the increasing CD4 counts during the first 8 weeks, that are generally observed on cART [19, 20].
The current study sought to clarify KS incidence during the initial months on cART, whether the risk was elevated compared to pre-cART, and whether the risk reflected contemporaneous changes in CD4 count. Pneumocystis jiroveci pneumonia (PJP), an uncommon manifestation of IRIS [9, 13], was included as a comparison condition.
METHODS
Patients
The FHDH-ANRS CO4 cohort is described in detail elsewhere [21]. Briefly, FHDH-ANRS CO4 is an open hospital cohort study conducted since 1992 by a clinical epidemiological network of 69 teaching hospitals belonging to 26 HIV Treatment and Information Centers (COREVIH) located in both mainland France and French overseas territories. The cohort includes patients who have documented HIV-1 or HIV-2 infection and who have given their written informed consent to participate. The FHDH-ANRS CO4 database, and its use, were approved by the French computer watchdog (CNIL) in accordance with French law. Data at each visit are recorded prospectively by trained research assistants. Visits are typically scheduled, and data are collected, at months 1 and 3 after starting cART, then every 3–4 months. Intervals up to 6 months between visits are accepted for patients whose viral load has been well controlled for several years. For the current analysis, the follow-up was through June 2009. The standardized FHDH-ANRS CO4 data collection form includes baseline characteristics, standard biological markers such as CD4 cell count and plasma HIV RNA level, clinical manifestations, treatments, clinical trials in which the patients are enrolled, deaths, and causes of death, as reported in the medical records. From 1992 through December 2009, more than 117,000 HIV-infected subjects had attended at least one follow-up visit, with a mean follow-up of 81 months. The current analysis defined cART as three antiretroviral drugs (not counting ritonavir as a booster) else one or more boosted protease inhibitors (PI) with or without a non-nucleoside reverse transcriptase inhibitor (NNRTI). There were three exclusions: patients with a history of KS or PJP prior to their first visit in FHDH-ANRS CO4, those who had received single or dual antiretroviral medications prior to cART, and those with only HIV-2 infection.
Variables
Numbers of patients evaluated each month were aggregated (person-time) and analyzed in strata. We considered the following explanatory variables, some of which were fixed and some of which were time-varying. Use and duration of cART for each patient in each stratum was divided into 5 time-interval categories: “no cART and year <1996”, “no cART and year ≥1996” (reference category), “≤3 months since starting cART”, “4–6 months since starting cART” and “>6 months since starting cART”. A patient was presumed to stay on cART once it was started. KS (PJP) risk in each of the first months after cART initiation was also examined. CD4 cell counts (per µL) were categorized as follows: “0–49”, “50–99”, “100–199”, “200–349”, “350–499” and “≥500”. For each patient, time was accumulated between each CD4 count measurement. As expected with follow-up every 3–4 months, median time between two CD4 values was 3.2 months (interquartile range, 2.0 – 5.2 months). Plasma HIV RNA (copies per mL) was classified as follows: “<500”, “500–4999”, “5000–49,999” and “≥50,000”; and time between HIV RNA values was accumulated as for CD4 counts. History of an AIDS-defining clinical condition (European definition for AIDS stage[22]) for each patient in each stratum was a binary variable. Sex and exposure group were combined as follows: “Men who have sex with men (MSM)”, “Injecting drug users (IDU)”, “Other men” and “Other women”. Patients also were classified by geographical origin: migration from sub-Saharan Africa “Yes” or “No”. Age in each stratum was divided into three groups: 15–34 years, 35–49 years, and ≥50 years. Calendar years were grouped into three periods: “<1996” (single and dual antiretroviral therapy available)"1996–1999” (early cART period) and “≥2000” (current cART period).
Age, calendar period, CD4 cell count, AIDS, HIV RNA and duration of cART treatment were included as time-dependent variables. The first cART regimen received by each patient was classified as follows: “no cART or calendar period before cART”, “regimen exclusively containing nucleoside reverse transcriptase inhibitors (NRTIs)”, “PI-containing regimen” and “NNRTI-containing regimen without PI”. Patients who developed KS or PJP during follow-up were censored at that visit. Thus, CD4 count and other values were the most recent before KS or PJP diagnosis.
KS (ICD-9: 173.x, ICD-10 B210, C46x) and PJP (ICD-9: 136.3, ICD-10: B59, B206) were coded with the International Classification of Disease version 9 [23] until the end of 1996, and with version 10 [24] thereafter.
Statistical Analyses
KS and PJP incidence rates (number of KS or PJP cases, respectively, divided by person-time) were computed for each stratum of the different variables. The effect of the variables, including duration of cART exposure with “no cART and year ≥1996” at the reference category, on difference in incidence (risk) of KS (PJP) was assessed using Poisson regression modeling to calculate relative risk (RR) and 95% confidence intervals (CI). The crude effect of cART exposure on risk of KS (PJP) was assessed in a univariable Poisson regression model (Model 0). Potential confounding variables were taken into account in multivariable Poisson regression models adjusted for age, sex and exposure group, migration from sub-Saharan Africa, and AIDS stage (Model 1). Model 1 was also used to estimate KS (PJP) risk during months 1, 2, 3, and 4–6 on cART. Model 2 adjusted for the variables in Model 1 plus CD4 cell count. Model 3 adjusted for the variables in Model 2 plus plasma HIV RNA since 1997 when this assay became available in France. Models 2 and 3 for PJP were also adjusted for PJP prophylaxis, that is, use of trimethoprim-sulfamethoxazole, dapsone or aerosolized pentamidine. Model 3 was used to assess differences among first cART regimens on the risk of KS (PJP).
Median and interquartile range (IQR) within-patient changes in CD4 count (crude and per month), from cART initiation to the next CD4 value within 3 months, were calculated for incident KS and PJP cases, as well as for participants without these incident conditions. Paired and unpaired Wilcoxon rank sum tests were used to compare changes and groups, respectively.
Two sensitivity analyses were performed. First, because CD4 count is a major predictor of both KS and PJP, models with categorical CD4 were compared with the models with linear CD4, each fitted with the same adjustment variables (age, sex and exposure group, migration for sub-Saharan Africa, AIDS stage and cART duration) using the likelihood ratio test. This was repeated using cubic splines for the CD4 values. Second, the primary analyses for KS were repeated with restriction to MSM, who comprised the largest exposure category and had the highest risk for KS.
Analyses were performed using Matlab® 7, version 2009b (Mathworks, Inc., Natick, Massachusetts, USA).
RESULTS
The population included 66,369 HIV-infected patients and more than 310,000 person-years (PY) of follow-up, during which 1811 cases of KS (PY=319,844) and 1718 cases of PJP (PY=314,540) were diagnosed, with crude incidence rates of 5.66 and 5.46 per 1000 PY, respectively. The KS cases included 467 with visceral involvement, 1127 without visceral involvement, and 217 unspecified sites. Table 1 presents the demographic and HIV characteristics of enrolled patients.
Table 1.
Description of 66,369 participants in the study at enrollment.*
| N (%) | ||
|---|---|---|
| Age | ||
| 15–34 | 25170 (37.9 %) | |
| 35–49 | 30833 (46.5 %) | |
| >=50 | 10366 (15.6 %) | |
| Group, by sex, exposure, sub-Saharan Africa immigration | ||
| MSM, not a migrant | 20890 (31.5 %) | |
| IDU, not a migrant | 8429 (12.7 %) | |
| Other men, not a migrant | 13982(21.1 %) | |
| Other women, not a migrant | 13073 (19.7 %) | |
| MSM, migrant | 180 (0.3 %) | |
| IDU, migrant | 83 (0.1 %) | |
| Other men, migrant | 3732 (5.6 %) | |
| Other women, migrant | 6000 (9.0 %) | |
| CD4 cell count | ||
| unknown | 2155 | |
| 0–49 | 3722 (5.8 %) | |
| 50–99 | 2203 (3.4 %) | |
| 100–199 | 4305 (6.7 %) | |
| 200–349 | 9439 (14.7 %) | |
| 350–499 | 34166 (53.2 %) | |
| >=500 | 10379 (16.2 %) | |
| HIV-RNA in copies/mL | ||
| Missing | 15594 | |
| <500 | 24972 (49.1 %) | |
| 500–4999 | 6844 (13.5 %) | |
| 5000–49999 | 9836 (19.4 %) | |
| >=50000 | 9123 (18.0 %) | |
| AIDS | ||
| No | 56134 (84.6%) | |
| Yes | 10235 (15.4%) | |
| PJP prophylaxis | ||
| No | 40805 (61.5 %) | |
| Yes | 25564 (38.5 %) | |
| Calendar year | ||
| 1992–1995 | 12498 (18.8 %) | |
| 1996–1999 | 7029 (10.6 %) | |
| 2000–2009 | 46842 (70.6 %) | |
| Duration of cART | ||
| no cART, year<1996 | 13788 (20.8 %) | |
| no cART, year ≥1996 | 12498 (18.8 %) | |
| ≤1 month | 681 (1.0 %) | |
| 2 months | 774 (1.2 %) | |
| 3 months | 686 (1.0 %) | |
| 4–6 months | 1771 (2.7 %) | |
| >6 months | 36171 (54.5 %) | |
Abbreviations: PY, person-years; MSM, men who have sex with men; IDU, injection drug use; AIDS, acquired immunodeficiency syndrome; HIV-RNA, human immunodeficiency virus plasma viral load; PJP, Pneumocystis jiroveci pneumonia;cART, combination antiretroviral therapy. Prophylaxis is use of trimethoprim-sulfamethoxazole, dapsone or aerosolized pentamidine with no prior history of PJP.
Crude incidence rates for KS and PJP by demographic and HIV categories are presented in Table 2. In both African immigrants and non-immigrants, KS rates were markedly elevated in MSM, relatively low in IDU, and higher in other men than in other women. PJP rates differed little by demographic and HIV categories, except in the small group of IDU immigrants (19.07 per 1000 PY). Incidence rates of both KS and PJP were strongly related to lower current CD4 count and higher current HIV-RNA level. Less than 11% of the PY were in the pre-cART era (1992–1995), but about half of the KS and of the PJP cases occurred during this time. The incidence of KS fell 6.6-fold from the pre-cART to the early cART era (1996–1999); it then fell 1.9-fold more from the early to the current cART era (2000–2009). The corresponding declines in PJP incidence were 4.7-fold and 1.6-fold.
Table 2.
Incidence of Kaposi sarcoma and Pneumocystis jiroveci pneumonia by variables of interest.
| Kaposi sarcoma | Pneumocystis jiroveci pneumonia | ||||||
|---|---|---|---|---|---|---|---|
| PY of follow_up |
No. of cases |
Incidence per 1000 PY |
PY of follow_up |
No. of cases |
Incidence per 1000 PY |
||
| All | 319844 | 1811 | 5.66 | 314540 | 1718 | 5.46 | |
| Age | |||||||
| 15–34 | 119691 | 672 | 5.61 | 118539 | 676 | 5.70 | |
| 35–49 | 153833 | 869 | 5.65 | 150763 | 835 | 5.54 | |
| >=50 | 46320 | 270 | 5.83 | 45238 | 207 | 4.58 | |
| Group, by sex, exposure, sub-Saharan Africa immigration | |||||||
| MSM, not a migrant | 102019 | 1263 | 12.38 | 102734 | 590 | 5.74 | |
| IDU, not a migrant | 41220 | 73 | 1.77 | 40006 | 298 | 7.45 | |
| Other men, not a migrant | 66856 | 293 | 4.38 | 63960 | 441 | 6.89 | |
| Other women, not a migrant | 65522 | 54 | 0.82 | 63724 | 291 | 4.57 | |
| MSM, migrant | 837 | 10 | 11.95 | 850 | 3 | 3.53 | |
| IDU, migrant | 394 | 1 | 2.53 | 367 | 7 | 19.07 | |
| Other men, migrant | 16010 | 64 | 4.00 | 16033 | 44 | 2.74 | |
| Other women, migrant | 26986 | 53 | 1.96 | 26865 | 44 | 1.64 | |
| Current CD4 cell count | |||||||
| unknown | 5221 | 128 | 24.52 | 5224 | 169 | 32.35 | |
| 0–49 | 10282 | 739 | 71.87 | 9364 | 617 | 65.89 | |
| 50–99 | 7987 | 228 | 28.55 | 7500 | 225 | 30.00 | |
| 100–199 | 23866 | 224 | 9.39 | 22836 | 228 | 9.98 | |
| 200–349 | 61936 | 212 | 3.42 | 60554 | 247 | 4.08 | |
| 350–499 | 76905 | 154 | 2.00 | 76080 | 116 | 1.52 | |
| >=500 | 133647 | 126 | 0.94 | 132981 | 116 | 0.87 | |
| Current HIV-RNA in copies/mL | |||||||
| Missing | 45016 | 1134 | 25.19 | 44802 | 961 | 21.43 | |
| <500 | 165325 | 173 | 1.05 | 161465 | 95 | 0.59 | |
| 500–4999 | 34677 | 58 | 1.67 | 34322 | 58 | 1.69 | |
| 5000–49999 | 46211 | 114 | 2.47 | 45959 | 152 | 3.31 | |
| >=50000 | 28615 | 332 | 11.60 | 27952 | 452 | 16.17 | |
| AIDS | |||||||
| No | 273651 | 1248 | 4.56 | 273701 | 1310 | 4.79 | |
| Yes | 46193 | 563 | 12.19 | 40840 | 408 | 9.99 | |
| PJP Prophylaxis | |||||||
| No | Not applicable | 189040 | 1049 | 5.55 | |||
| Yes | Not applicable | 125501 | 669 | 5.33 | |||
| Calendar year | |||||||
| 1992–1995 | 33173 | 991 | 29.87 | 33054 | 766 | 23.17 | |
| 1996–1999 | 58823 | 266 | 4.52 | 58279 | 285 | 4.89 | |
| 2000–2009 | 227848 | 554 | 2.43 | 223207 | 667 | 2.99 | |
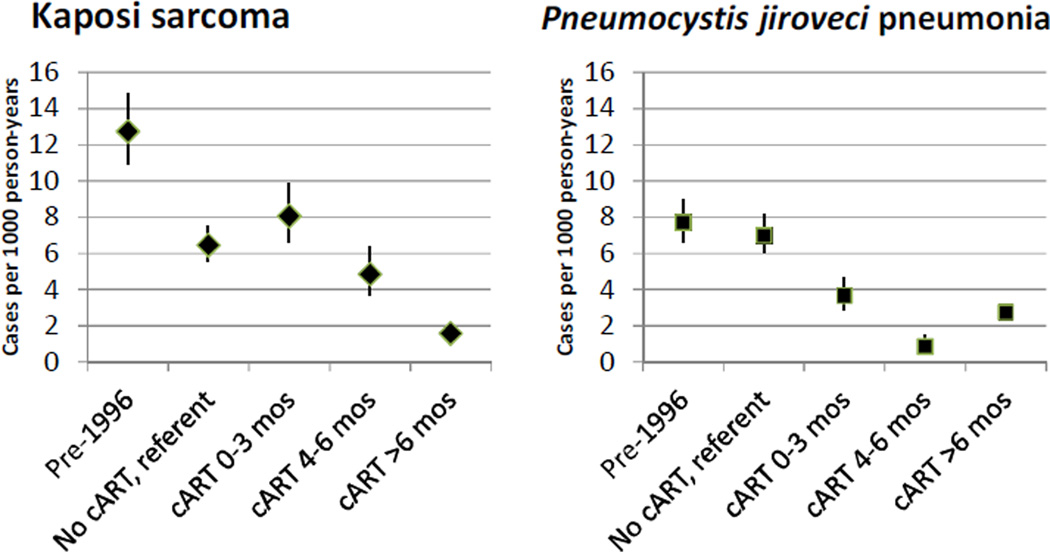
Considering KS and PJP risk by time on cART, the referent groups were PY of follow-up between 1996–2009 while not receiving cART, during which 335 KS cases and 462 PJP cases occurred. As shown in Figure 1 and Table 3, KS risk was very high during the first three months on cART (N=160, RRModel 1 3.33), and this elevated risk was largely but incompletely attenuated by adjustment for current CD4 count (RRModel 2 1.25) or current CD4 and HIV RNA (RRModel 3 1.47). KS risk was significantly elevated during each of the first six months on cART (RRModel 1 4.13 in month 1, RRModel 1 3.83 in month 2, RRModel 1 2.08 in month 3, and RRModel 1 1.50 in months 4–6). Figure 1 presents KS and PJP incidence rates by time on cART, adjusted for current CD4 count and potential confounding variables. The increased risk during months 1–3 accounted for 1.6 KS cases per 1000 patient-years after starting cART.
Figure 1.
Incidence of Kaposi sarcoma and Pneumocystis jiroveci pneumonia from Poisson Model 2, which adjusted for current CD4 count and potential confounding variables.
Table 3.
Relative risk (RR) of Kaposi sarcoma and Pneumocystis jiroveci pneumonia associated with cART use and duration derived from Poisson models.
| Kaposi sarcoma | ||||||
|---|---|---|---|---|---|---|
| No. of cases |
Incidence per 1000 PY |
Crude RR Model 0 95%CI |
Adjusted RR Model 1* 95%CI |
Adjusted Model 2** 95%CI |
Adjusted Model 3*** 95%CI |
|
| Duration of cART | P=<0.0001 | P=<0.0001 | P=<0.0001 | P=<0.0001 | ||
| no cART, year<1996 | 991 | 29.87 | 7.32 [6.47;8.28] |
7.20 [6.28;8.24] |
1.98 [1.69;2.30] |
- |
| no cART, year ≥1996 | 335 | 4.08 | 1 | 1 | 1 | 1 |
| ≤3 months | 160 | 16.07 | 3.94 [3.26;4.76] |
3.33 [2.74;4.05] |
1.25 [1.02;1.53] |
1.47 [1.16;1.87] |
| 4–6 months | 71 | 7.51 | 1.84 [1.42;2.38] |
1.50 [1.15;1.96] |
0.75 [0.57;0.98] |
1.27 [0.95;1.71] |
| >6 months | 254 | 1.37 | 0.34 [0.29;0.40] |
0.25 [0.21;0.29] |
0.24 [0.20;0.29] |
0.40 [0.32;0.48] |
| Pneumocystis jiroveci pneumonia (PJP) | ||||||
|---|---|---|---|---|---|---|
| No. of cases |
Incidence per 1000 PY |
Crude RR Model 0 95%CI |
Adjusted RR Model 1* 95%CI |
Adjusted Model 2** 95%CI |
Adjusted Model 3*** 95%CI |
|
| Duration of cART | P=<0.0001 | P=<0.0001 | P=<0.0001 | P=<0.0001 | ||
| no cART,year<1996 | 766 | 23.17 | 4.77 [4.31;5.41] |
4.48 [3.92;5.12] |
1.10 [0.95;1.28] |
- |
| no cART,year ≥1996 | 462 | 5.63 | 1 | 1 | 1 | 1 |
| ≤3 months | 84 | 8.63 | 1.80 [1.42;2.30] |
1.59 [1.25;2.03] |
0.52 [0.41;0.67] |
0.43 [0.32;0.56] |
| 4–6 months | 13 | 1.40 | 0.30 [0.18;0.53] |
0.27 [0.15;0.46] |
0.12 [0.07;0.21] |
0.19 [0.11;0.33] |
| >6 months | 393 | 2.18 | 0.46 [0.40;0.53] |
0.38 [0.32;0.43] |
0.39 [0.34;0.45] |
0.63 [0.53;0.74] |
Abbreviations: PY, person-years; cART, combination antiretroviral therapy; RR, relative risk; CI, confidence interval.
adjusted for age, sex and exposure group, migration for subsaharan Africa, AIDS stage; PJP also adjusted for prophylaxis.
adjusted for variables in Model 1 and concurrent CD4 cell count (time-dependent covariate).
adjusted for variables in Model 2 and concurrentplasma HIV RNA (time-dependent covariate), which was not available until 1997.
In contrast to KS, PJP risk was minimally elevated during months 1–3 on cART (N=84, RRModel 1 1.59), and it was markedly reduced during this interval with adjustment for current CD4 count (RRModel 2 0.52, Figure 1 and Table 3). Without CD4 adjustment, PJP risk was elevated in month 1 on cART (RRModel 1 3.56), neutral in month 2 (RRModel 1 0.98), and significantly reduced in all subsequent months. PJP prophylaxis, which was included in all of the adjusted models, significantly reduced PJP incidence, even with adjustment for current CD4 (RRModel 2 0.26, P<0.001) or current CD4 and HIV load (RRModel 3 0.33, P<0.001).
To compare the effects of different initial cART regimens, recipients of a PI-containing regimen were considered as the reference group. Compared to PI recipients, KS risk did not differ for initial regimens that contained only NRTIs (RRModel 3 1.12) or an NNRTI (RRModel 3 1.15). Likewise, PJP risk did not differ with initial regimens that contained only NRTIs (RRModel 3 1.02), or an NNRTI (RRModel 3 0.94) compared to PI.
Current HIV load and especially current CD4 count strongly affected PJP and KS risk in these models (Supplemental Table). To investigate further, Table 4 presents median CD4 counts, at cART initiation, as well as the change in CD4 count between cART initiation and the next test within 3 months. As expected, CD4 at cART initiation was substantially and significantly lower in participants who subsequently developed KS (median 82, IQR 28–278, cells/µL) or PJP (median 61, IQR 8–185 cells/µL) compared to those who developed neither of these ADC (P<0.0001 for each). During a median of about 50 days (as per protocol), CD4 count increased by 68 cells/µL in participants who developed KS, versus 0 cells/µL in participants who developed PJP. The median rate of increase was 52.6 cells/month in participants who did not develop KS, versus 43.8 cells/month in participants who developed KS (P=0.052). CD4 count increased 39.5 cells/month in those who did not develop PJP, whereas it did not increase at all in participants who developed PJP [median 0, (IQR −8.2, 28.6) cells/month, P=0.0009]. Rate of CD4 change during the first months on cART was significantly higher in participants who developed KS than in participants who developed PJP (P=0.0003).
Table 4.
Change in CD4 count among patients who did or did not develop Kaposi sarcoma (KS) or Pneumocystis jiroveci pneumonia (PJP) during the first 3 monthson cART.
| Group | Baseline CD4* | Next test (days)* |
CD4 change* | CD4 change per month* |
|---|---|---|---|---|
| KS(N=140) | 82 (28, 278) | 50 (38, 66) | 68 (10, 125) | 43.8 (16.4, 64.8)‡ |
| No KS(N=27,615) | 258 (134, 392) | 45 (33, 62) | 75 (10, 157) | 52.6 (31.0, 68.1) |
| P value for KS† | <0.0001 | 0.23 | 0.13 | 0.052 |
| PJP(N=73) | 61 (8, 185) | 58 (45, 79) | 0 (−8, 49) | 0 (−8.2, 28.6) |
| No PJP(N=26,956) | 265 (146, 398) | 55 (43, 68) | 72 (8, 154) | 39.5 (21.6, 61.4) |
| P value for PJP† | <0.0001 | 0.16 | 0.0006 | 0.0009 |
Median (interquartile) values.
KS versus no KS.PJP versus no PJP.
Change from baseline P=0.0002.
In sensitivity analyses, modeling CD4 count in six categories (≤50 … >500 cells/µL) predicted KS and PJP significantly better than did linear CD4 count (P<0.0001 for both KS and PJP), and cubic spline terms did not improve the fit of the CD4 categories (P=0.26 for KS, P=0.34 for PJP). Poisson modeling was repeated with restriction to MSM, the subpopulation at highest risk for KS, and the relative risks for KS by use and duration of cART were nearly identical to those presented in Table 3.
DISCUSSION
We sought to quantify the magnitude of incident KS and its association with cART-mediated CD4 reconstitution. For comparison, we used incident PJP, which has been a common ADC in developed countries but a minor manifestation of IRIS in most reports [9, 13]. Very low CD4 count strongly predicted KS and PJP, but we observed stark differences between these ADC during the first months on cART. PJP was associated with failure to increase CD4, whereas KS was associated with CD4 increases at approximately the same rate as in patients who developed neither PJP nor KS. By Poisson modeling, PJP crude risk was elevated only during the first month on cART, and PJP adjusted risk was directly related to the success or failure of CD4 reconstitution during the first six months on cART. In contrast, KS adjusted risk during months 1–3 on cART was incompletely related to concurrent CD4 or HIV-RNA level, and KS crude risk remained elevated for three months on cART. The elevated risk of KS was similar with all three types of cART regimen. These findings support the hypothesis that some incident KS cases occur during immune reconstitution. The absolute effect, however, was rather small, as months 1–3 on cART accounted for 1.6 additional KS cases per 1000 PY, after adjustment for CD4 count and HIV-RNA level.
We excluded cohort members who had a prior diagnosis of PJP or KS, and thus we did not address the effect of immune reconstitution on progressing KS or recurrent (paradoxical) PJP following cART initiation. In the deferred-ART initiation study (ACTG A5164), four patients with recurrent IRIS-associated PJP had robust increases in CD4 count during the initial weeks on cART [25], which contrasts with the zero median change in CD4 count in our incident PJP cases. IRIS-associated progressing KS cases have had increasing CD4 counts [6–8], and Bower et al. noted a higher CD4 reconstitution in progressing KS patients than that observed in patients with stable or improving KS [5]. Because of non-standardized time scales, those observations cannot be directly compared to the median increase in CD4 cell count in our incident KS cases, 43.8 cells/month during a median of 50 days on cART.
A robust, dysregulated, specific CD4+ T-cell response against one or more antigens of an opportunistic pathogen is likely to play a major role in IRIS [12, 26]. However, the mechanisms underlying the clinical manifestations of IRIS are unsettled [27]. Immunopathogenesis studies have been small and have included almost no KS cases. In one worsening (paradoxical) and one incident (unmasking) KS case, no clear differences in CD4+ or CD8+ T-cell responses against KSHV/HHV8 peptide pools were found [12]. Longer term studies might be revealing, as reconstitution of T-cell responses against KSHV/HHV8 peptides and clearance of KSHV/HHV8 viremia generally requires more than 6 months on cART [28]. However, this longer interval would be incompatible with clinical recognition of IRIS-KS cases and our finding that KS risk was only elevated during the first 3 months on cART.
Notable strengths of our study include its large size and uniformity. The FHDH is a large network of centers that follow a common protocol for monitoring and treating patients with HIV. Because the FHDH staff is vigilant for ADC and complications of cART, over-ascertainment of KS and PJP during the initial months on cART might be a weakness. This appears to have been minimal, as the cART-associated relative risks that we found for KS and PJP show the strong predictive effect of current CD4 count, immediate potency of prophylaxis and cART against PJP, and delayed potency of cART against KS.
Another possible weakness is enrichment of our cART-unexposed referent group with patients at lowest risk for KS or PJP. Our CD4-adjusted estimates of PJP risk suggest that this bias was well controlled, as PJP risk was 2-fold lower with only 3 months on cART, and as it was nearly identical in our referent group and the pre-cART-era population. It seems very unlikely that clinicians could defer cART based on low risk for KS but not PJP. Rather, the lower KS risk in the cART era, even without cART, likely reflects the dramatically falling risk of KS that antedated single-agent zidovudine and that possibly reflects falling KSHV/HHV8 incidence in MSM that started in the mid-1980s [29–31].
In summary, our study clarifies the dynamic effects of cART initiation and CD4 reconstitution on KS and PJP incidence. We showed that, even with CD4 adjustment, KS risk increased significantly during the first three months on cART, following which KS risk fell significantly with more than six months on cART. This dynamic high-then-low change in risk would have been missed if KS incidence had been averaged over the first year on cART [32]. Rapid, strong CD4 reconstitution on cART was associated with an equally rapid fall in PJP risk, but with a much slower fall in KS risk. These findings suggest that clinicians should be vigilant for PJP in patients who have poor CD4 reconstitution, and likewise should be vigilant for KS in patients who have robust CD4 reconstitution.
Supplementary Material
Acknowledgements
The authors are grateful to all the participants and research assistants of the French Hospital Database on HIV. J.-M.L., D.C. and J.J.G. designed and conducted the study and drafted the manuscript. J.-M.L. and D.C. conducted the statistical analyses. F.B., S.Gr., N.V., S. Ga, A.-S. L.-C., J.P., M.P., O.L., S.M., E.Ros., E.Rou., P.T., and P.deT. supervised recruitment, retention, and ascertainment and collection of end points and other data, and provided critical clinical interpretations on the findings. All authors have reviewed the latest version of the manuscript and have approved its content.
The French Hospital Database on HIV is supported by Agence Nationale de Recherches sur le SIDA et les hépatites (ANRS), INSERM, and the French Ministry of Health.
This work, specifically the French Hospital Database on HIV, was supported by Agence Nationale de Recherches sur le SIDA et les hépatites (ANRS), INSERM, and the French Ministry of Health; and the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Contributor Information
Jean-Marc Lacombe, INSERM UMR-S 943; UPMC Univ Paris 06 UMR-S 943; INSERM-TRANSFERT; Paris, France.
François Boue, AP-HP, Hôpital Antoine Béclère, Service de médecine interne et d’immunologie clinique ; Université Paris-Sud, Clamart, France.
Sophie Grabar, INSERM UMR-S 943; AP-HP, Groupe Hospitalier Cochin Hôtel-Dieu, Unité de biostatistique et épidémiologie; Université Paris Descartes, Paris, France.
Nathalie Viget, Centre Hospitalier de Tourcoing, Service universitaire des maladies infectieuses et du voyage, Tourcoing, France.
Sandrine Gazaignes, AP-HP, Hôpital Saint Louis, Service des maladies infectieuses et tropicales, Paris, France.
Anne-Sophie Lascaux-Cametz, AP-HP, Hôpital Henri-Mondor, Service d’immunologie clinique, Créteil, France.
Jérome Pacanowski, AP-HP, Hôpital Saint Antoine, Service des maladies infectieuses et tropicales, Paris, France.
Marialuisa Partisani, Hôpitaux Universitaires de Strasbourg, Le Trait d’Union - Centre de soins de l’infection par le VIH, Strasbourg, France.
Odile Launay, Université Paris Descartes; AP-HP, Hôpital Cochin, Paris, France.
Sophie Matheron, Université Denis Diderot Paris 7; AP-HP Hôpital Bichat-Claude Bernard, Service de Maladies infectieuses et Tropicales, Paris, France.
Eric Rosenthal, Hôpital l’Archet, Département de médecine interne; Université de Nice-Sophia Antipolis, Nice, France.
Elisabeth Rouveix, Hôpital Ambroise Paré, Service de médecine interne, Boulogne, France.
Pierre Tattevin, CHU Pontchaillou, Service des Maladies Infectieuses et de Réanimation Médicale, Rennes, France.
Pierre de Truchis, AP-HP, Hôpital Raymond Poincaré, Service de médecine aigue spécialisée, Garches, France.
Dominique Costagliola, INSERM UMR-S 943; UPMC Univ Paris 06 UMR-S 943, Paris, France.
James J Goedert, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD USA.
Reference List
- 1.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 2.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect.Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, Musher DW, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81:213–227. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Leidner RS, Aboulafia DM. Recrudescent Kaposi's sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient.Care STDS. 2005;19:635–644. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- 5.Bower M, Nelson M, Young AM, Thirlwell C, Newsom-Davis T, Mandalia S, et al. Immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma. J.Clin.Oncol. 2005;23:5224–5228. doi: 10.1200/JCO.2005.14.597. [DOI] [PubMed] [Google Scholar]
- 6.Letang E, Almeida JM, Miro JM, Ayala E, White IE, Carrilho C, et al. Predictors of immune reconstitution inflammatory syndrome-associated with kaposi sarcoma in mozambique: a prospective study. J Acquir Immune Defic Syndr. 2010;53:589–597. doi: 10.1097/QAI.0b013e3181bc476f. [DOI] [PubMed] [Google Scholar]
- 7.Connick E, Kane MA, White IE, Ryder J, Campbell TB. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma during potent antiretroviral therapy. Clin Infect Dis. 2004;39:1852–1855. doi: 10.1086/426078. [DOI] [PubMed] [Google Scholar]
- 8.Feller L, Anagnostopoulos C, Wood NH, Bouckaert M, Raubenheimer EJ, Lemmer J. Human immunodeficiency virus-associated Kaposi sarcoma as an immune reconstitution inflammatory syndrome: a literature review and case report. J Periodontol. 2008;79:362–368. doi: 10.1902/jop.2008.070225. [DOI] [PubMed] [Google Scholar]
- 9.Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin.Infect.Dis. 2012;54:424–433. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore PS, Chang Y. Kaposi's sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology. Am J Epidemiol. 1998;147:217–221. doi: 10.1093/oxfordjournals.aje.a009440. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA, Biggar RJ, Marshall VA, Walters MA, Gamache CJ, Whitby D, et al. Detection and quantification of Kaposi's sarcoma-associated herpesvirus to predict AIDS-associated Kaposi's sarcoma. AIDS. 2003;17:1847–1851. doi: 10.1097/00002030-200308150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Mahnke YD, Greenwald JH, Dersimonian R, Roby G, Antonelli LR, Sher A, et al. Selective expansion of polyfunctional pathogen-specific CD4+ T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak RM, Richardson JT, Buchacz K, Chmiel JS, Durham MD, Palella FJ, et al. Immune reconstitution inflammatory syndrome: incidence and implications for mortality. AIDS. 2012;26:721–730. doi: 10.1097/QAD.0b013e3283511e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 15.Porter BO, Ouedraogo GL, Hodge JN, Smith MA, Pau A, Roby G, et al. d-Dimer and CRP levels are elevated prior to antiretroviral treatment in patients who develop IRIS. Clin Immunol. 2010;136:42–50. doi: 10.1016/j.clim.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe HW, De Stavola BL, Carpenter LM, Porter K, Cox DR. Immune reconstitution and risk of Kaposi sarcoma and non-Hodgkin lymphoma in HIV-infected adults. AIDS. 2011;25:1395–1403. doi: 10.1097/QAD.0b013e3283489c8b. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi S, Maso LD, Rickenbach M, Polesel J, Hirschel B, Cavassini M, et al. Kaposi sarcoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. Br.J.Cancer. 2008;99:800–804. doi: 10.1038/sj.bjc.6604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Ding AA. Population HIV-1 dynamics in vivo: applicable models and inferential tools for virological data from AIDS clinical trials. Biometrics. 1999;55:410–418. doi: 10.1111/j.0006-341x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 20.Bennett KK, DeGruttola VG, Marschner IC, Havlir DV, Richman DD. Baseline predictors of CD4 Tlymphocyte recovery with combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:20–26. doi: 10.1097/00126334-200209010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Tassie JM, Gasnault J, Bentata M, Deloumeaux J, Boue F, Billaud E, et al. Survival improvement of AIDS-related progressive multifocal leukoencephalopathy in the era of protease inhibitors. Clinical Epidemiology Group. French Hospital Database on HIV. AIDS. 1999;13:1881–1887. doi: 10.1097/00002030-199910010-00010. [DOI] [PubMed] [Google Scholar]
- 22.Ancelle-Park R. Expanded European AIDS case definition. Lancet. 1993;341:441. doi: 10.1016/0140-6736(93)93040-8. [DOI] [PubMed] [Google Scholar]
- 23.ICD-9: International Statistical Classification of Diseases (Revision 1995) Geneva: Word Health Organisation; 1977. [Google Scholar]
- 24.ICD-10: International Statistical Classification of Diseases and Related Health Problems. Geneva: Word Health Organisation; 1993. [Google Scholar]
- 25.Grant PM, Komarow L, Andersen J, Sereti I, Pahwa S, Lederman MM, et al. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PLoS.One. 2010;5:e11416. doi: 10.1371/journal.pone.0011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL, DerSimonian R, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116:3818–3827. doi: 10.1182/blood-2010-05-285080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price P, Murdoch DM, Agarwal U, Lewin SR, Elliott JH, French MA. Immune restoration diseases reflect diverse immunopathological mechanisms. Clin.Microbiol.Rev. 2009;22:651–663. doi: 10.1128/CMR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flores R, Goedert JJ. Reconstitution of immune responses against Kaposi sarcoma-associated herpesvirus. AIDS. 2010;24:2279–2281. doi: 10.1097/QAD.0b013e32833c7bb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi's sarcoma and non- Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94:1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien TR, Kedes D, Ganem D, Macrae DR, Rosenberg PS, Molden J, et al. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi's sarcoma. J.Infect.Dis. 1999;180:1010–1017. doi: 10.1086/315039. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien TR, Engels EA, Rosenberg PS, Goedert JJ. Relationship between Kaposi sarcomaassociated herpesvirus and HIV. JAMA. 2002;287:1525–1526. doi: 10.1001/jama.287.12.1525. [DOI] [PubMed] [Google Scholar]
- 32.Lodi S, Guiguet M, Costagliola D, Fisher M, de Luca A, Porter K. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst. 2010;102:784–792. doi: 10.1093/jnci/djq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.