Abstract
Objective
Contingency management (CM) can effectively treat addictions by providing abstinence incentives. However, CM fails for many who do not readily meet the abstinence criterion and earn incentives. Shaping may improve outcomes in these hard-to-treat (HTT) individuals, as shaping sets intermediate criteria for incentive delivery between the present behavior and total abstinence. This should result in HTT having improving rather than poor outcomes throughout treatment. We examined if shaping improved outcomes in HTT smokers (those never abstinent during a 10-visit baseline).
Method
Smokers were stratified into HTT (n=96) and easier-to-treat (n=50; ETT – those abstinent at least once during baseline), and randomly assigned to either standard CM or shaping (CMS). CM provided incentives for breath carbon monoxide (CO) levels < 4 ppm (approximately 1-day of abstinence). CMS shaped abstinence by providing incentives for CO levels lower than the 7th lowest of the participant’s last 9 samples or < 4 ppm. Interventions lasted for 60 successive weekdays visits.
Results
Cluster analysis identified four groups of participants: stable successes; improving; deteriorating; and poor outcomes. In comparison to ETT, HTT were more likely to belong to one of the two unsuccessful clusters (odds ratio (OR)=8.1, 95% CI [3.1, 21]). This difference between the HTT and the ETT was greater with CM (OR=42 [5.9, 307]) than with CMS where the difference between HTT and ETT was not significant. Assignment to CMS predicted membership in the improving (P=0.002) as compared to the poor outcomes cluster.
Conclusion
Shaping can increase the effectiveness of CM for HTT smokers.
Keywords: Smoking Cessation, Contingency Management, Shaping, Percentile Schedules
Smoking can be effectively treated using contingency management (CM) to reinforce abstinence (Volp et al 2009; see Sigmon, Lamb and Dallery 2008). However, those who do not readily initiate abstinence in such programs frequently do not earn incentives and do not do well (Higgins et al 2006; Lamb, Morral, Kirby, Iguchi and Galbicka, 2004). Lamb, Kirby, Morral, Galbicka and Iguchi (2004) suggested that shaping might be used to set intermediate criteria for incentive delivery between the present behavior and total abstinence, and thus make CM more effective for hard-to-treat (HTT) smokers (i.e., those who do not stop smoking immediately before or immediately after treatment begins). This is because, HTT smokers are less likely to be abstinent during CM, and if CM works by reinforcing abstinence and abstinence never occurs, it is never reinforced. Thus, CM should not be as effective in HTT smokers. Shaping may improve CM in HTT smokers, as it works by reinforcing successive approximations of the desired behavior until the desired behavior ultimately occurs. Thus, by reinforcing reduced smoking or shorter periods of abstinence, the longer periods of abstinence typically reinforced in CM might be shaped, and CM’s effectiveness in HTT smokers increased.
Over the last several years, we developed a means to shape smoking cessation that uses percentile schedules (Lamb, Kirby et al 2004; Lamb, Morral et al 2004; Lamb et al 2007). These provide incentives if the current behavior is more similar to the desired behavior than some percentage of recent behaviors (Galbicka, 1994). For example, rather than requiring complete abstinence for incentive delivery, one could require that the current CO sample (a measure of smoking in the last day) be less than any of the previous nine days. A sample meeting this criterion would be the sample nearest to abstinence of the last ten samples collected, i.e., it was better than 9 of the last ten samples and such a sample would occur 10% of the time. This is a fairly stringent criterion, but does not require complete abstinence for incentive delivery. By adjusting the number of previous samples the present sample must be better than, any probability of earning an incentive can be achieved. Thus, if the current sample must be better than only 3 of the last 9 samples (i.e., among the 7 best of the last 10 samples), it will do so 70% of the time (producing an incentive on this same 70%). Percentile schedules, thus, deliver incentives following an individual’s better behavior. As the individual’s behavior changes, the percentile criterion remains constant, but the CO level that corresponds to that criterion changes as the samples comprising the comparison distribution changes. We have found that with HTT smokers, making it more likely that an individual earns incentives (e.g., as with the 70th percentile schedule just described) is more effective than making it more difficult for the individual to earn an incentive (e.g., requiring the current sample be better than all of the last 9; Lamb, Morral et al 2004). Recently, we showed that percentile schedules could shape reduced smoking and increase abstinence in smokers without plans to quit (Lamb et al 2007).
In the present study, we examine if CM with shaping (CMS) improves outcomes of HTT smokers compared to CM alone. We stratified smokers into HTT and easier-to-treat (ETT – those abstinent for at least one day during a 10-visit pre-intervention period) groups, and randomly assigned them to either CMS or CM. After the 60-visit intervention, we analyzed daily CO samples using cluster analysis to identify four types of treatment response: stable success, improving, deteriorating, or poor (see Morral, Iguchi, Belding and Lamb, 1997). As ETT were able to be abstinent prior to randomization we expected them to perform well with either CM or CMS. On the other hand, HTT have difficulty initiating abstinence and thus earning an incentive. By bringing the HTT into contact with incentives for reduced smoking, we expected CMS to improve outcomes for HTT in comparison to CM. In particular, relative to CM, CMS should increase the proportion of HTT having improving rather than poor outcomes. Because HTT start off poorly, they are not expected to be found in either the stable success or the deteriorating groups.
We hypothesize: (1) that relative to CM, CMS increases the odds that individuals belong to the improving as opposed to the poor outcomes cluster and (2) any improved outcomes in CMS relative to CM, are due to the improvements in the HTT; (3) that ETT do better than HTT with a greater proportion of ETT belonging to one of the two successful clusters; (4) that with CMS this difference between ETT and HTT is smaller. We do not expect CMS and CM to differ in the ETT; however, this experiment was not designed to test the equivalency of CMS and CM in ETT.
Methods
Participants
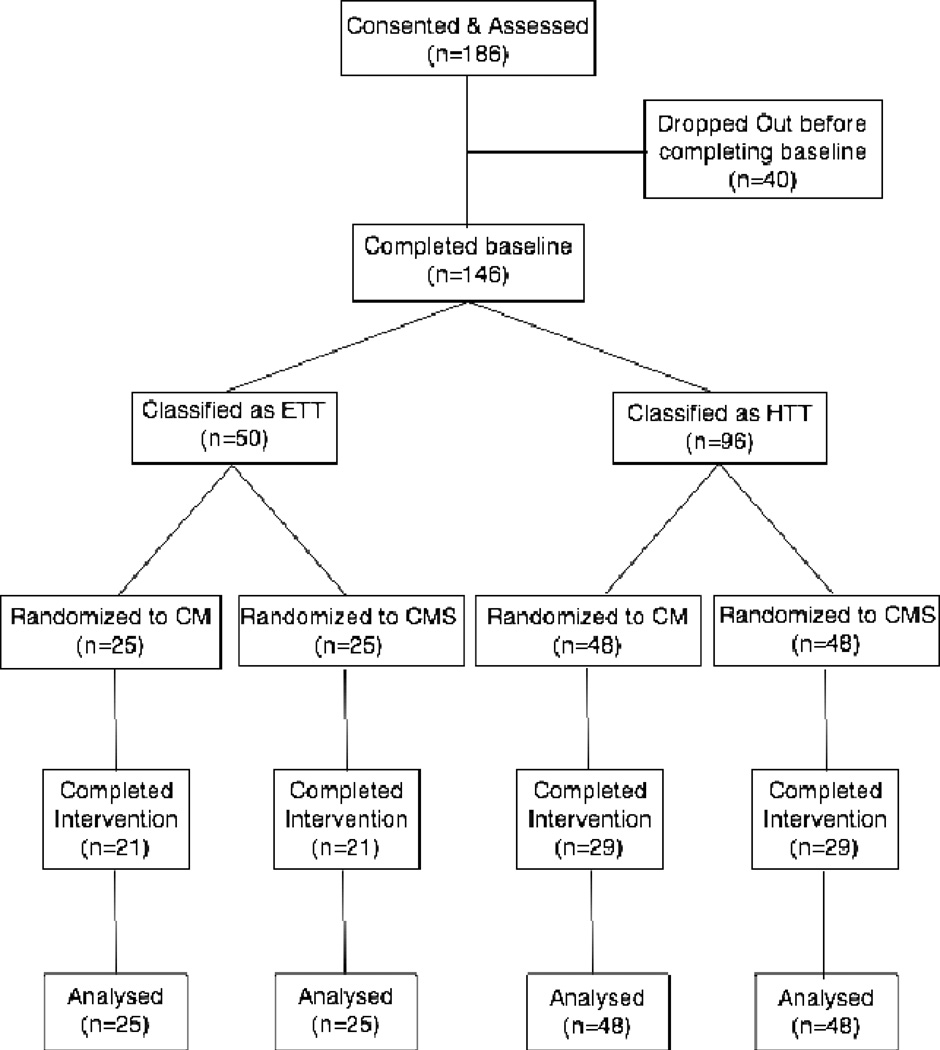
Participants were ≥ 18 years, smoking ≥ 15 cigarettes per day, seeking to quit smoking, and willing to deliver a breath sample each weekday for up to four months. A breath carbon monoxide (CO) ≥ 15 ppm at intake was also required. Participants were recruited through flyers, by word of mouth and occasional news stories between January 2002 and January 2005. One hundred and eighty-six individuals provided informed consent (Figure 1). Of these, 146 completed the 10-visit baseline and were randomized. Participants leaving before randomization were similar to those randomized. About half left without explanation, while the remainder cited difficulties with daily attendance. One individual decided the study was not likely to be helpful. The University of Texas Health Science Center at San Antonio Institutional Review Board approved this study.
Figure 1.
Consort Flow Chart. Outcomes were based on during intervention smoking behavior and analyzed on an intent to treat basis.
Measures
Breath COs were measured using a CO monitor (Vitalograph Inc. Lenexa, KS). Participants took a deep breath, held it for 20 sec and then expired into a disposable mouthpiece. The peak reading was recorded. We used a CO abstinence criterion of 4 ppm because previous work (Javors, Hatch, and Lamb, 2005) indicated that this criterion has a specificity of 0.945.
During a half hour intake session immediately following informed consent, the Fagerstrøm Test of Nicotine Dependence (FTND), the Contemplation Ladder (CL), and the Confidence Questionnaire (CQ) were collected. The FTND consists of six items that produces a score ranging from 0 to 10 (Heatherton, Kozlowski, Frecker, and Fagerstrøm, 1991). The CL (Biener & Abrams, 1991) is an 11-point anchored visual analog scale assessing readiness to quit smoking, and demonstrates good predictive validity (Biener & Abrams, 1991), convergent validity (Amodei & Lamb, 2004), and reliability (Rustin & Tate, 1993). The CQ (Condiotte & Lichtenstein, 1981) is a 14-item self-efficacy measure. It is a short version of the original (Baer & Lichtenstein, 1988), which has high internal reliability (alpha-.99), and requires smokers to rate the probability that they would be able not to smoke in a range of situations. The score is the average of these 14 ratings and can range from 0 to 100. In addition, demographic information, and smoking histories were collected using internally developed instruments.
Baseline Assessment, Randomization, and Interventions
The study consisted of 70 visits on successive weekdays, excluding holidays: A 10-visit pre-intervention period followed by a 60-visit intervention period. This took around 3 to 3.5 months and was similar in length to many smoking cessation trails (e.g., Yudkin, Jones, Lancaster, and Fowler 1996) and CM trials (e.g., Higgins, Budney, Bickel, Foerg, Donham, and Badger, 1994). Excused absences could be pre-arranged with 24-hours advance notice. In emergencies, shorter advanced notice was allowed. Visits were scheduled at a mutually determined time between 11:00 am and 5:30 pm. Participants met with a research assistant and 1) delivered a CO sample; 2) reported the number of cigarettes smoked in the last day; and 3) signed a receipt for money received. Participants earned $1.00 for each visit, which took about 5 min. No counseling was provided at these visits or by us at any other time.
10-visit pre-intervention period
On visit 1, participants could earn $2.50 for a CO < 4 ppm. They were told this could be obtained by not smoking for a day. For visits 2–10, no contingencies were in effect. Participants were told that this was so we could see how they were doing on their own. Participants were classified as ETT (N=50) or HTT (N=96) based on whether or not they delivered one or more CO < 4 ppm during this period. The mean baseline CO in the HTT was 18.1 (7.5) compared to 6.3 (5.3) in the ETT of whom 9 delivered a single CO < 4 ppm, 20 delivered 2–5 COs < 4 ppm, 15 delivered 6–9 COs < 4 ppm and 6 delivered 10 COs < 4 ppm during baseline. Previous work showed that those delivering a CO < 4 ppm during this period are more likely to do well during the intervention (Lamb, Morral et al 2004).
Randomization
Following the 10-visit pre-intervention period, participants were randomized to either CM or CMS using a multi-step procedure. First, participants were categorized as either ETT or HTT. Second, HTT and ETT were categorized on whether at intake they reported intending to use a smoking cessation medication; creating four categories: HTT-medication, HTT-no medication; ETT-medication; ETT-no medication. Individuals within each of these categories were then randomly assigned to CMS or CM using a blocking procedure accounting for order of study entry. A research assistant told each participant on visit 10 which group they had been assigned to, as it was not possible to blind participants to treatments given the nature of the interventions.
CM
Incentives were available on each of the 60 intervention visits for delivering a CO < 4 ppm. Incentive amount was set using an escalating payment schedule (Higgins et al. 1994). Incentives began at $2.50 and increased by $0.50 with each criterion sample. The delivery of 5 consecutive criterion samples resulted in a $10.00 bonus. When a sample did not meet criterion, the next available incentive reset to $2.50. After a reset, the delivery of 5 consecutive criterion samples resulted in the incentive being returned to the highest amount earned. The maximum that could be earned under this schedule with either CM or CMS was $1,157.50.
CMS
CMS was identical to CM, except that the incentive criteria were set using a percentile schedule (Galbicka 1994). Participants earned incentives whenever a CO was < 4 ppm, or when it was below the 7th lowest of the last 9 observations. This moving criterion kept the criterion within the range of recently observed behavior for a given person (i.e., not exceeding their demonstrated abilities) while providing incentives for submitting lower CO levels. Participants were told on each visit the CO criterion for their next visit. They were given no specific information about how these criteria were set.
Data Analysis
Three sets of analyses were conducted. The first compared characteristics of participants in the two interventions, and examined attendance, incentive delivery rates and amounts earned. The second developed the outcome clusters central to our hypotheses. The third examined our specific hypotheses: (1) that relative to CM, CMS increases the odds that individuals belong to the improving as opposed to the poor outcomes cluster and any improved outcomes in CMS are because (2) outcomes are generally better for the HTT-CMS than the HTT-CM; (3) that ETT do better than HTT with a greater proportion of ETT belonging to one of the two successful clusters; (4) that with CMS this difference between ETT and HTT is smaller. Finally, in the ETT we also compared outcomes between CMS and CM, but did not have specific hypotheses about these differences. These outcome comparisons were conducted both using cluster analysis, which can produce powerful and meaningful outcome measures, but can be sample dependent, and with outcome measures that were not sample dependent. All analyses were conducted using STATA version 10 (STATA; College Station, TX) on a Macintosh computer.
Participant Characteristics, attendance, incentive delivery rates and amounts earned
We compared participant characteristics using t-tests with a Welch correction, median tests, Fisher’s Exact tests for 2x2 tables and chi-square tests for larger tables. Incentive contact was compared between the CM and CMS groups for the HTT and ETT subgroups separately to determine if 1) CMS improved contact in the HTT and insured contact on ≥ 70% of visits for the HTT as it was theoretically designed to do; and 2) to examine if in ETT, contact was similar between groups. We used Fisher’s Exact and median tests. Amounts earned were examined similarly.
Clustering Procedure
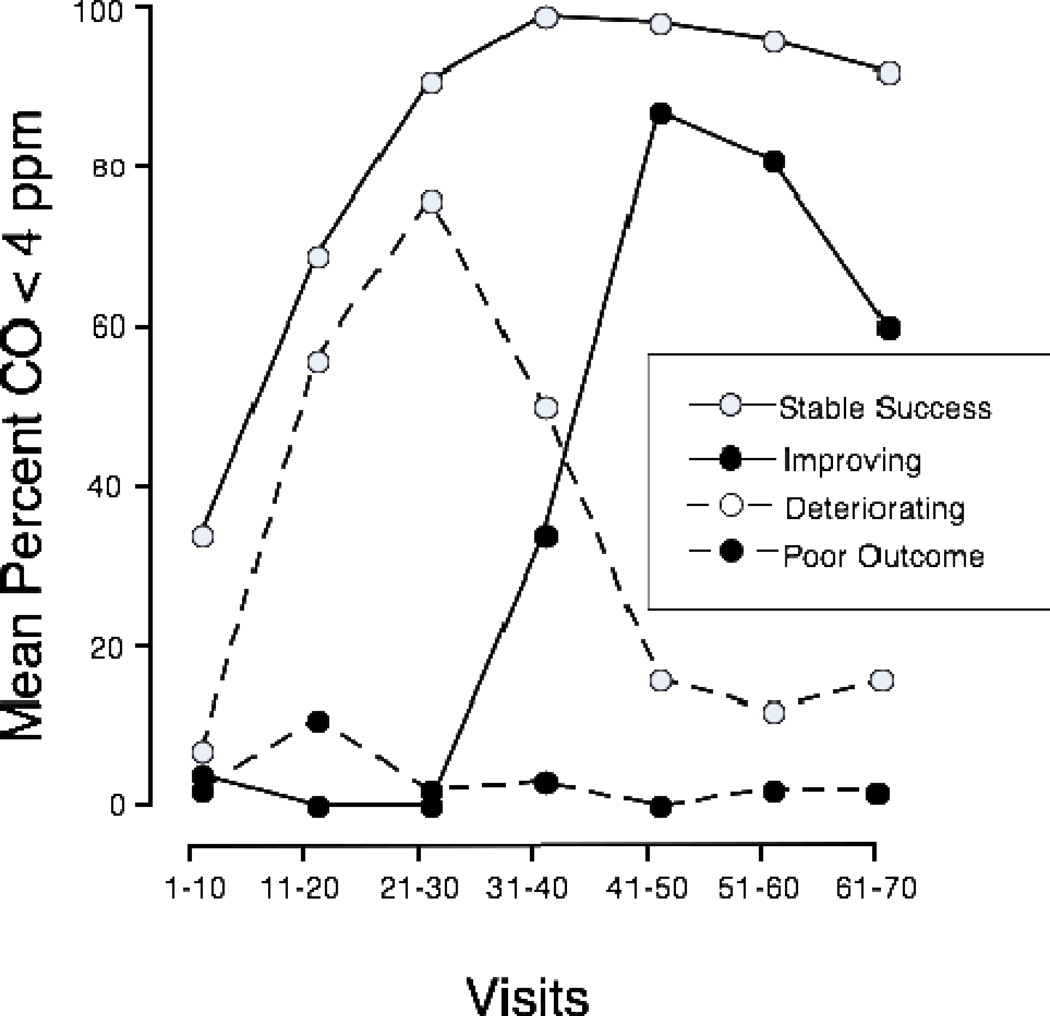
We expected CMS to change the cluster membership of the HTT, such that instead of falling in the poor outcomes cluster, more HTT would be classed among those with improving outcomes. We categorized participants into different outcome groups using cluster analysis based on the number of COs < 4 ppm during each set of ten sequential visits during the 60-visit intervention phase, i.e., during visits 11–20, 21–30, 31–40, 41–50, 51–60, 61–70. This provided a way to examine the temporal pattern of delivery of COs < 4 ppm. We used methods similar to those described in Morral and coworkers (1997) forming clusters using Ward’s agglomeration and squared Euclidean distances. We examined solutions having from 2 to 6 clusters, as solutions with more than 6 clusters had too few members to be useful. The 4-cluster solution appeared optimal. As can be seen in Figure 2, it consisted of a stable success (n=66), an improving (n=9), a deteriorating (n=19), and a poor outcome cluster (n=52), essentially replicating another study of CM in methadone maintenance patients (Morral et al 1997). The 4-cluster solution also provided the clusters needed to test our primary hypothesis that relative to CM, CMS would increase the odds of belonging to the improving rather than the poor outcomes cluster. The four clusters did not differ from each other on any subject characteristic in Table 1, except longest time without smoking, and FTND. While the overall ANOVA indicated differences in longest time without smoking, none of the between groups comparisons were significant. Those in the stable success cluster had lower FTND scores than those in the deteriorating (t (82)=2.9 P<0.05) or poor outcomes clusters (t (114)=2.8, P<0.05).
Figure 2.
Outcome trajectories for the four-cluster solution. The mean percentage of breath samples meeting the criterion for 1-day abstinence (i.e., CO < 4 ppm) are shown for each cluster in each block of 10 study visits. Dashed lines represent unsuccessful outcomes (poor outcomes –filled points, N=52; and deteriorating – open points, N=19) and solid lines represent successful outcomes (stable success – open points, N=66; and improving – filled points, N=9).
Table 1.
Participant Demographics and Smoking Histories
| ETT | HTT | ||||||
|---|---|---|---|---|---|---|---|
| total | ETT | HTT | CM | CMS | CM | CMS | |
| N=146 | N=50 | N=96 | N=25 | N=25 | N=48 | N=48 | |
| Demographics | |||||||
| Age (yrs) 1 | 39.2 (11.7) | 36.0 (12.2) * | 40.9 (11.2) | 39.7 (12.9)* | 32.4 (10.5) | 38.7 (10.9) | 43.1 (11.1) |
| Caucasian2,3 | 99 (68%) | 32 (64%) | 67 (70%) | 15 (60%)* | 17 (68%) | 35 (73%) | 32 (67%) |
| Hispanic | 23 (16%) | 9 (18%) | 14 (15%) | 2 (8%) | 8 (32%) | 8 (16%) | 14 (29%) |
| Other | 24 (17%) | 9 (18%) | 15 (15%) | 8 (32%) | 1 (4%) | 4 (8%) | 4 (8%) |
| Female2 | 63 (43%) | 20 (40%) | 43 (45%) | 13 (52%) | 7 (28%) | 22 (46%) | 21 (44%) |
| Married | 60 (41%) | 18 (36%) | 42 (44%) | 11 (44%) | 7(28%) | 16 (33%) | 26 (54%) |
| Employed Full Time | 92 (63%) | 29 (58%) | 63 (66%) | 18 (72%) | 11 (44%) | 34 (71%) | 29 (60%) |
| Annual income | |||||||
| <15,0002,4 | 37 (26%) | 16 (32%) | 21 (22%) | 6 (24%) | 10 (40%) | 10 (21%) | 11 (23%) |
| 15,000–25,000 | 44 (30%) | 14 (28%) | 30 (32%) | 6 (24%) | 8 (32%) | 15 (31%) | 15 (32%) |
| 25,001–35,000 | 32 (22%) | 11 (22%) | 21 (22%) | 6 (24%) | 5 (20%) | 10 (21%) | 11 (23%) |
| >35,000 | 32 (22%) | 9 (18%) | 23 (24%) | 7 (28%) | 2 (8%) | 13 (27%) | 10 (21%) |
| Education | |||||||
| HS/GED2,5 | 50 (34%) | 17 (34%) | 33 (34%) | 9 (36%) | 8 (32%) | 15 (31%) | 18 (38%) |
| BA+ | 38 (26%) | 14 (28%) | 24 (25%) | 5 (20%) | 9 (36%) | 14 (29%) | 10 (21%) |
| VoTech/AA/Other | 58 (40%) | 19 (38%) | 39 (41%) | 11 (44%) | 8 (32%) | 19 (40%) | 20 (42%) |
| Smoking History | |||||||
| Cigarettes per day1 | 23.9 (8.4) | 22.3 (6.2) | 24.7 (9.3) | 23.8 (7.7) | 20.7(3.8) | 23.1 (5.8) | 26.4 (11.7) |
| intake Breath CO (ppm) | 24.1 (8.1) | 22.3 (6.8) * | 25.0 (8.5) | 22.7(6.5) | 21.9 (7.3) | 24.6 (7.3) | 25.4 (9.6) |
| Fagerstrøm Score (FTND) | 5.7 (2.1) | 5.2 (2.3) * | 6.0 (2.0) | 5.5 (2.5) | 4.9 (2.1) | 5.9 (1.9) | 6.2 (2.0) |
| Contemplation ladder (CL) score6 | 8.6 (1.9) | 8.9 (1.8) | 8.5 (1.9) | 8.9 (1.7) | 8.8 (1.8) | 8.5 (1.7) | 8.4 (2.1) |
| Confidence Questionnaire (CQ) score6 | 48.1 (18.0) | 54.0 (18.4) * | 45.1 (17.1) | 57.9 (19.5) | 50.2 (16.8) | 45.2 (18.1) | 44.9 (16.3) |
| age first smoked | 14.4 (3.2) | 14.9 (2.3) | 14.2 (3.5) | 14.6 (2.6) | 15.2 (2.1) | 14.5 (3.6) | 14.0 (3.5) |
| age smoked regularly | 17.1 (3.3) | 17.4 (2.8) | 16.9 (3.6) | 17.6 (3.5) | 17.2 (1.9) | 17.5 (4.0) | 16.3 (3.0) |
| live with smoker2 | 59 (40%) | 19 (38%) | 40 (42%) | 9 (36%) | 10 (40%) | 22 (46%) | 18 (38%) |
| smoking allowed7 | 54/141 (38%) | 11/48 (23%)* | 43/93 (46%) | 6/24 (25%) | 5/24 (21%) | 21/45 (47%) | 22/48 (46%) |
| times tried quit8 | 3 (2–5) | 3 (2–6) | 3 (2–5) | 5 (3–10) * | 2 (1–4) | 3 (2–4) | 4 (2–6) |
| longest without | 90 (7–270) | 90 (7–180) | 90 (7–330) | 90 (7–300) | 60 (7–97.5) | 105 (3–365) | 90 (14–330) |
| days without last year | 2 (0–14) | 6 (0–30) * | 0.5 (0–14) | 10 (0–30) | 3.3 (0.8–27.5) | 0.5 (0–8.5) | 0.5 (0–14) |
| plans to use cessation medication2 | 53 (36%) | 36 (38%) | 17 (34%) | 9 (36%) | 8 (32%) | 18 (38%) | 18 (38%) |
mean (standard deviation)
N (Percent of sample)
self-reported race/ethnicity
income data from one participant was not collected
HS= High School, GED=Graduate Equivalency Degreee, BA+=bachelors degree or higher, VoTech=completed trade school, AA=Associates Degree
data missing for 4 subjects
number for whom smoking was allowed at work/number responding (percent)
median (inter-quartile range)
P<0.05; Means compared using Welch test. Medians compared using median test. 2x2 tables examined using Fishers Exact test, and larger tables using chi squared test.
ETT=Easier to Treat; HTT=Harder to Treat; CM=Contingency Management without Shaping; CMS=Contingency Management with Shaping
The 2 and 3 cluster solutions were suboptimal, because they produced a highly heterogenous cluster consisting of what would become the improving, deteriorating and/or poor outcomes clusters. The 5 and 6 cluster solutions were potentially useful. The 5-cluster solution broke out from the stable successes an early improving cluster. To insure that our conclusions were not an artifact of selecting the 4 over the 5-cluster solution, we re-ran all our analyses using the 5-cluster solution with essentially identical results. The 6-cluster solution broke out from the poor outcomes cluster a rapid deteriorating cluster. For the questions posed, the 6-cluster solution would have identical results to the 5-cluster solution.
Comparisons of Outcomes
Intent to treat analyses were conducted including all randomized participants. As the outcome measures were all based upon whether a scheduled CO sample was < 4 ppm, there were no missing data: a sample that was scheduled, but not delivered was counted as not < 4 ppm. Probabilities of < 0.05 of the null hypothesis being true were considered significant.
To examine how membership in the improving versus the poor outcomes cluster or in the stable success and improving versus the deteriorating and the poor outcomes clusters were influenced by either treatment (CM v CMS) or group membership (ETT v HTT), Odds Ratios (OR) were calculated using logistic regression procedures. When necessary differences in proportions were tested using Fisher’s Exact Test. Additional analyses were conducted to confirm these results were not sample dependent. In particular, we also examined two non-cluster outcomes: number of visits with a breath CO < 4 ppm and delivery of a CO < 4 ppm on each visit during visits 66–70. These were examined using the median test and by calculating ORs. We looked at the influence of covariates on these outcomes as described below. In doing this, ANOVA procedures were substituted for the median test.
We examined the influence of variables listed in Table 1 on these outcomes. This was done in a multi-staged process. First, we examined the influence of all the variables in Table 1 together. Any having a P<0.15 were included in subsequent analyses, as were variables that differed between any of the sub-groups reported in Table 1 that had a P<0.30. Membership in the improving as opposed to poor outcomes was treated slightly differently because of the limited number of participants. We examined demographic, current smoking and smoking history variables in three separate analyses and chose variables for subsequent analyses according to the criteria above. This permitted a manageable number of covariates to be examined. We then included all these covariates in the analyses testing our hypotheses, and the adjusted results are reported. The unadjusted results were similar to the adjusted results. The only outcome measure for which the effects of covariates could not be examined directly was membership in the improving rather than the poor outcomes cluster comparing CMS v CM. This was because all those in the improving cluster received CMS. However, none of the potential covariates predicted membership in the improving as compared to the poor outcomes cluster.
Results
Participants
Average participant age was just under 40 (Table 1). About two-thirds were Caucasian and Non-Hispanic, about 15% were Hispanic, and about 40% were female. Approximately 40% were married, and two-thirds had some education beyond High School. On average, participants smoked just over a pack per day, started between ages 14 and 15, and began smoking regularly between 16 and 18. Half had between 2 and 5 previous quit attempts. About a third planned on using cessation medications.
As expected, HTT were different from ETT in several ways. They were older, had lower CQ scores and were more likely to have smoking permitted at work. HTT also had fewer smoke-free days in the past year and higher intake COs and FTND scores. HTT CM and CMS did not differ, which strengthens conclusions that can be drawn from hypothesis testing focusing on these two subgroups alone. Some differences were observed between ETT CM and CMS (see Table 1).
Attendance & Incentive contact
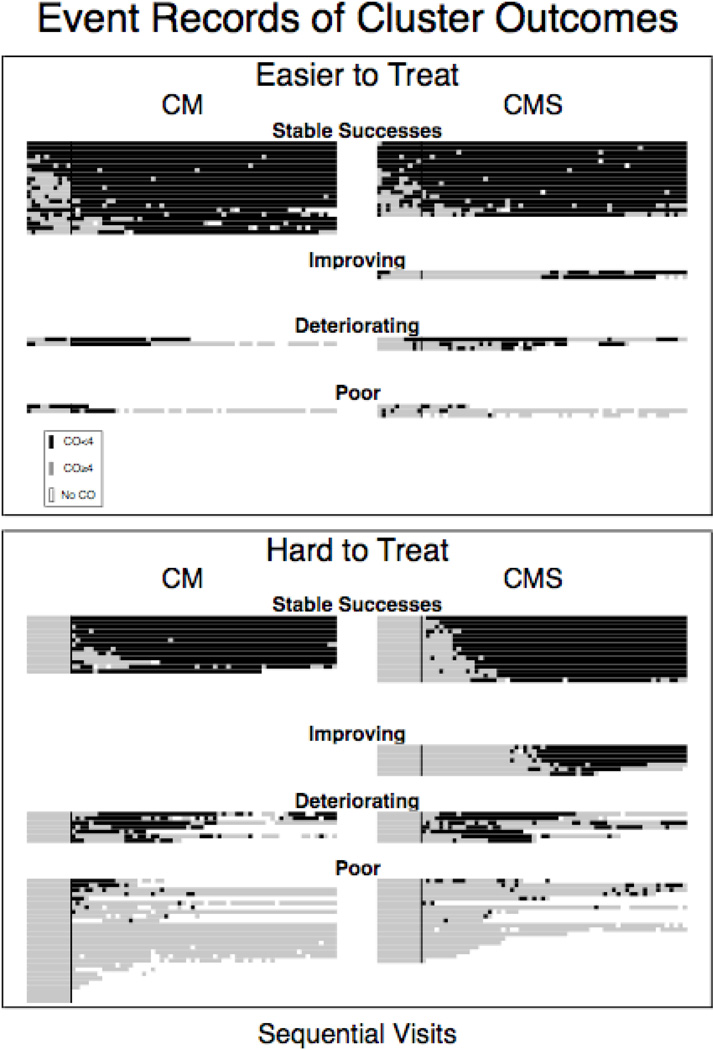
The median number of visits attended was 69 out of 70 (IQR 40 – 70). For all sub-groups, the medians were 70 except for the HTT-CM (65.5). Non-attendance for individuals can be seen by looking for blank spaces in Figure 3.
Figure 3.
Event records for each individual grouped together into clusters. Top panel presents data for the Easier to Treat, who had a CO < 4 ppm before the start of the interventions at visit 11. Bottom panel presents data for the Hard to Treat, who did not have a CO < 4 ppm before the start of the intervention. Dark spaces represent breath CO levels < 4 ppm, while lighter spaces represent breath CO levels ≥ 4 ppm. Empty spaces represent missed visits. The Horizontal axis is sequential visits and each ‘line’ of data represents an individual subject. The vertical line in each column separates the tenth and eleventh visit and indicates when the experimental contingencies went into effect. The left column is participants receiving CM, and the right column is participants receiving CMS.
CMS is designed to provide incentives on 70% or more of visits, and increased incentive contact in the HTT is the mechanism by which CMS is postulated to improve outcomes in the HTT. Thus, it is important to assess whether CMS improves contact over CM in the HTT and that this level of contact is close to what CMS was designed to provide. Every HTT-CMS participant earned an incentive (48/48), but only 63% (30/48) HTT-CM did (Fisher’s Exact Test P<0.001). The median number of incentives earned by HTT-CM was 3.5, and 44 in HTT-CMS (median test χ2(1, N=96) = 12.0 P<0.001). CMS was designed to provide incentives on 70% or more of visits, and 44 of 48 (92%) HTT-CMS met this criterion compared to only 17 of 48 (35%) of HTT-CM (Fisher’s exact test, P<0.001). Of the four HTT-CMS earning incentives on fewer than 70% of visits, all earned an incentive on > 60% of visits. Among HTT-CM, 24 of 48 (50%) earned incentives on 10% or fewer visits.
While CMS increased incentive contact among the HTT, incentive contact was similar in ETT-CM and ETT-CMS. Every ETT participant earned an incentive. The median earned for ETT-CM was 57 (interquartile range 48–59) and for ETT-CMS 58 (50–59). Only 2 of 25 in CM and 1 out of 25 in CMS earned an incentive on fewer than 70% of their visits.
Maximum possible incentive earnings were $1,157.50, and the median was $486 (Table 2). Three points directly related to number of incentives earned can also be discerned: 1) ETT earned more the HTT; 2) CMS earned more than CM; and 3) as would be expected from the incentive contact data reviewed above, HTT-CMS earned more that HTT-CM, while ETT-CMS and ETT-CM earned similar amounts.
Table 2.
Median Incentive Earnings
| All | CM | CMS | |
|---|---|---|---|
| All | $486.00 | $221.00 | $644.00 |
| (55.00–987.00)1 | (2.50–930.50) | (234.50–992.00) | |
| N=146 | n=73 | n=73 | |
| ETT | $884.502 | $878.002 | $893.002 |
| (538.50–1056.00) | (538.50–1001.00) | (549.00–1155.00) | |
| n=50 | n=25 | n=25 | |
| HTT | $231.75 | $9.753 | $461.25 |
| (9.00–869.25) | (0.00–439.00) | (139.50–983.00) | |
| n=96 | n=48 | n=48 |
Median and inter-quartile range
ETT>HTT in that column; median test using Fisher’s Exact P<0.05
CM<CMS in that row; median test using Fisher’s Exact P<0.05
ETT=Easier to Treat; HTT=Harder to Treat;
CM=Contingency Management without Shaping; CMS=Contingency Management with Shaping
Treatment Outcomes
CMS v CM
Individuals in CM and CMS had similar likelihoods of being abstinent at end of treatment (38% vs 47%) and numbers of COs < 4 ppm (Medians: 30 [IQR 1–59] for CM vs 35 [6–54] for CMS) during treatment. When outcome clusters were collapsed, those receiving CMS were significantly more likely than those receiving CM (see Table 4) to belong to the stable success or improving cluster (56% versus 47%; OR = 2.6, 95% CI [1.1, 6.2]). This was specifically due to significantly higher membership of CMS in the improving category (0% CM vs 12% CMS), as can be seen in Figure 3, and Tables 3 and 4.
Table 4.
Outcome Analysis
| Good v. bad outcomes Clusters membership | ||||
|---|---|---|---|---|
| Variable | alone | CMSvCM | ETTvHTT | HTT: CMSvCM |
| Comparison | 2.6 (1.1, 6.2)* | 8.1 (3.1, 21)* | 5.9 (1.6, 22)* | |
| employment | 3.0 (0.95, 9.3) | 3.8 (1.2, 12.5) | 3.4 (0.94, 12.3) | 6.8 (1.1, 43)* |
| salary | 0.7 (0.4, 1.1) | 0.7 (0.4, 1.1) | 0.7 (0.4, 1.2) | 0.7 (0.3, 1.4) |
| FTND1 | 0.7 (0.6, 0.9)* | 0.7 (0.6, 0.9)* | 07 (0.6, 0.9)* | 0.6 (0.4, 0.9)* |
| CL1 | 1.2 (0.98, 1.5) | 1.3 (1.01, 1.6)* | 1.2 (0.9, 1.5) | 1.4 (1.01, 2)* |
| Age regular | 1.1 (0.96, 1.2) | 1.1 (0.95, 1.4) | 1.1 (0.95, 1.2) | 1.1 (0.95, 1.4) |
| Smoking allowed | 0.7 (0.3, 1.6) | 0.6 (0.2, 2.2) | 0.9 (0.4, 2.4) | 0.6 (0.2, 2.2) |
| No. quit attempts | 1.1 (1.01, 1.2)* | 1.1 (0.9, 1.4) | 1.1 (0.97, 1.2) | 1.1 (0.9, 1.4) |
| Longest without | 1.001 | 1.001 | 1.002 | 1.002 |
| smoking | (1.0002, 1.002)* | (1.0003, 1.003)* | (1.0004, 1.003)* | (1.001, 1.003)* |
| Model LRχ2(df, N) | 34.4*(9,132) | 39.5*(10,132) | 55.0*(10,132) | 37.5*(10,132) |
| Adjusted R2 (N) | 0.19 (132) | 0.22 (132) | 0.30 (132) | 0.34 (85) |
| Improving v. poor outcomes cluster membership | ||||
| Variable | alone | CMSvCM | ETTvHTT | HTT: CMSvCM |
| Comparison | Fisher’s Exact | 2.8 (0.2–47) | Fisher’s Exact | |
| Test P=0.002 | 2.8 (0.2–47) | Test P=0.004 | ||
| gender | 5.5 (0.6, 47) | 5.1 (0.6, 44) | ||
| married | 4.4 (0.5, 38) | 3.9 (0.4, 35) | ||
| salary | 0.6 (0.2, 1.5) | 0.6 (0.2, 1.6) | ||
| CQ3 | 1.0 (0.7, 1.4) | 1.0 (0.9, 1.1) | ||
| Age regular smoking | 1.0 (0.7, 1.4) | 1.0 (0.8, 1.4) | ||
|
Number of
quit attempts |
1.1 (0.97, 1.2) | 1.1 (0.9, 1.2) | ||
| Model LRχ2 (df, N) | 12.0 (6,57) | (1,61) | 12.5 (7,57) | (1,54) |
| Adjusted R2 (N) | 0.28 (57) | (61) | 0.29 (57) | (54) |
| Number of visits with CO < 4 ppm | ||||
| Variable | alone | CMSvCM | ETTvHTT | HTT: CMSvCM |
| Comparison | F (1,130)=0.20 | F (1,130)=51.57* | F (1,83)=1.84 | |
| FTND1 | F (1,131)=14.28* | F (1,130)=14.12* | F (1,130)=8.78* | F (1,83)=9.99* |
| CL1 | F (1,131)=4.64* | F (1,130)=4.67* | F (1,130)=2.10 | F (1,83)=2.65 |
|
Longest
without smoking (days) |
F (1,131)=7.41* | F (1,130)=7.39* | F (1,130)=16.49* | F (1,83)=13.55* |
| Days last yr | F (1,131)=4.76* | F (1,130)=4.75* | F (1,130)=1.62 | F (1,83)=2.78 |
| Model | F (4,131)=7.50* | F (5,130)=6.00* | F (5,130)=18.62* | F (5,83)=6.77 |
| Adjusted R2 (N) | 0.16 (136) | 0.16 (136) | 0.39 (136) | 0.25 (89) |
| Every CO < 4 ppm on visits 66–70 | ||||
| Variable | alone | CMSvCM | ETTvHTT | HTT: CMSvCM |
| Comparison | 1.8 (0.8, 3.9) | 3.4 (1.5, 7.5)* | 5.1 (1.4, 19)* | |
| FTND | 0.8 (0.7, 0.98)* | 0.8 (0.7, 0.99)* | 0.9 (0.7, 1.04) | 0.6 (0.5, 0.9) |
| CL | 1.2 (0.97, 1.5) | 1.2 (0.97, 1.5) | 1.2 (0.9, 1.4) | 1.2 (0.9, 1.7) |
|
Age regular smoking began |
1.1 (0.95, 1.2) | 1.1 (0.96, 1.2) | 1.0 (0.9, 1.2) | 1.1 (0.97, 1.3) |
| Longest withoutsmoking (days) | 1.001 (1.0001, 1.001)* |
1.001 (1.0001, 1.001)* |
1.001 (1.0002, 1.001)* |
1.002 (1.001, 1.003)* |
|
Number of
quit attempts |
1.1 (0.99, 1.1) | 1.1 (0.99, 1.14) | 1.03 (0.96, 1.1) | 1.1 (0.92, 1.3) |
| Model LRχ2 (df, N) | 19.75*(7,136) | 21.96*(8,136) | 28.61*(8,136) | 36.12*(8,136) |
| Adjusted R2 (N) | 0.11 (136) | 0.12 (136) | 0.15 (136) | 0.32 (88) |
OR and 95% CL unless specified otherwise;
P<0.05;
ETT=Easier to Treat;
HTT=Harder to Treat;
CM=Contingency Management without Shaping;
CMS=Contingency Management with Shaping
Fagerstrøm Test of Nicotine Dependence
Contemplation Ladder
Confidence Questionaire
Reports planning to use cessation medications in this quit attempt
days without smoking in the past year
Table 3.
Outcome Results
| ETT | HTT | ||||||
|---|---|---|---|---|---|---|---|
| total | ETT | HTT | CM | CMS | CM | CMS | |
| N=146 | N=50 | N=96 | N=25 | N=25 | N=48 | N=48 | |
| Cluster Outcomes | |||||||
| Stable success1 | 66 | 38 (76%)) | 28 (29%) | 21 (84%) | 17 (68%) | 13 (27%) | 15 (31%) |
| Improving | 9 | 2 (4%) | 7 (7%) | 0 (0%) | 2 (8%) | 0 (0%) | 7 (15%) |
| Deteriorating | 19 | 5 (10%) | 14 (15%) | 2 (8%) | 3 (12%) | 7 (15%) | 7 (15%) |
| Poor Outcomes | 52 | 5 (10%) | 47 (49%) | 2 (8%) | 3 (12%) | 28 (58%) | 19 (40%) |
| Non-Cluster Outcomes | |||||||
| 32 | 30 | 17 | 15 | 11 | 19 | ||
| CO < 4 Visits 66–701 | (42%) | (64%)* | (31%) | (68%) | (60%) | (23%) | (40%) |
| 30.5 | 61 | 10.5 | 61 | 61 | 3.5 | 21 | |
| Visits CO <4 ppm2 | (2–57) | (43–67)* | (0–43.5) | (51–67) | (28–67) | (0–42) | (1–47) |
N (Percent of sample)
median (inter-quartile range)
P<0.05, Fisher’s Exact or Median Test; ETT=Easier to Treat; HTT=Harder to Treat;
CM=Contingency Management without Shaping;
CMS=Contingency Management with Shaping
HTT-CM v HTT-CMS
The differences between CMS and CM were a result of differences between the HTT-CMS and HTT-CM. In the HTT relative to CM, CMS increased the odds of being abstinent at the end of treatment (Table 4). The HTT-CMS and HTT-CM did not differ in the number of visits with a CO < 4 (Tables 3 & 4). Among the HTT, CMS had a higher proportion belonging to the stable success and improving clusters than CM (Tables 3 & 4). As can be seen in Figure 3, this was a result of those receiving CMS being more likely than those receiving CM to belong to the improving rather than the poor outcomes cluster (Table 4).
ETT v HTT
A larger proportion of ETT than HTT were abstinent at the end of treatment (see tables 3 & 4). This was the case with CM (OR=6.9 95% CI [1.9, 25]), but was significantly less and not significant with CMS (1.6 [0.5, 5.3]). The ETT had more visits with a CO < 4 ppm than the HTT (Table 3 & 4). This difference was large: Seventy-five percent of the ETT had 43 or more visits delivering a CO < 4 ppm, while 75% of the HTT had less than 44 visits delivering a CO < 4 ppm. This difference between ETT and HTT was significant in both the CM and CMS groups (Table 3). As can be seen by comparing the top and bottom panels of Figure 3, when outcome clusters were collapsed, the ETT were more likely than the HTT to belong to stable success or improving clusters (Table 4). This OR was greater in those receiving CM (42 [5.9, 307]) than in those receiving CMS (4.0 [0.9, 17]) for whom the OR was non-significant. Thus, the ETT did better in treatment than the HTT in those receiving CM, but this difference was smaller and often not significant in those receiving CMS.
ETT-CMS v ETT-CM
None of the outcomes examined differed between the ETT-CMS and the ETT-CM. They did not differ in their likelihood of being abstinent at the end of treatment, or the number of visits with a CO < 4 ppm, or in their likelihood of belong to the stable success or improving clusters.
Discussion
Shaping makes CM more effective for HTT smokers who are least likely to respond well to abstinence-oriented CM. Abstinence-oriented CM is effective at facilitating smoking cessation (Sigmond et al 2008); however, like all effective treatments, it fails for many. These HTT individuals can often be identified early on by their failure to meet the abstinence criterion. In abstinence-oriented CM, this means they do not receive the available incentives designed to reinforce abstinence and increase its future frequency. As reinforcement of abstinence is the presumed active ingredient of CM, treatment failure is both understandable and predictable for these smokers. Shaping provides incentives for successive approximations of the desired behavior, in this case longer and longer periods of abstinence (as reflected by lower CO levels). This insures incentive delivery following behavior closer to the desired behavior (Galbicka 1994) even in the absence of complete abstinence early in treatment. Shaping improved outcomes for HTT but not ETT, as ETT were able to meet the abstinence criterion even before treatment began.
Treatment success in general (Yudkin et al 1996) and with CM in particular can often be predicted based upon the rapid initiation of abstinence. In the present study, the ETT-CM had 42-fold greater odds of a successful outcome than did the HTT-CM. Individuals rapidly initiating abstinence are more likely to come into contact with the programmed incentives of CM, as well as with the natural positive consequences of abstinence and to do so sooner than those in whom abstinence is delayed or fails to occur. The units of abstinence that are reinforced (usually a day, a week or two weeks) are much shorter than the units of abstinence that are taken as a measure of success (1 month, 3 months, a year, etc.). Reinforcing these smaller units increases their frequency and builds the larger units by which we measure success. Those who succeed in CM often exhibit these smaller units that will earn incentives even before incentive delivery begins. However, without effective treatment, the likelihood that these smaller units increase in frequency to become the larger units by which we measure success is small (Morral et al. 1997). In large part, effective treatments work by facilitating the longer-term success of ETTs.
The HTT differed from the ETT in several ways. The HTT were more dependent and had fewer days without smoking in the past year. They also were more likely to have workplaces permitting smoking and were less confident in their ability to refrain from smoking. Thus, the HTT in this study were similar to those who in other studies are less successful in stopping their smoking. Further, other studies of CM (e.g., Higgins et al 2006) and of nicotine replacement therapies (e.g., Yudkin et al 1996) found that smokers who did not rapidly initiate abstinence at the beginning of treatment were very likely to do poorly. Thus, it is remarkable that CMS improved outcomes for the HTT. Still, CMS moved only 7 individuals into the improving cluster, and this might at first seem to be a modest effect. However, the result of this was that 46% of the HTT receiving CMS had a good outcome as opposed to 27% of the HTT receiving CM, resulting in a 70% improvement in the number of HTT having good outcomes.
One element of CMS that likely enhanced the effectiveness of the percentile schedule was its use in conjunction with an escalating payment schedule. Each sequential incentive delivery increased the magnitude of the next available incentive, and failure to meet the criterion reset incentive value to its starting amount. Roll and colleagues (Roll and Higgins, 2000; Roll, Higgins, and Badger, 1996) showed that escalating payment schedules increase the likelihood of continuing to meet the reinforcement criterion once it has been met. As percentile schedules guarantee that an individual meets the reinforcement criterion and the escalating payment schedule increases the probability that once it is met, it continues to be met, these two elements of CMS work together to facilitate continued smoking reductions. When the percentile schedule reduces smoking such that further reduction results in abstinence the escalating payment schedule dictates that the incentive available for abstinence be larger than it was initially. Numerous studies show that larger incentives are more effective at promoting abstinence (e.g., Lamb, Kirby, et al 2004; Stitzer and Bigelow, 1984).
There are limitations of the CMS intervention. As with CM, some individuals were in the deteriorating cluster. The deteriorating cluster consisted of similar numbers of those receiving CMS or CM. Participants initiated abstinence under both interventions, but failed to maintain abstinence over time in treatment. This pattern of behavior suggests that both groups might benefit from additional relapse prevention interventions, from a different escalating schedule (Roll and Higgins, 2000; Roll, et al. 1996) or possibly from delaying their quit attempt while gradually reducing their smoking. All of these possibilities merit further investigation.
There are potential limitations to this study. First, this study was not designed to detect differences in abstinence rates at post-treatment follow-up. Detecting such differences would have required substantially more participants, and we decided to first test the utility of shaping abstinence during treatment, given the strong relationship between outcomes during treatment and at follow-up for CM treatments (Higgins, Badger, and Budney, 2000; Higgins et al 2006). Given the positive results of this study, larger studies examining post-treatment follow-up abstinence rates of CMS and CM are merited, as the cost-effectiveness of these interventions and their relative cost-effectiveness depends upon how long the effects of these treatments endure.
Second, while we believe this study provides compelling evidence that shaping can improve outcomes among HTT smokers, contingently reinforcing breath CO levels may not be practical in many settings as a smoking cessation treatment. The intervention involved 70 daily visits and may appear burdensome limiting its general application, and exerting a selection bias on participation. While the visits were short, about 5 minutes, the near daily nature of these means that most participants would need to live or work near the treatment site. We did not track the number of individuals who decided not to schedule a meeting for informed consent because of this potential burden. Of the 186 participants providing informed consent, 22% dropped out in the approximately 2-weeks of daily visits before the intervention began. Almost all of those who provided a reason cited the burden of the daily visits.
While self-reports of smoking are typically more easily collected, their reliability in the context of a CM intervention is doubtful, making in-person deliveries of more objective breath samples important. Using a low CO cut-off (e.g. < 4 ppm) on afternoon samples results in these being adequate measures of smoking in the last day (Javors et al 2005), but requires near daily measurement in CM. Less frequent monitoring is necessary when cotinine levels are measured. However, monitoring cotinine is more costly, takes more time to analyze, is confounded by nicotine replacement therapies, and still requires measurement at least twice a week. The requirement for frequent visits suggests some contexts in which this intervention might be practical. In particular, worksite implementation or implementation in more densely populated urban areas would seem promising. Alternatively, remote monitoring technology has been used as a way to reduce the burden of participating in CM interventions for smoking cessation (Dallery and Glenn, 2005).
An alternative to shaping is using high initial payments. We and others have shown that smoking abstinence (Lamb, Kirby, et al. 2004; Stitzer & Bigelow, 1983) increases as the incentive available increases. With sufficient incentives, abstinence initiation rates can be quite high (e.g., 75%). Stitzer and her colleagues (e.g., Robles, et al. 2000) suggested that combining high payment amounts with easier initial response requirements might result in higher initial abstinence rates. Kirby, Marlowe, Festinger, Lamb, and Platt (1998) showed that using initially high incentive amounts and short abstinence requirements that changed to smaller incentive amounts and longer abstinence requirements as these were met could result in an effective treatment for cocaine addiction. These procedures and the shaping procedure described here all increase the likelihood that some period of abstinence earns an incentive that can potentially reinforce abstinence and increase its future probability. Given the results of Kirby et al (1998) and this study, shaping procedures may further improve CM outcomes.
Shaping improved outcomes for those least likely to do well in CM in this smoking cessation study. Shaping is likely to prove useful in the treatment of other addictions as well. Preston, Umbricht, Wong, and Epstein (2001) and Kirby et al. (1998) showed its potential utility in treating cocaine addiction. Extending shaping to other interventions focusing on increasing or decreasing a single behavior that can be measured ordinally would be straightforward. For instance, Athens, Vollmer, and Pipkin (2007) showed that shaping could increase on-task engagement in students. Further extension to things like workplace productivity or quality improvement would also seem straightforward. Addressing broader goals (e.g., education and health promotion) that are made up of multiple behaviors may be more difficult. What role formalized shaping procedures, such as percentile schedules, can play in facilitating these is an important area of future endeavor. Shaping can play an important role in facilitating abstinence in CM by capturing small increases in abstinence and increasing the future probability of prolonged abstinence.
Acknowledgments
We would like to thank Floyd Jones, and Gilbert Holguin for their help in conducting this study. We would also like to thank the participants that made this study possible. Finally, we would like to thank the National Institutes of Health for their support of this work through grant DA013304.
Contributor Information
R.J. Lamb, University of Texas Health Science Center at San Antonio
Kimberly C. Kirby, Treatment Research Institute
Andrew R. Morral, RAND
Greg Galbicka, Sanofi-aventis Pharmaceuticals.
Martin Y. Iguchi, University of California, Los Angeles
References
- Amodei N, Lamb RJ. Convergent and concurrent validity of the contemplation ladder and URICA scales. Drug and Alcohol Dependence. 2004;73:301–306. doi: 10.1016/j.drugalcdep.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Athens ES, Vollmer TR, Pipkin CC. Shaping academic task engagement with percentile schedules. Journal of Applied Behavior Analysis. 2007;40(3):475–488. doi: 10.1901/jaba.2007.40-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. Journal of Consulting & Clinical Psychology. 1988;56(1):104–10. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Condiotte MM, Lichtenstein E. Self-efficacy and relapse in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1981;49:648–658. doi: 10.1037//0022-006x.49.5.648. [DOI] [PubMed] [Google Scholar]
- Dallery J, Glenn IM. Effects of an internet-based voucher reinforcement program for smoking abstinence: a fesibility study. Journal of Applied Behavior Analysis. 2005;38:349–357. doi: 10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbicka G. Shaping in the 21st century: moving percentile schedules into applied settings. Journal of Applied Behavior Analysis. 1994;27(4):739–760. doi: 10.1901/jaba.1994.27-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrøm KO. The Fagerstrøm Test for Nicotine Dependence: a revision of the Fagerstrøm Tolerance Questionnaire. British Journal of Addiction. 1991;86:9, 1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental Clinical Psychopharmacology. 2000;8(3):377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg F, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug and Alcohol Dependence. 2006;85(2):138–141. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Evaluation of cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Marlowe DB, Festinger DS, Lamb RJ, Platt JJ. Schedule of voucher delivery influences cocaine abstinence. Journal of Consulting and Clinical Psychology. 1998;66(5):761–776. doi: 10.1037//0022-006x.66.5.761. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Improving contingency management programs for addiction. Addictive Behavior. 2004;29(3):507–523. doi: 10.1016/j.addbeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76(3):247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Javors MA, Galbicka G, Iguchi M. Contingencies for change in complacent smokers. Experimental and Clinical Psychopharmacology. 2007;15(3):245–255. doi: 10.1037/1064-1297.15.3.245. [DOI] [PubMed] [Google Scholar]
- Morral AR, Iguchi MY, Belding MA, Lamb RJ. Natural classes of treatment response. Journal of Consulting and Clinical Psychology. 1997;65(4):673–685. doi: 10.1037//0022-006x.65.4.673. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. Journal of Consulting and Clinical Psychology. 2001;69(4):643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Robles E, Silverman K, Preston KL, Cone EJ, Katz E, Bigelow GE, Stitzer ML. The brief abstinence test: voucher-based reinforcement of cocaine abstinence. Drug and Alcohol Dependence. 2000;58:205–212. doi: 10.1016/s0376-8716(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58(1–2):103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29(4):495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin TA, Tate JC. Measuring the stages of change in cigarette smokers. Journal of Substance Abuse Treatment. 1993;10:209–220. doi: 10.1016/0740-5472(93)90046-5. [DOI] [PubMed] [Google Scholar]
- Sigmond SC, Lamb RJ, Dallery J. Tobacco. In: Higgins ST, Silverman K, Heil S, editors. Contingency Management in the Substance Abuse Treatment. NY, NY: Guilford Press; 2008. pp. 99–119. [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for carbon monoxide reduction: within-subject effects of pay amount. Journal of Applied Behavior Analysis. 1984;17(4):477–483. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch D, et al. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine. 2009;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- Yudkin PL, Jones L, Lancaster T, Fowler GH. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. British Journal of General Practice. 1996;46:145–148. [PMC free article] [PubMed] [Google Scholar]