Abstract
OBJECTIVE
To examine the rates of long-term biochemical recurrence-free survival (BRFS) with respect to isotope in intermediate-risk prostate cancer treated with external beam radiotherapy (EBRT) and brachytherapy.
METHODS
A total of 242 consecutive patients with intermediate-risk prostate cancer were treated with iodine-125 (125I) or palladium-103 (103Pd) implants after EBRT (range 45.0–50.4 Gy) from 1996 to 2002. Of the 242 patients, 119 (49.2%) were treated with 125I and 123 (50.8%) with 103Pd. Multivariate Cox regression analysis was used to analyze BRFS, defined according to the Phoenix definition (prostate-specific antigen nadir plus 2 ng/mL) with respect to Gleason score, stage, pretreatment prostate-specific antigen level, and source selection. Late genitourinary/gastrointestinal toxicities were assessed using the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer scale.
RESULTS
At a median follow-up of 10 years, the BRFS rate was 77.3%. A statistically significant difference was found in the 10-year BRFS rate between the 125I- and 103Pd-treated groups (82.7% and 70.6%, respectively; P = .001). The addition of hormonal therapy did not improve the 10-year BRFS rate (77.6%) compared with RT alone (77.1%; P = .22). However, a statistically significant difference in the BRFS rate was found with the addition of hormonal therapy to 103Pd, improving the 10-year BRFS rate for (73.8%) compared with 103Pd alone (69.1%; P = .008). On multivariate analysis, isotope type (103Pd vs 125I), pretreatment prostate-specific antigen level > 10 ng/mL, and greater tumor stage increased the risk of recurrence by 2.6-fold (P = .007), 5.9-fold (P < .0001), and 1.7-fold (P = .14), respectively.
CONCLUSION
125I renders a superior rate of BRFS compared with 103Pd when used with EBRT. Hormonal therapy does not provide additional benefit in patients with intermediate-risk prostate cancer treated with a combination of EBRT and brachytherapy, except for the addition of hormonal therapy to 103Pd.
Prostate brachytherapy as a boost to external beam radiotherapy (EBRT) in the treatment of men with clinically localized prostate cancer has increasingly regained popularity in the past decade.1 More than one third of men with prostate cancer treated with RT receive brachytherapy as a component of their treatment, and approximately one half of the men treated with brachytherapy also received supplemental EBRT according to the 1999 Patterns of Care Study.1 Although combining EBRT with brachytherapy offers several potential advantages in the outcomes compared with using either treatment alone, such combination therapy can lead to an increase in the risk of normal tissue injury compared with either modality alone. However, improved outcomes in tumor control should not be eclipsed by an increased rate in normal tissue complications.
Advances in treatment techniques and technology represent a step into modernity with regard to improving outcomes. The use of transrectal ultrasound guidance for preplanned or intraoperatively planned implants2–4 and postimplant dosimetry have led to improved prostate coverage, while respecting the tolerances of the adjacent critical structures.5 The enhanced quality of the implants has been demonstrated to result in improved biochemical relapse-free survival (BRFS) outcomes in several modern studies.6–8
A number of reports have demonstrated the effectiveness of various radioactive isotopes9–14; however, no role of any commonly used isotope in prostate brachytherapy combined with EBRT has been examined with respect to BRFS. Although hormonal therapy (HT) has demonstrated improved outcomes in patients with intermediate-risk prostate cancer (IRPC) when used with EBRT, few reports have supported the utility of HT combined with EBRT and brachytherapy.15 The purpose of the present study was to estimate the rates of long-term BRFS with respect to the choice of radioactive isotope and the influence of the addition of HT.
MATERIAL AND METHODS
Study Design and Patient Population
After obtaining institutional review board approval from New York Presbyterian Hospital, we conducted a retrospective review study of >1000 patients with clinically localized prostate adenocarcinoma and selected 287 patients with IRPC treated with combination therapy who had complete follow-up data available. Combination therapy included an interstitial implant with radioactive isotope 125I or 103Pd performed approximately 2–6 weeks after EBRT from 1996 to 2002. Patients were excluded from the study if they did not have ≥3 post-treatment prostate-specific antigen (PSA) levels (n = 31), lacked adequate information on late toxicity (n = 13), or lacked adequate pretreatment staging (n = 1), leaving 231 patients evaluable for the present study. Of the 231 patients, 137 received treatment at Weill Cornell Medical Center (New York, NY) and 105 at New York Hospital Queens (Queens, New York).
Staging
All patients were initially evaluated with medical history and physical examination, including the digital rectal examination (DRE), serum PSA level, and ultrasound-guided transrectal prostate biopsy with Gleason score histologic grading. Other staging modalities were performed at the discretion of the treating physician as clinically indicated and included radiographic studies, such as bone and computed tomography scanning of the pelvis, magnetic resonance imaging of the prostate or pelvis, positron emission tomography, and bone scanning. The 1992 American Joint Committee on Cancer staging system was used to assign clinical stage.16 The T stage was assigned according to the DRE results. The pathologic biopsies were reviewed and the Gleason score was assigned primarily by the pathology staff.
Risk Classification
Intermediate risk was defined according to the Memorial Sloan-Kettering Cancer Center risk grouping described by Zelefsky et al17 as having 1 of the following unfavorable risk factors: Gleason score >6 or Stage T2b or initial serum PSA level >10 ng/mL. Biochemical failure and BRFS after RT with and without hormonal therapy in men with localized prostate cancer was defined according to the Consensus American Society for Radiation Oncology Conference Definition, known as the Phoenix Definition: an increase of ≥2 ng/mL greater than the nadir PSA level.18
Treatment
The patients first received EBRT to 45.0–50.4 Gy in daily 1.8-Gy fractions 5 times weekly. The initial target volume included the prostate and seminal vesicles. The planned target volume margin was 0.5 cm posteriorly and 1 cm elsewhere. Megavoltage photon (10–15 MV) beams delivered intensity-modulated RT using 5 fields (2 anterior oblique fields, 2 posterior oblique fields, and 1 posterior field) with B-mode acquisition and targeting ultrasound or cone-beam image guidance, with the patients treated in a custom alpha-cradle immobilization device. A preplanning transrectal ultrasound volume study was performed in preparation for the subsequent implant. Brachytherapy was performed 4 weeks after EBRT if there was no pubic arch interference, no significant urinary symptoms (American Urological Association score <12), and prostate gland volume <60 cm3. The minimal peripheral dose included the outlined volumes and was derived using standard radiation planning software. The prescription dose for the boost 90–100 Gy for an iodine-125 (125I) implant (National Institute of Standards and Technology 99) and 80–90 Gy for a palladium-103 (103Pd) implant (Task Group Report 43). A modified uniform peripheral loading format to limit the dose to the urethra to <150% of the prescription dose was followed. Quality assurance was performed using the postimplant computed tomography scan dosimetry 30 days after implantation procedure. A dose-volume histogram analysis was subsequently performed.
Follow-Up
Patients were evaluated with physical examination, DRE, and PSA measurement every 3–6 months for the first 2 years after implant, every 6 months for the next 3 years, and yearly thereafter. Late genitourinary (GU)/gastrointestinal (GI) toxicities were assessed using the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer scale.
Statistical Analysis
Descriptive statistics (including the mean, median, standard deviation, range, frequency, and percentage) were calculated to characterize the study population. The 2-sample t test or Wilcoxon rank sum test was used, as appropriate, to evaluate differences in continuous demographic/clinical variables between the isotope and HT groups. The chi-square test was used to evaluate differences in categorical demographic/clinical variables between the isotope and HT groups. BRFS was estimated using Kaplan-Meier survival analysis. The log-rank test was used to compare the BRFS between the 125I and 103Pd isotope groups and between the use and nonuse of HT with RT. Patients were classified as having biochemical recurrence by the presence of a date of biochemical failure as determined by the PSA level, the observation of local or distant failure, intervention with androgen ablation, or the date of death. Patients were censored at their last follow-up date if none of these criteria were present.
Multivariate Cox proportional hazards regression analysis was used to determine the independent effect of Gleason score, tumor stage, pretreatment PSA level, and radioactive source selection on BRFS. All P values are 2-sided, with statistical significance evaluated at the 0.05 α-level. The 95% confidence intervals (CIs) were calculated to assess the precision of the obtained estimates. All analyses were performed in SAS, version 9.2 (SAS Institute, Cary, NC) and Stata, version 11.0 (StataCorp, College Station, TX).
RESULTS
The patient and tumor characteristics are summarized in Table 1. The median age at diagnosis was 64.0 years (range 43.0–82.0). The vast majority of patients had Stage T1 disease (79%), and 21.1% of the patients presented with Stage T2b disease as assessed by the DRE findings. Gleason score >6 was present in 53.3% of patients. The median pretreatment PSA level was 7.1 ng/mL (range 0.6–46.5). Of the 242 patients, 119 (49.2%) were treated with 125I and 123 (50.8%) with 103Pd. The median follow-up period was 10 years (range 8.0–15.0).
Table 1.
Patient and tumor characteristics of 2 cohorts stratified by isotope use (n = 242)
| Variable | 125I (n = 119) | 103Pd (n = 123) | P Value |
|---|---|---|---|
| T stage | NS | ||
| T1 | 92 | 99 | |
| T2b | 27 | 24 | |
| T3 | 0 | 0 | |
| Gleason score | NS | ||
| 2–6 | 55 | 57 | |
| 7 | 56 | 56 | |
| 8–10 | 8 | 10 | |
| Pretreatment PSA (ng/mL) | NS | ||
| Median | 6.8 | 7.1 | |
| Range | 0.6–40.1 | 0.6–46.5 | |
| HT use | NS | ||
| Yes | 27 | 30 | |
| No | 92 | 93 | |
| Prostate postbrachytherapy | NS | ||
| V100 (%) | 95.5 | 95 | |
| D90 (%) | 110 | 109 | |
| Rectal postbrachytherapy (rectal dose 10%) | 68.1 | 50.6 | NS |
| Urethra postbrachytherapy (urethral dose 10%) | 131 | 125.4 | NS |
| Prostate size (cm3) | .01 | ||
| Median | 55 | 28 | |
| Range | 41–80 | 10–40 | |
| Prescribed dose (Gy) | .03 | ||
| Median | 108 | 85 | |
| Range | 100–120 | 80–90 | |
| Seeds (n) | .04 | ||
| Median | 53 | 69 | |
| Range | 34–81 | 45–112 | |
| Seed source strength (U) | 0.57 | 2 | .01 |
125I, iodine-125; 103Pd, palladium-103; NS, not significant; PSA, prostate-specific antigen; HT, hormonal therapy; V100, percentage of target volume receiving 100% of the prescribed dose; D90, minimal dose received by 90% of the target volume.
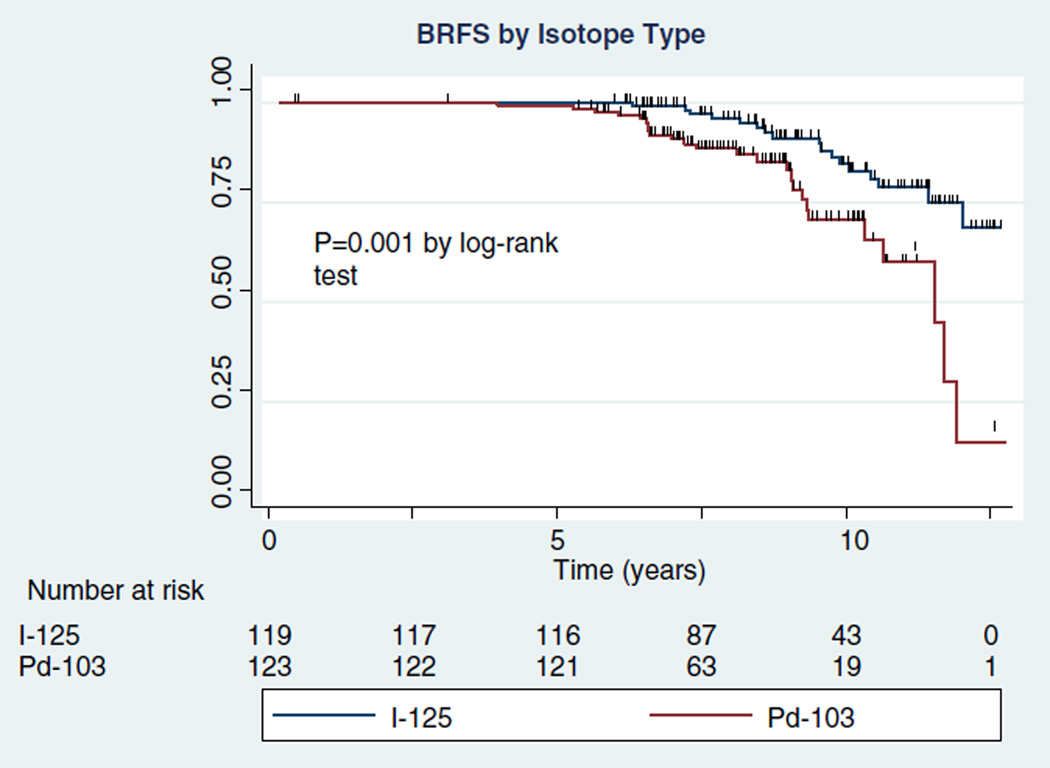
The overall BRFS rate was 77.3% (95% CI 68.9%–83.6%) at 10 years. A total of 42 recurrences developed. There were 7 local failures (2.9%), determined by positive biopsy findings, positive DRE findings, or the use of androgen ablation therapy for suspected local recurrence. The remaining 35 (83%) of the 42 recurrences were distal and were treated with HT and palliative RT. Of these 42 patients, 12 (29%) died, 9 of progressive disease and 3 of other causes (2 cerebral vascular accidents and 1 myocardial infarction). When stratified by the radioactive isotope type, of the 119 patients treated with 125I, 17 developed recurrence. Of the 123 subjects treated with 103Pd, 25 developed recurrence. This translated into a statistically significant difference in BRFS at 10 years between the 125I and 103Pd groups (82.7%, 95% CI 71.7%–89.8%; vs 70.6%, 95% CI 56.3%–81.0%; respectively; P = .001; Fig. 1).
Figure 1.
BRFS as assessed by use of different isotopes.
Of the 242 patients, most (n = 185 [76.4%]) did not receive HT, and 57 (23.6%) were treated with HT in addition to RT. A total of 25 recurrences developed in 185 patients who did not receive HT, and 17 in the 57 patients treated with HT. The use of HT in addition to RT did not improve the BRFS rate at 10 years (77.6%, 95% CI 61.8%–87.4%) compared with RT alone (77.1%, 95% CI 66.5%–84.7%; P = .22). However, when administration of HT was examined with respect to the isotope used, a statistically significant difference in BRFS was found with the addition of HT. Specifically, the 10-year BRFS rate for 103Pd was improved to 73.8% (95% CI 46.7%–88.6%) with HT compared with 103Pd without HT (69.1%, 95% CI 50.9%–81.7%; P = .008). Of the 97 patients treated with 103Pd and no HT, 16 patients developed a recurrence. In contrast, of the 26 patients treated with 103Pd plus HT, 9 developed a recurrence. A total of 88 patients were treated with 125I without HT, with 9 developing a recurrence. Of the 31 patients treated with 125I plus HT, 8 developed recurrence. The 10-year BRFS rate for patients treated with 125I and HT was 80.5% (95% CI 58.9%–91.5%). The 10-year BRFS rate for patients treated with 125I without HT was 83.8% (95% CI 69.5%–91.7%).
On multivariate analysis, the following factors increased the risk of biochemical recurrence: isotope type (103Pd vs 125I [referent]) by 2.6-fold (95% CI 1.3–5.1; P = .007), pretreatment PSA (>10 vs ≤10 ng/mL, referent) by 5.9-fold (95% CI 2.9–12.0; P < .0001), and higher tumor stage (T2a-T2b vs T1a-T1c, referent) by 1.7-fold (95% CI 0.8–3.3; P = .14; Table 2). No treatment-related deaths, deep venous thromboses, cardiopulmonary events, serious bleeding, sepsis, cerebral vascular events, or other serious perioperative complications occurred. No grade 4–5 late GU/GI toxicities were observed. The only late grade 3 toxicity was GU/GI toxicity in 10 (4.1%) of 242 patients.
Table 2.
Multivariate analysis of biochemical recurrence
| Variable | Multivariate HR (95% CI) | P Value |
|---|---|---|
| Isotope selection (103Pd vs 125I, referent) | 2.57 (1.29–5.11) | .007 |
| Pretreatment PSA (<10 vs ≤10 ng/mL, referent) | 5.89 (2.90–11.97) | >.0001 |
| T stage (T2a/T2b vs T1a/T1c, referent) | 1.68 (0.85–3.31) | .14 |
| Gleason score (<6 vs ≤6, referent) | 0.66 (0.34–1.26) | .21 |
| Gland size (<40 vs ≤ 40 cm3, referent) | 0.53 (0.43–1.02) | .32 |
HR, hazard ratio; CI, confidence interval; other abbreviations as in Table 1.
COMMENT
A few advantages are attributable to combining EBRT with brachytherapy, including a greater intraprostatic dose than that achieved with either modality alone, optimization of the dose to the periprostatic region that cannot be accomplished with brachytherapy alone, and dose compensation in the low-dose regions from suboptimal source placement. Although the benefits will be answered by a prospective RTOG-0232 trial, the present study has presented mature long-term outcomes at 10 years. Our data have demonstrated an overall BRFS rate of 77% at 10 years. The RTOG-0019 Phase II trial provided a 48-month estimate of biochemical recurrence of 19% and 14%, according to the American Society for Radiation Oncology consensus and Phoenix definitions, respectively.19 A number of retrospective experiences have reported long-term follow-up for a mixed cohort of patients (low-, intermediate-, and high-risk groups), ranging from 2 to 15 years and with patient numbers of 65–1469.7–14,20–23 On examining patients with IRPC only, the number of patients reported in these retrospective series was rather small (<200) with the BRFS rate at 2–15 years demonstrating a wide range of 59%–92% (Table 3).
Table 3.
Comparison of biochemical recurrence-free survival
| Series | IRPC (n) |
Follow-Up (mo) |
EBRT Dose (Gy) |
Implant Dose (Gy) |
Radioactive Source |
HT (%) | BRFS (%) | Definition of PSA Failure (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 y | 10 y | 15 y | ||||||||
| Sylvester et al,7,8,27 | 90 | 63 | 45 | 120 | 125I | 0 | — | 84 | — | Modified ASTRO consensus definition (2 consecutive increases in serum PSA) |
| — | — | — | 90 | 103Pd | — | — | — | — | ||
| 91 | 108 | 45 | 120 | 125I | — | — | — | 80% | ||
| — | — | — | 90 | 103Pd | — | — | — | — | ||
| 90 | 44 | 45 | 120 | 125I | 23 | 77 | — | — | ||
| — | — | 90 | 103Pd | — | — | — | — | |||
| Dattoli et al,9,22 | 71 | 24 | 41 | 80 | 103Pd | 13.7 | 70 | — | — | PSA <1.0 |
| 119 | 114 | 41 | 80 | 103Pd | 37 | — | — | 87%* | ASTRO consensus definition, nadir + 2 and PSA >0.2 ng/mL at last follow-up | |
| Critz et al,10,20 | 1029 | 45 | 45 | 80 | 125I | 0 | 79 | 72 | — | PSA >0.5 ng/mL |
| 1469 | 72 | 45 | 80 | 125I | 0 | — | 80 | — | PSA nadir >0.2 ng/mL or subsequent PSA increase >0.2 ng/mL | |
| Ragde et al,11 | 82 | 122 | 45 | 120 | 125I | 0 | — | 79 | — | ASTRO† |
| Grado et al,12 | 72 | 47 | 45 | 120 | 125I | 13.9 | 72‡ | — | — | Two successive increases in PSA |
| 100 | 103Pd | 88§ | — | — | ||||||
| Sigh et al,13 | 65 | 36 | 50.4 | — | 103Pd | 86 | 87† | — | — | ASTRO† |
| Merrick et al,14,21 | 66 | 53.7 | 45 | 110 | 125I | 53.7 | 80 | — | — | ASTRO consensus definition |
| — | 90 | 103Pd | — | — | — | |||||
| 58.6 | — | — | 98.4∥ | — | ||||||
| Stock et al,15 | 432 | 56 | 45 | 125 | 125I | 81 | — | 92∥ | — | Phoenix |
| 100 | 103Pd | — | — | — | — | |||||
| Jani et al,23 | 54 | 49 | 45 | 108 | 125I | 39 | — | 59 | — | Two successive PSA increases after nadir to <1.0 ng/mL |
| 90 | 103Pd | |||||||||
| RTOG 001921 | 130 | 48 | 45 | 108 | 125I | 27 | 81/86 | — | — | ASTRO consensus/Phoenix |
| Present series | 242 | 120 | 45 | 100 | 125I | 24 | — | 77 | — | Phoenix |
| 80 | 103Pd | |||||||||
IRPC, intermediate-risk prostate cancer; EBRT, external beam radiotherapy; ASTRO, American Society for Radiation Oncology; RTOG, Radiation Therapy Oncology Group; other abbreviations as in Table 1.
Actuarial 14-year data.
Three consecutive increases in serum PSA level measured 6 months apart.
Hormone-naive group.
Patients exposed to previous deprivation.
Eight-year biochemical recurrence free survival.
The issue of which radioactive isotope to use in patients treated with combination RT remains unsettled. In our experience, a statistically significant difference was seen in the 10-year BRFS rate with respect to radioisotope selection, with the combination of EBRT and an 125I implant rendering significantly more superior BRFS. Stone et al24 reported a 10-year BRFS rate of 78% in patients with cT1-T2 disease treated with brachytherapy (125I) with or without EBRT and with or without 6 months of androgen ablation. Datoli et al22 reported a 10-year BRFS rate in low-, intermediate-, and high-risk patients of 93%, 80%, and 61%, respectively, in a cohort of 1469 men treated with 125I plus EBRT. A statistically significant difference in isotope selection was found by Sylvester et al.8 In contrast, in other publications no difference by isotope selection was noted, rendering equivalent BRFS for either 125I or 103Pd.21 One hypothesis regarding the reason 125I might improve BRFS when used with EBRT is its long half-life (~60 days) compared with that of 103Pd (17 days). Thus, even resistant cells surviving brachytherapy with 103Pd will likely be affected by 125I for a longer period and cell resistance overcome.
In general, HT did not affect BRFS in all patients in our series. Data supporting HT in conjunction with brachytherapy have been limited by retrospective designs and small sample sizes. The American Brachytherapy Society recognizes the use of HT with brachytherapy and EBRT and recommends its use only in the context of downstaging large prostate glands before brachytherapy. 25 Stone et al26 reported the results with neoadjuvant HT and brachytherapy in 115 patients treated with leuprolide and flutamide for 3 months before implantation and an additional 3 months after implantation. They suggested a benefit in local control in high-risk patients (PSA >10 ng/mL, greater than Stage T2a, Gleason score >6) with a rate of positive prostate biopsy findings at 2 years of 3.4% versus 21.2% in the hormone-naive group (P = .003). Sylvester et al27 performed a matched-pair analysis of 98 patients with IRPC, with 21 treated with EBRT and brachytherapy plus HT. No statistically significant difference was observed between the overall rates of BRFS at 5 years, with 77% in the HT group and 58% in the no-HT group (P = .08). A recent publication addressing the effect of HT on IRPC treated with combination RT reported 350 patients who received HT and 82 who did not.15 The 8-year BRFS rate with and without HT was 92% and 92%, respectively (P = .4; Table 3). Our study supports previous reports in that no strong evidence exists for the addition of HT or neoadjuvant HT to prostate brachytherapy combined with EBRT; thus, HT should not be routine in this disease setting.
When hormones were assessed, we found that the addition of HT to 103Pd compared with 103Pd alone improved the BRFS rate from 69.1% to 73.8%. Merrick et al21 reported on 223 patients for whom the isotope selection (125I vs 103Pd) did result in a significant difference in the univariate analysis outcome (P = .007). Because none of their patients received HT, its role with regard to isotope selection and BFRS in the setting of EBRT plus brachytherapy is unknown. Dattoli et al22 used 103Pd as a boost after neoadjuvant EBRT for 282 patients. Of these, 103 received neoadjuvant/adjunctive HT and had a 14-year BRFS rate of 87% for IRPC, with HT not affecting the failure rate (P ≤ .3). Jani et al23 examined 54 patients treated with RT plus either 125I (44% of patients) or 103Pd (56% of patients); only 21 (39%) of the 54 patients received HT. On multivariate analysis, HT and the choice of isotope (125I vs 103Pd) were not significant as predictive factors. Some studies examined HT with respect to isotope selection and EBRT but failed to demonstrate advantages to using HT.26,27 The main criticism is the very limited number of patients and retrospective nature of the reports.
Our multivariate analysis demonstrated that the isotope type and a pretreatment PSA level >10 ng/mL significantly increased the odds of biochemical recurrence, but that increasing stage demonstrated a trend toward increasing the risk of failure. A number of studies have corroborated our findings.7–10,12–14,21,22 Sylvester et al8 demonstrated that both greater Gleason score and increasing stage were predictive of poor BRFS.8 Other institutional experiences revealed that a greater Gleason score and PSA level conferred a poorer outcome.22 Institutions that have reported their experience using a combination approach have treated patients with a wide “entry criteria,” making the task of drawing inferences from the studies more challenging. Thus, our study has provided clinically useful information, although we cannot underscore enough the need for level 1 evidence to shed definitive light on the posed questions.
Some have suggested that the morbidity associated with a combination of EBRT and brachytherapy is not dramatically different from that observed with either modality administered alone.28 Others have reported increased morbidity after combination therapy.28 Although our rate of long-term GU/GI toxicity was 4.1%, consistent with the RTOG 0019 trial 18-month estimate of late grade 3 GU/GI toxicity of 3.3% (95% CI 0.1–6.5), we report a much lower long-term GU/GI complication rate than the 48-month late grade 3 GU/GI toxicity at 15% (95% CI 8%–21%) from the RTOG trial.19,29 The results of our study and those from RTOG-0019 are encouraging owing to the complete absence of grade 4–5 late GU/GI toxicities.
Currently, 2 main isotopes are used for EBRT and permanent brachytherapy, 125I and 103Pd. No clinical trials have compared the benefits and problems of the 2 isotopes; however, comparisons can be made between the isotopes by observing the data for complications and success for each isotope. The isotopes have many differences, as well as advantages and disadvantages. Both are capsulated in titanium on a 4-mm by 0.8-mm diameter silver rod. The main difference is present in the half-lives of the isotopes; 103Pd has a half-life of 17 days and has lower photon energy, and 125I has a half-life of approximately 60 days and greater photon energy. With a shorter half-life, the original dose of 103Pd is greater, meaning that 103Pd is better for more quickly growing tumors. 125I is usually used for patients with low-grade tumors. Studies comparing 125I and 103Pd on rat cells support the use of iodine for slow-growing tumors and palladium for more quickly growing tumors. However, studies at Yale have compared the side effects of the 2 isotopes, suggesting that 103Pd is the better isotope. The Yale studies have shown that complications with 125I are greater than with 103Pd, with 18% of patients experiencing side effects with 125I and those treated with 103Pd experiencing no side effects. Once completed, a prospective RTOG 0232 trial should provide definitive answers.
CONCLUSIONS
To our knowledge, our series has presented the longest follow-up of patients with IRPC treated with combination EBRT and brachytherapy in published studies to date. Excellent BRFS and a low rate of late GU/GI toxicities are achieved with this treatment approach. 125I isotope as a source for an implant renders a superior rate of BRFS compared with 103Pd when used with EBRT. As per our investigation, HT did not provide any additional benefit in the setting of patients with IRPC treated with a combination approach, except for the addition of HT to 103Pd.
Acknowledgments
Financial Disclosure: P. Christos was partially supported by a grant from the Clinical Translational Science Center (grant UL1-RR024996).
Footnotes
This study was presented at the 50th Annual Meeting of the American Society for Radiation Oncology, Boston, Massachusetts, 2008.
References
- 1.Lee WR, Moughan J, Owen JB, et al. The 1999 patterns of care study of radiotherapy in localized prostate carcinoma: a comprehensive survey of prostate brachytherapy in the United States. Cancer. 2003;98:1987–1994. doi: 10.1002/cncr.11774. [DOI] [PubMed] [Google Scholar]
- 2.Nori D, Donath D, Hilaris BS, et al. Precision transperineal brachy-therapy in the treatment of early prostate cancer. Endocurietherapy/Hyperthermia Oncol Int J. 1990;6:119–130. [Google Scholar]
- 3.Grimm PD, Blasko JC, Ragde H. Ultrasound-guided transperineal implantation of iodine-125 and palladium-103 for the treatment of early-stage prostate cancer: technical concepts in planning, operative technique, and evaluation. In: Schellhammer PF, editor. New Techniques in Prostate Surgery. vol 2. Philadelphia: WB Saunders; 1994. pp. 113–126. [Google Scholar]
- 4.Nori D, Moni J. Current issues in techniques of prostate brachy-therapy. Semin Surg Oncol. 1997;13:444–453. doi: 10.1002/(sici)1098-2388(199711/12)13:6<444::aid-ssu9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Merrick GS, Wallner K, Butler WM, et al. A comparison of radiation dose to the bulb of the penis in men with and without prostate brachytherapy-induced erectile dysfunction. Int J Radiat Oncol Biol Phys. 2001;50:597–604. doi: 10.1016/s0360-3016(01)01475-4. [DOI] [PubMed] [Google Scholar]
- 6.Potters L, Cao Y, Calugaru E, et al. A comprehensive review of CT-based dosimetry parameters and biochemical control in patients treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2001;50:605–614. doi: 10.1016/s0360-3016(01)01473-0. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester JE, Blasko JC, Grimm PD, et al. Ten-year biochemical relapse-free survival after external beam radiation and brachytherapy for localized prostate cancer: the Seattle experience. Int J Radiat Oncol Biol Phys. 2003;57:944–952. doi: 10.1016/s0360-3016(03)00739-9. [DOI] [PubMed] [Google Scholar]
- 8.Sylvester JE, Grimm PD, Blasko JC, et al. 15-Year biochemical relapse free survival in clinical stage T1-T3 prostate cancer following combined external beam radiotherapy and brachytherapy: Seattle experience. Int J Radiat Oncol Biol Phys. 2007;67:57–64. doi: 10.1016/j.ijrobp.2006.07.1382. [DOI] [PubMed] [Google Scholar]
- 9.Dattoli M, Wallner K, Sorace R, et al. 103Pd brachytherapy and external beam irradiation for clinically localized, high-risk prostatic carcinoma. Int J Radiat Oncol Biol Phys. 1996;35:875–879. doi: 10.1016/0360-3016(96)00214-3. [DOI] [PubMed] [Google Scholar]
- 10.Critz FA, Levinson AK, Williams WH, et al. Simultaneous radiotherapy for prostate cancer: 125I prostate implant followed by external-beam radiation. Cancer J Sci Am. 1998;4:359–363. [PubMed] [Google Scholar]
- 11.Ragde H, Korb LJ, Elgamal AA, et al. Modern prostate brachytherapy: Prostate specific antigen results in 219 patients with up to 12 years of observed follow-up. Cancer. 2000;89:135–141. [PubMed] [Google Scholar]
- 12.Grado GL, Larson TR, Balch CS, et al. Actuarial disease-free survival after prostate cancer brachytherapy using interactive techniques with biplane ultrasound and fluoroscopic guidance. Int J Radiat Oncol Biol Phys. 1998;42:289–298. doi: 10.1016/s0360-3016(98)00209-0. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Zelefsky MJ, Raben A, et al. Combined 3-dimensional conformal radiotherapy and transperineal Pd-103 permanent implantation for patients with intermediate and unfavorable risk prostate cancer. Int J Radiat Oncol Biol Phys. 2000;90:275–280. [PubMed] [Google Scholar]
- 14.Merrick GS, Butler WM, Lief JH, et al. Biochemical outcome for hormone-naïve patients with high-risk prostate cancer on managed with permanent interstitial brachytherapy and supplemental external-beam radiation. Brachytherapy. 2002;8:322–327. doi: 10.1097/00130404-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Stock RG, Yalamanchi S, Hall SJ, et al. Impact of hormonal therapy on intermediate risk prostate cancer treated with combination brachytherapy and external beam irradiation. J Urol. 2010;183:546–550. doi: 10.1016/j.juro.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.American Joint Committee on Cancer. Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 17.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. [PubMed] [Google Scholar]
- 18.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Lee WR, Bae K, Lawnton C, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy analysis of Radiation Therapy Oncology Group Study 0019. Cancer. 2007;109:1506–1512. doi: 10.1002/cncr.22560. [DOI] [PubMed] [Google Scholar]
- 20.Critz FA, Levinson K. 10-Year disease-free survival rates after simultaneous irradiation for prostate cancer with a focus on calculation methodology. J Urol. 2004;172:2232–2238. doi: 10.1097/01.ju.0000144033.61661.31. [DOI] [PubMed] [Google Scholar]
- 21.Merrick GS, Butler WM, Wallner KE, et al. Impact of supplemental external beam radiotherapy and/or androgen deprivation therapy on biochemical outcome after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;61:32–43. doi: 10.1016/j.ijrobp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Dattoli M, Wallner K, True L, et al. Long-term outcomes after treatment with brachytherapy and supplemental conformal radiation for prostate cancer patients having intermediate and high-risk features. Cancer. 2007;110:551–555. doi: 10.1002/cncr.22810. [DOI] [PubMed] [Google Scholar]
- 23.Jani AB, Feinstein JM, Pasciak R, et al. Role of external beam radiotherapy with low-dose-rate brachytherapy in treatment of prostate cancer. Urology. 2006;67:1007–1011. doi: 10.1016/j.urology.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Stone NN, Stock RG, Unger P. Intermediate term biochemical-free progression and local control following 125-iodine brachytherapy for prostate cancer. J Urol. 2005;173:803–807. doi: 10.1097/01.ju.0000152558.63996.29. [DOI] [PubMed] [Google Scholar]
- 25.Nag S, Bice W, DeWyngaert K, et al. The American Brachytherapy Society recommendations. Semin Urol Oncol. 2000;46:221–230. doi: 10.1016/s0360-3016(99)00351-x. [DOI] [PubMed] [Google Scholar]
- 26.Stone NN, Stock RG, Under P. Effects of neoadjuvant hormonal therapy on prostate biopsy results after (125)I and (103)Pd seed implantation. Mol Urol. 2000;4:163–168. [PubMed] [Google Scholar]
- 27.Sylvester JM, Blasko JC, Grimm PD, et al. Short-course androgen ablation combined with external beam radiotherapy and low-dose-rate permanent brachytherapy in early stage prostate cancer: a matched subset analysis. Mol Urol. 2000;4:155–159. [PubMed] [Google Scholar]
- 28.Sarosdy MF. Urinary and rectal complications of contemporary permanent transperineal brachytherapy for prostate carcinoma with or without external beam radiation therapy. Cancer. 2004;101:754–760. doi: 10.1002/cncr.20446. [DOI] [PubMed] [Google Scholar]
- 29.Lee WR, DeSilvio M, Lawnton C, et al. A phase II study of external beam radiotherapy combined with permanent source brachytherapy for intermediate-risk, clinically localized adenocarcinoma of the prostate: preliminary results of RTOG P-0019. Int J Radiat Oncol Biol Phys. 2006;64:804–809. doi: 10.1016/j.ijrobp.2005.09.002. [DOI] [PubMed] [Google Scholar]