SUMMARY
Targeted intervention of the B-Raf V600E gene product that is prominent in melanoma has been met with modest success. Here, we characterize the pharmacological properties of PLX4032, a next-generation inhibitor with exquisite specificity against the V600E oncogene and striking anti-melanoma activity. PLX4032 induces potent cell cycle arrest, inhibits proliferation, and initiates apoptosis exclusively in V600E-positive cells in a variety of in vitro experimental systems; follow-up xenograft studies demonstrate extreme selectivity and efficacy against melanoma tumors bearing the V600E oncoproduct. The collective data support further exploration of PLX4032 as a candidate drug for patients with metastatic melanoma; accordingly, validation of PLX4032 as a therapeutic tool for melanoma patients is now underway in advanced human (Phase III) clinical trials.
INTRODUCTION
Patients afflicted with metastatic melanoma display 5-year survival rates of ~ 10–15%. A major factor in this dismal prognosis is the relative absence of effective drugs to combat this deadly disease. Dacarbazine represents the sole FDA-approved drug for metastatic melanoma, but elicits durable responses in less than 5% of patients. Thus, most melanoma patients are enrolled in clinical trials, where the promise of efficacy supersedes the poor response rates observed with dacarbazine.
Since the identification of a highly prevalent somatic mutation (V600E) of the BRaf gene in 2002 (Davies et al., 2002), several inhibitors of the Raf/MEK/ERK signal transduction cascade have been developed (Shepherd et al., 2010). Sorafenib represented a first-generation Raf inhibitor designed to inhibit aberrant MAPK signaling, but failed to display measurable responses in melanoma clinical trials (Eisen et al., 2006; Flaherty et al., 2008). Likewise, inhibitors of MEK also exhibited subpar results in the clinic (Dummer R, 2008). These early failures led to the development of next-generation, oncogene-specific small molecules capable of inhibiting signals initiating from mutant, but not wild-type, B-Raf (Tsai et al., 2008).
PLX4032, a highly-specific and potent inhibitor of the V600E mutant form of B-Raf, was developed from a scaffold-based screening platform (Flaherty KT et al, in press). This small molecule is currently under evaluation in clinical trials for patients with metastatic melanoma. Results from these studies indicate that PLX4032 is effective in ~ 80% of patients harboring the V600E mutation without yielding substantial toxicities. Phase III trials are currently under accrual.
Here, we report the preclinical characterization of PLX4032. PLX4032 displays potent anti-melanoma effects in a variety of in vitro and in vivo melanoma models and represents a highly-exciting drug candidate for patients with few therapeutic alternatives.
MATERIALS & METHODS
Cell Lines and Reagents
Human melanoma cell lines were isolated and cultured as previously described (Roesch et al., 2010). After establishment of continuous growth, cells were maintained in 2% melanoma media, a 4:1 mixture of MCDB153 and L15, supplemented with 2 mM Ca+2, heat-inactivated fetal bovine serum (2%), and insulin (5 μg/ml). All cells were incubated at 37°C and 5% CO2 at constant humidity. All molecular reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Cell Cycle Analysis
Melanoma cells were seeded onto 100-mm dishes at 60% confluence and allowed to adhere overnight. Increasing concentrations of PLX4032 were administered for 24 h before supernatant and cells were collected, pelleted, and fixed with 70% ethanol. Before staining with propidium iodide (10 μg/ml), cells were incubated for 1 h at 37°C in 0.5 mg/ml RNase I to rid samples of residual RNA contamination. Samples were then analyzed by using the EPICS XL (Beckman-Coulter) apparatus.
Cell Proliferation Assays
Cells were plated into a 96-well plate at a density of between 1.8 ×104 and 2.5 × 104 per ml and allowed to adhere overnight. The following day, cells were treated with increasing concentrations of PLX4032 in quadruplicate. Cells were left to grow for 72 h before being treated with 10% (vol/vol) 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent for 3 h. Medium containing MTT was quickly removed, and the crystallized precipitate was solubilized using DMSO. Absorbance was subsequently read in a plate reader at 540 nm. Relative growth was calculated as the absorbance of cells in the absence of PLX4032 (UAF) divided by the absorbance of cells in a given concentration of PLX4032 (TAF) after the initial absorbance (IA) was subtracted from both.
Data show the mean of at least three independent experiments ± SE.
Apoptosis Staining
Melanoma cells were plated into 100-mm dishes at 60% confluence and subsequently treated with 1 μM PLX4032 for 24-hour intervals. Media and cells were harvested and pelleted before staining with annexin-FITC and propidium iodide. Samples were subsequently analyzed by using the EPICS XL apparatus.
Western Blot Analysis
Whole cell crude extracts were collected using RIPA lysis buffer and quantitated using a bicinchoninic acid (BCA) kit (Pierce, Rockford, IL). 30 μg protein was loaded into 10% SDS-PAGE gel and electrophoretically separated before transfer to a PVDF membrane (Millipore, Bedford, MA). Membranes were blocked in TBST plus 5% milk for 1 hour. Blocked membranes were incubated in primary antibody (phospho-p44/42 MAPK, Cell Signaling, Danvers, MA) overnight, washed in TBST, and probed with secondary HRP-conjugated antibody diluted in TBST plus 5% milk (Amersham Biosciences, Piscataway, NJ). Membranes were stripped and re-probed for β-actin to ensure equal protein loading.
Xenograft Studies
Metastatic melanoma cells, C8161 or 1205Lu (1 × 106), were s.c. injected into the flanks of SCID mice and allowed ≈2 weeks to reach 0.125 cm3 in volume. Subsequently, the animals received either 100 mg/kg PLX4032 (oral gavage) or vehicle control twice daily for 15 days. Tumor volume was recorded every 72 h. The average tumor size for each respective group was normalized to the tumor volume at the first day of treatment. After 15 days of treatment, animals were sacrificed and tumors were excised, fixed in formalin, paraffin-embedded, and analyzed by immunohistochemistry.
Live/Dead Spheroid Staining
Melanoma spheroids were prepared by using the liquid overlay method (Smalley et al., 2006). Briefly, 2,000 melanoma cells in 200 μl (10,000 cells per ml) were added to a 96-well plate coated with 1.5% agar (Difco, Becton-Dickinson, Franklin Lakes, NJ). Plates were incubated for 72 h, by which time cells had organized into three-dimensional spheroids. Spheroids were then harvested by using a P1000 pipetteman. The medium was removed, and the spheroids were implanted into a gel of bovine collagen I containing EMEM, L-glutamine, and 2% FBS. Normal 2% melanoma medium was overlaid on top of the solidified collagen. Spheroids were treated with increasing concentrations of PLX4032 and allowed to grow for 72 h. Spheroids were then washed twice in PBS before being treated with calcein-AM and ethidium bromide for 1 h at 37°C according to the manufacturer’s directions. Photographs of the invading spheroids were then taken by using a Nikon-300 inverted fluorescence microscope.
Artificial Skin Reconstructs
Human skin reconstructs were generated as described previously (Meier et al., 2000). Three milliliters of fibroblast-containing bovine type I collagen (7.5 × 104 cells per ml) plus Matrigel (4:1 ratio) was added to each insert of tissue culture trays and allowed to constrict in DMEM plus 10% FBS for 4 days at 37°C. For epidermal reconstruction, keratinocytes were mixed with melanoma cells at a ratio of 10:1 in keratinocyte serum-free medium containing 2% dialyzed FCS, 60 μg/ml bovine pituitary extract, 4.5 ng/ml bFGF, 100 nM human endothelin-3, and 10 ng/ml human SCF. A total of 5 × 106 cells were seeded on each contracted collagen gel. Cultures were kept submerged in medium containing 1 ng/ml EGF for 2 days, 0.2 ng/ml EGF for another 2 days, and were raised to the air-liquid interface via feeding from below with high calcium (2.4 mM) medium in EGF-free medium. After 15 days, skin reconstructs were treated with either 1 μM PLX4032 or vehicle control for 72 h and subsequently embedded in paraffin for subsequent sectioning and staining.
Immunohistochemical Staining
Seven-micrometer paraffin sections were processed by deparaffinization and rehydration followed by endogenous peroxidase blocking (1% H2O2 in methanol for 20 min) and antigen retrieval (boiled in 10 mM citrate buffer for 10 min). Tissue sections were blocked with 2% goat or horse serum (Vector Laboratories) and incubated with rabbit polyclonal antibody against phospho-MAPK/ERK, Ki67, or rabbit monoclonal antibody control for 30 min at 37°C followed by overnight exposure at 4°C. Samples were subsequently incubated with biotinylated secondary antibodies (Vector Laboratories). Immunoreactivity was detected by using the ABC Elite kit (Vector Laboratories). AEC and/or biotin was used as a final chromogen and hematoxylin as the nuclear counterstain. Negative controls for all antibodies were made by replacing the primary antibody with nonimmunogenic IgG.
RESULTS
Specificity of MAPK Inhibition
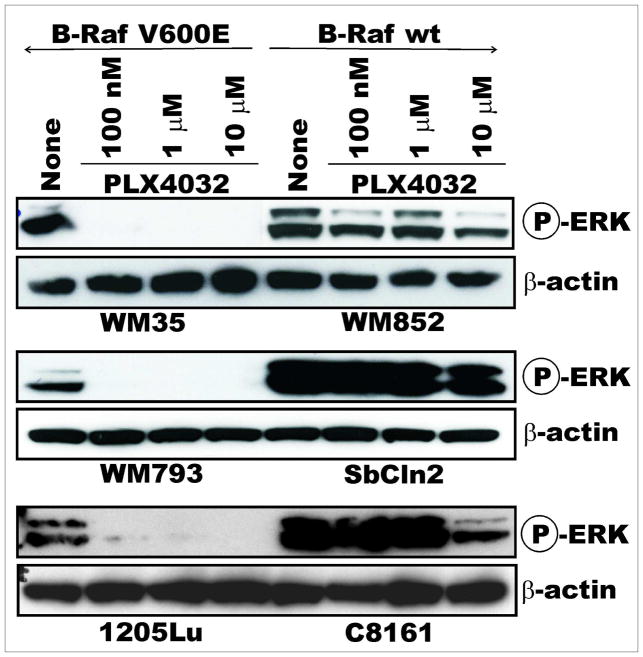
We previously published a scaffold- and structure-based discovery platform that identified PLX4720, a first-generation inhibitor from which PLX4032 was subsequently derived (Tsai et al., 2008). First, the specificity of B-Raf V600E inhibition was tested via immunoblotting analysis. A panel of melanoma cell lines expressing either the V600E oncogene product, mutant N-Ras, or wild-type B-Raf/N-Ras (Table 1) were assayed for activated ERK after treatment with varying concentrations of PLX4032. The compound displayed dose- and V600E-dependent inhibition of pERK for periods up to 72 hours (Figure 1). As a comparison to a clinically-relevant inhibitor of Raf, a panel of cells was also treated with Sorafenib; Sorafenib did not display the specificity for the mutant B-Raf that was observed with PLX4032 (data not shown).
Table 1. Mutational Status of Various Melanoma Cell Lines.
The BRAF and NRAS mutation status of all relevant melanoma cell lines is depicted.
| Cell Line | B-Raf | N-Ras |
|---|---|---|
| WM35 | V600E | wild-type |
| WM793 | V600E | wild-type |
| 1205Lu | V600E | wild-type |
| WM3434 | V600E | N.D. |
| SbCln2 | wild-type | Q61K |
| WM852 | wild-type | Q61R |
| WM1361A | wild-type | Q61R |
| C8161 | wild-type | wild-type |
| WM3451 | wild-type | N.D. |
| WM NCI1 | wild-type | N.D. |
N.D. indicates not determined.
Figure 1. PLX4032 Inhibits Raf Signaling in a V600E-dependent Manner.
A panel of melanoma cell lines with either mutant (left panel) or wild-type (right panel) B-Raf was treated with increasing doses of PLX4032 for 2 hours before lysates were collected for immunoblotting. pERK levels were measured to determine activity of the Raf/MEK/ERK signaling cascade both pre- and post-treatment. β-actin serves as a loading control.
Selectivity Profile and Cellular Effects of PLX4032
As a functional correlate to ERK inhibition, multiple cell-based assays were performed to ascertain the effects of PLX4032 on proliferation, survival, cell cycle progression, and apoptosis. MTS assays were carried out to investigate the effects of PLX4032 on the survival of various melanoma cell lines; the compound displayed strong inhibition of growth exclusively in cells harboring the V600E oncogene (Figure 2A, left panel). Colony formation assays exhibited similar selectivity when exposed to PLX4032 for extended periods of time, indicating that prolonged exposure to drug does not decrease the selectivity profile (Figure 2B). Flow cytometric-based analysis of cell cycle status after treatment with PLX4032 demonstrated potent G0/G1 arrest in cells harboring mutant, but not wild-type B-Raf (Figure 2C). Similar V600E-specific responses were observed in an apoptosis assay measuring Annexin IV and propidium iodide incorporation (Figure 2D). Collectively, these data suggest that PLX4032 exhibits potent anti-melanoma effects in a V600E-specific manner.
Figure 2. In vitro activity of PLX4032 in Melanoma.
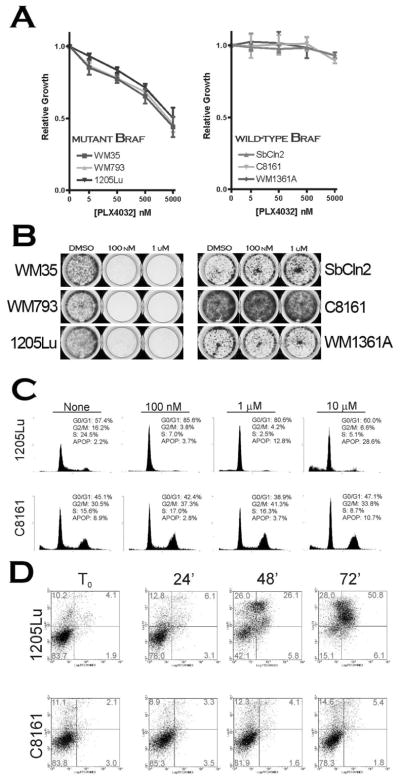
A) Proliferation of V600E+ (left panel) or wild-type B-Raf (right panel) cells in increasing concentrations of PLX4032. B) Colony formation assay; cells were seeded in 6-well dishes and exposed to 100 nM and 1 μM PLX4032 for 14 days. Surviving cells were stained in methylene blue and photographed. C) Representative cell cycle analysis of mutant (1205Lu) and wild-type (C8161) melanoma cells in response to PLX4032 treatment. D) Annexin V/PI staining of mutant and wild-type B-Raf cells at 24-hour intervals after treatment with 1 μM PLX4032.
PLX4032 in 3D Melanoma Tumor Models
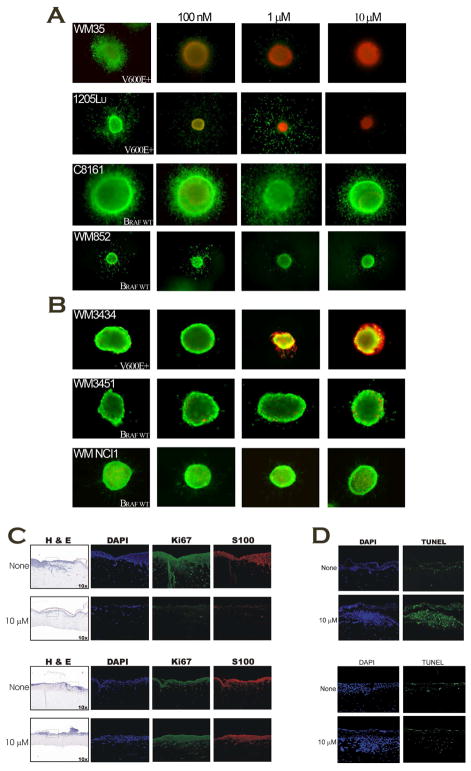
Because an overwhelming majority of preclinical drug candidates fail during clinical development, it would appear that preclinical characterization of drug potential merits improvement. For some time, we have argued that three-dimensional (3D) based assays are more predictive of clinical efficacy because these models incorporate many of the tumor microenvironmental features (i.e., stromal cells and extracellular matrices) otherwise not present in traditional 2D approaches. First, melanoma spheroids were embedded into a collagen matrix and overlayed with increasing concentrations of PLX4032. The results again demonstrate specificity against mutant B-Raf, while leaving wild-type B-Raf unperturbed (Figure 3A). Next, primary spheroids were generated from freshly-harvested human tumor specimens to minimize genotypic artifacts associated with prolonged in vitro culture. These experiments demonstrated similar effects against the V600E oncogene product (Figure 3B).
Figure 3. PLX4032 Exhibits Anti-melanoma Activity in 3D-based Cellular Models.
A) Collagen-embedded melanoma spheroids from established lines were treated with indicated doses of PLX4032 for 72 hours and stained for viability with calcein-AM (green) and ethidium bromide (red). B) Primary spheroids from freshly-isolated human melanomas were embedded in collagen and exposed to PLX4032 for 72 hours, followed by staining as described in 3A. C&D) Artificial skin reconstructs were generated with either mutant or wild-type B-Raf cells and treated with 1 μM PLX4032 for 72 hours before harvesting and immunostaining for the indicated protein markers; Ki67 indicates proliferation, TUNEL represents apoptosis.
The in vitro and ex vivo data from the spheroid experiments suggested that PLX4032 may be effective in complex, heterogenous tumors. Before testing the compound in vivo, we employed the use of human artificial melanoma skin reconstructs. This complex model incorporates extracellular matrices, as well as the primary cellular entities found within a melanotic tumor including fibroblasts, keratinocytes, and melanoma cells. Melanoma skin reconstructs were generated for ~ 21 days and subsequently overlayed with PLX4032 for 72 hours before harvesting. The reconstructs were paraffin-embedded, sectioned, and stained for markers of proliferation (Ki67; Figure 3C) and apoptosis (TUNEL; Figure 3D). Results from this human model showed that PLX4032 is capable of decreasing proliferation, as well as inducing apoptosis in mutant B-Raf melanomas; non-transformed cells, however, appear to be unaffected by the compound indicating that therapeutic toxicities in patients may be minimal.
Xenograft Studies with PLX4032
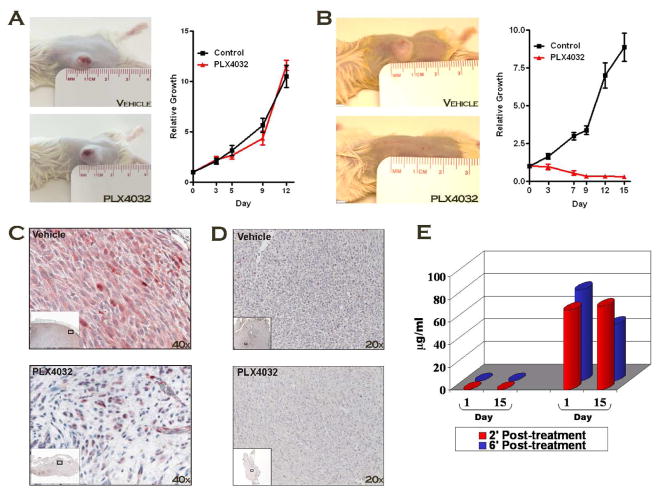
SCID mice were subsequently used in xenograft studies to ascertain the effects of PLX4032 in vivo. Highly-tumorigenic B-Raf mutant (1205Lu) or B-Raf wild-type (C8161) metastatic melanoma cells were subcutaneously injected into the flanks of animals and tumors were allowed to reach palpability before drug intervention. PLX4032-treated animals displayed no visible signs of toxicity at regimens as high as 100 mg/kg/BID. In control-treated animals, tumor volume increased rapidly (Figure 4A/B). B-Raf wild-type xenografts treated with PLX4032 grew at similar rates to control tumors (Figure 4A). V600E-positive tumors, however, were largely abolished by PLX4032 treatment indicating that its activities are largely cytotoxic, not cytostatic (Figure 4B).
Figure 4. PLX4032 Initiates Potent Tumor Regression in V600E-positive Xenografts.
A,B) Xenograft tumors established from either B-Raf wild-type (A) or mutant (B) cell lines were administered 100 mg/ml PLX4032 BID and tumor volume was recorded every 72 hours for 15 days. C) 1205Lu tumors from control and treated mice were harvested, paraffin-embedded, and stained with pERK to measure target efficacy. Photos are depicted at 40x magnification. D) 1205Lu tumors control and treated mice were harvested, paraffin-embedded, and stained with Ki67 to indicate relative proliferation within respective tumors. E) Pharmocokinetic analysis of plasma drug levels from xenografts on the first and last day of treatment at 2- and 6-hours post treatment.
Tumors were harvested from mice, paraffin-embedded, and immunohistochemistry performed to determine the effects of PLX4032 on key indicators of cell proliferation. As expected, pERK levels were significantly diminished, exclusively in mutant B-Raf tumors (Figure 4C); likewise, Ki67 was equally reduced, suggesting that the proliferative index of these tumors was severely compromised (Figure 4D). Lastly, plasma drug levels were obtained at various time points on the first and last day of treatment to assess the pharmacokinetic profile of PLX4032 in this xenograft model. After 15 days of treatment, plasma drug levels were ~ 100 μM, a number congruent with the levels currently achieved in the ongoing clinical trials (Flaherty KT, 2010). Taken together, the data strongly support advancement of PLX4032 into clinical trials for further development.
DISCUSSION
The B-Raf oncogene is mutated in approximately half of melanoma patients (Nathanson, 2010) and is consequently a high-priority target for drug discovery and development. Previous generation Raf inhibitors (i.e., Sorafenib) demonstrated preclinical promise (Karasarides et al., 2004; Sharma et al., 2006), but suboptimal response rates in clinical trials (Eisen et al., 2006; Flaherty et al., 2008). Here, we present preclinical data demonstrating that PLX4032 is a strong candidate for further clinical development. Early data from Phase I/II clinical trials supports our findings and could potentially lead to the first FDA-approved drug for advanced melanoma patients in over 30 years.
A number of Raf and MEK inhibitors have demonstrated promising preclinical results in vitro and in xenograft models, but subsequently failed in human trials. Functional redundancy between isoforms of these kinases has been implicated in the lack of clinical response (Montagut et al., 2008). More recent evidence suggests that broad inhibition of MEK impairs T lymphocyte function, when compared against inhibition of the V600E oncoproduct (Boni et al., 2010); this phenomenon may explain why broadly-acting inhibitors of Raf (sorafenib) and MEK (AZD6244) exhibit little activity in the clinic, where immunomodulation of disease is more prominent than in immune-deficient xenograft models. The recent success of ipilimumab in Phase III clinical trials underscores the involvement of the immune system in achieving optimal therapeutic response in melanoma (Hodi et al., 2010). Future trials will likely implement both a small molecule arm and an immune effector component to determine if synergistic activity is possible.
The degree of selectivity for mutant B-Raf displayed by PLX4032 is remarkable and most currently envisage a clinical scenario whereby patients are preselected for therapy based on their B-Raf status. However, predicting response from within patients expressing mutant B-Raf may be more complicated than originally anticipated. Our small panel of V600E-positive melanoma lines exhibited an approximate IC50 value of ~ 5 μM, several-fold above what has been reported elsewhere (Joseph et al., 2010; Yang et al., 2010). Subsequent studies in our laboratory have demonstrated that the response to PLX4032 across additional mutant B-Raf lines is not uniform; in fact, IC50 values range from low nanomolar to nearly 10 micromolar (data not shown). Thus, the anti-melanoma activity of PLX4032 is not solely predicated on the ability to inhibit the mutant B-Raf gene product—other molecular entities appear to be involved. Relevant studies in other cancer types suggest that activation of the PI3K pathway is one mechanism by which tumor cells may bypass apoptotic signals initiated through anti-tumor agents (Halilovic et al., 2010; Park et al., 2010; Smalley and Sondak, 2010; Wee et al., 2009). In our study, each of the V600E+ lines used in our studies harbor either mutant or deleted PTEN, the negative regulator of the PI3K pathway, which likely explains the elevated IC50 values. Prior studies support this hypothesis, demonstrating that dual inhibition of the MAPK and PI3K pathways is an effective therapy for advanced melanoma (Krasilnikov et al., 2003; Meier et al., 2007).
Despite our promising preclinical data and encouraging results from early human trials, there are concerns now associated with Raf inhibitors. Multiple groups have published mechanistic details surrounding the pro-tumorigenic potential of Raf inhibitors in cancers that do not harbor oncogenic mutations in B-Raf (Halaban et al., 2010; Hatzivassiliou et al., 2010; Heidorn et al., 2010; Poulikakos et al., 2010). These studies demonstrate that the use of Raf inhibitors in tumors lacking Raf mutations can actually increase flux through the MAPK pathway through formation of Raf heterodimers. However, our in vitro data do not demonstrate increased pERK levels after PLX4032 treatment in all cells expressing wild-type B-Raf ; we attribute this discrepancy to the substantially decreased amount of serum in our melanoma culture conditions (2%) compared to those previous studies (5–10%). This hypothesis is supported by the fact that we indeed observe increased pERK activity in high growth factor-containing melanocyte culture conditions (Supplementary Figure 1). Because approximately 10–15% of patients treated with B-Raf-specific drugs develop squamous cell carcinomas (Flaherty K, 2009), prospective genotyping of melanoma patients for mutations in B-Raf will be necessary to stratify response rates, as well as to minimize potentially-dangerous side effects.
The ‘next wave’ of research in targeted therapy in melanoma will likely center on the emergence of Raf inhibitor-resistant clones. Indeed, data from clinical trials has indicated that, despite high response rates, nearly all patients eventually relapse. The mechanism(s) underlying this therapeutic escape will lead to next-generation treatment regimens that may render Raf-resistant melanomas susceptible to alternative therapies. We recently reported two plausible resistance mechanisms including upregulation of the receptor tyrosine kinase, IGF1R (Villanueva J, in press), and emergence of a minor subpopulation of melanoma cells expressing a histone demethylase, JARID1B (Roesch et al., 2010). In both cases, it appears that melanoma cells are extremely plastic and can readily adapt to extreme selective pressures.
SIGNIFICANCE.
Genotype-derived, patient-specific therapies are imminent. In malignant melanoma, the most frequent somatic mutation is the valine to glutamic acid base substitution at position 600 in BRAF (V600E); this mutation confers constitutive flux through the mitogen-activated protein kinase (MAPK) pathway and is believed to be an initiating event in malignant transformation. However, extensive efforts to pharmacologically inhibit the MAPK pathway in advanced melanoma for therapeutic benefit have largely failed. Next-generation inhibitors, tailored to inhibit only the mutant form of B-Raf, are now being investigated as alternatives to broadly-acting inhibitors of the MAPK pathway. Here, we demonstrate the striking specificity of PLX4032 for melanoma cells expressing the V600E oncoproduct using a variety of experimental approaches; we also reveal that PLX4032 is highly-efficacious in physiologically-relevant, human-derived 3D-based platforms, which might better predict clinical success than more conventional methods. Collectively, our findings suggest that minimally-toxic, but measurable responses can be achieved through tailored therapies that inhibit the mutant form of B-Raf, an oncogene product expressed in a majority of melanoma patients.
References
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–9. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dummer RRC, Chapman PB, Sosman JA, Middleton M, Bastholt L, Kemsley K, Cantarini MV, Morris C, Kirkwood JM. AZD6244 (ARRY-a428896) versus temozolomide (TMZ) in patients with advanced melanoma: An open-label, randomized, multicenter, phase II study. J Clin Oncol. 2008;26:Abstract 9033. [Google Scholar]
- Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KPI, Sosman J, Kim K, Ribas A, McArthur G, Lee RJ, Grippo JF, Nolop K, Chapman P. Phase I study of PLX4032: Proof of concept for V600EBRAF mutation as a therapeutic target in human cancer. J Clin Oncol. 2009;27:15s. [Google Scholar]
- Flaherty KTPI, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Schiller J, Schuchter LM, Liu G, Tuveson DA, Redlinger M, et al. A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clin Cancer Res. 2008;14:4836–42. doi: 10.1158/1078-0432.CCR-07-4123. [DOI] [PubMed] [Google Scholar]
- Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, et al. PIK3CA Mutation Uncouples Tumor Growth and Cyclin D1 Regulation from MEK/ERK and Mutant KRAS Signaling. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–8. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–8. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E, et al. Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J Dermatol. 2007;156:1204–13. doi: 10.1111/j.1365-2133.2007.07821.x. [DOI] [PubMed] [Google Scholar]
- Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, et al. Human melanoma progression in skin reconstructs : biological significance of bFGF. Am J Pathol. 2000;156:193–200. doi: 10.1016/S0002-9440(10)64719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–61. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson KL. Using genetics and genomics strategies to personalize therapy for cancer: focus on melanoma. Biochem Pharmacol. 2010;80:755–61. doi: 10.1016/j.bcp.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Rabinovsky R, Carey M, Hennessy BT, Agarwal R, Liu W, et al. Integrative analysis of proteomic signatures, mutations, and drug responsiveness in the NCI 60 cancer cell line set. Mol Cancer Ther. 2010;9:257–67. doi: 10.1158/1535-7163.MCT-09-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–94. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd C, Puzanov I, Sosman JA. B-RAF inhibitors: an evolving role in the therapy of malignant melanoma. Curr Oncol Rep. 2010;12:146–52. doi: 10.1007/s11912-010-0095-2. [DOI] [PubMed] [Google Scholar]
- Smalley K, Sondak V. Melanoma — An Unlikely Poster Child for Personalized Cancer Therapy. N Engl J Med. 2010;363:876–78. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva JVA, Cipolla AK, Lee JT, Somasundaram R, Wubenhorst B, Xu X, Gimotty PA, Sosman J, Kong J, Chen Y, Santiago-Walker AE, Hayden JE, Laquerre S, McArthur G, Nathanson KL, Herlyn M. Acquired resistance to BRAF inhibitors mediated by an A/B/C RAF kinase switch in melanoma can be overcome by co-targeting MEK and IGF-1R/PI3K. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–93. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–27. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]