Abstract
We evaluated the contributions of Mycobacterium tuberculosis efflux pumps towards intrinsic resistance to different classes of peptidoglycan synthesis inhibitors (PSI). Our study indicates that the efflux pump knockout strains are more susceptible to PSI than the wild type. Vancomycin and ceftriaxone exhibited up to 3 log increased kill on efflux pump mutants compared to the wild-type strain, strongly suggesting an important role for efflux pumps in the intrinsic resistance of M. tuberculosis to PSI.
TEXT
Intrinsic resistance of Mycobacterium tuberculosis to peptidoglycan synthesis inhibitors (PSI) is generally attributed to the poor permeability of the mycobacterial cell wall and to the presence of the β-lactamase encoded by blaC (1, 2, 3). It is of concern that although β-lactam antibiotics are the most successful and widely used antibacterial agents; they have little or no use in the treatment of mycobacterial infections, including tuberculosis (4).
The intrinsic resistance of mycobacteria to PSI could also be attributed to the presence of multiple drug efflux pumps (5). We have constructed knockout (KO) mutants lacking the different classes of efflux pumps of M. tuberculosis as follows. Rv1258c (KO1) and Rv0849 (KO6) are major facilitator superfamily (MFS) efflux pumps, Rv1218c (KO5) belongs to the ATP-binding cassette (ABC) superfamily, and Rv3065 (K07) belongs to the small multidrug resistance family (6, 7). We investigated the contributions of these efflux pumps of M. tuberculosis towards intrinsic resistance to various PSI by comparing the in vitro activities of selected drugs on wild-type (WT) M. tuberculosis and the efflux pump KO mutants. MICs were determined by the resazurin-based method as previously described (8). Bactericidal activities were evaluated by the CFU-based method as previously described (6).
The β-lactams exhibited increased potency against all four of the efflux pump KOs tested compared to the WT (Table 1). The MIC of penicillin was 64 μg/ml for WT M. tuberculosis. KO5 and KO7 were the most sensitive to penicillin, as measured by the MICs, which dropped 4-fold (16 μg/ml), while KO1 and KO6 exhibited a 2-fold drop (32 μg/ml) in the MIC. WT M. tuberculosis had an ampicillin MIC of 16 μg/ml; KO1, KO5, KO6, and KO7 were equally susceptible to this β-lactam, displaying 2- to 4-fold drops in the MICs (8 μg/ml). The MICs of meropenem for KO1 and KO5 dropped 2- to 4-fold, KO7 showed a 0- to 2-fold drop, and the MIC for KO6 remained the same as that for the WT, which was 2 to 4 μg/ml. Ceftriaxone had an MIC of 2 to 4 μg/ml for WT M. tuberculosis. The drop in the MICs for all four of the KOs was uniformly 4-fold (0.5 μg/ml). Cefotaxime had an MIC of 2 to 4 μg/ml for WT M. tuberculosis. The MICs for KO1, KO5, and KO7 dropped 2- to 4-fold, and that for KO6 dropped 0- to 2-fold. Vancomycin had an MIC of 2 μg/ml for WT M. tuberculosis. The MICs for KO1 and KO7 dropped 4-fold, while a MIC decrease of only 2-fold for the KO5 and KO6 strains was observed. Interestingly, the decrease in the bacitracin MIC was noticeable only for KO5 and not for any of the other KOs studied.
Table 1.
MICs of PSI for WT and efflux pump KO mutant strains of M. tuberculosis
| Antibiotic | MIC (μg/ml)a |
||||
|---|---|---|---|---|---|
| WT | KO1 | KO5 | KO6 | KO7 | |
| Penicillin G | 64 | 32 | 16 | 32 | 16 |
| Ampicillin | 16 | 4–8 | 8 | 8 | 4–8 |
| Meropenem | 2–4 | 1 | 1 | 2–4 | 2 |
| Ceftriaxone | 2–4 | 0.5–1 | 0.5–1 | 0.5–1 | 0.5–1 |
| Cefotaxime | 2–4 | 0.5–1 | 0.5–1 | 1–2 | 0.5 |
| Vancomycin | 2 | 0.5 | 1 | 1 | 0.5 |
| Bacitracin | >128 | >128 | 32 | >128 | 128 |
| Isoniazid | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
MICs were determined by the resazurin-based microplate assay. Each value is the average of at least three independent assays.
Either the KO strains complemented with the respective genes on plasmids as previously described (7) or the hyperexpression strains were used to verify if the MICs were restored to the WT values. The MIC of compounds that showed 2- to 4-fold drops for the KO strains reverted closer to WT values when a functional copy of the respective efflux pump gene was present (data not shown).
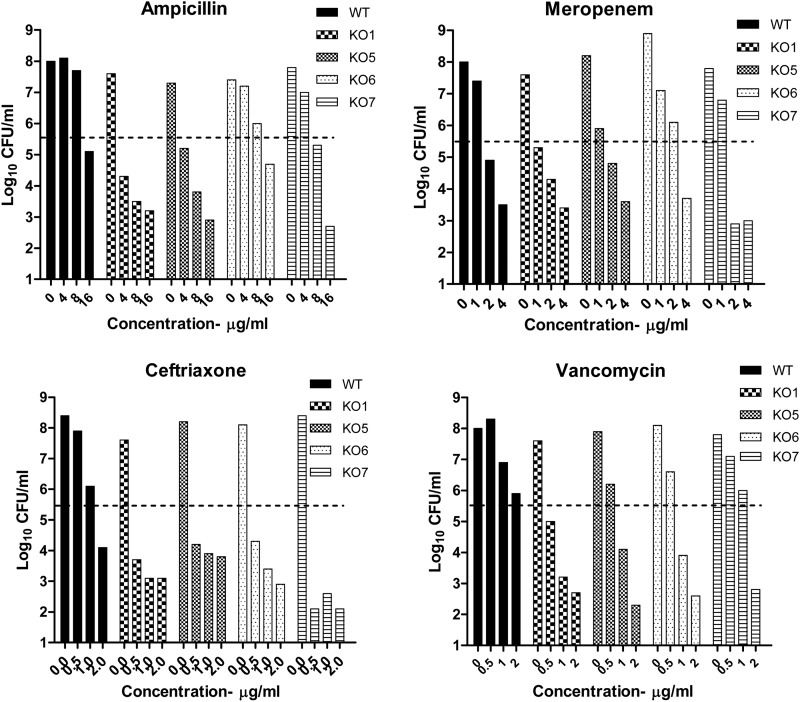
Measurement of bactericidal activities (log kill) revealed a slightly different picture (Fig. 1). The bactericidal activities of ampicillin for KO1, KO5, and KO7 showed good increase (1.8, 2.2, and 2.4 log10 CFU/ml, respectively) compared to that for the WT, and KO6 showed marginal kill of 0.5 log10 CFU/ml. Meropenem did not have an enhanced bactericidal effect on any of the efflux KOs tested. Ceftriaxone had increased kill values against the four KOs, ranging from 1 to 3 log10 CFU/ml. Vancomycin also had remarkable increases in its kill values, compared to that for the WT, for all four of the KOs that ranged from 2.8 to 3.5 log10 CFU/ml. The bactericidal activities of isoniazid remained the same across the WT and the KOs (6, 7) and are therefore not shown here.
Fig 1.
Bactericidal effects of β-lactams on WT and efflux pump KO mutant M. tuberculosis strains. Shown are the bactericidal activity profiles of PSI for WT M. tuberculosis and the efflux pump KO mutants. The three concentrations used cover the range of MICs for the different KO strains in this study (WT, KO1, KO5, KO6, and KO7). The y axis represents the number of bacteria surviving under each concentration shown on the x axis after exposure to antibiotics for 7 days. The dashed line indicates the cell number for all of the strains at the start of the experiment, which was 5.5 ± 0.2 log10 CFU/ml.
These results indicate the involvement of efflux pumps in the intrinsic resistance of M. tuberculosis to the β-lactam class of antibiotics and a few other drugs (vancomycin and bacitracin) that act on the peptidoglycan synthesis of M. tuberculosis. The differences in the n-fold drops in the MICs could imply that, depending upon the structure of the β-lactam studied, the recognition and extent of efflux by the different pumps could vary. An earlier study by Danilchanka et al. (9) had shown the involvement of an ABC transporter of M. tuberculosis (Rv0194) in resistance to ampicillin, which further corroborates the role of efflux in the resistance of this bacterium to β-lactam antibiotics. Using efflux pump KO mutants to study drug efflux in bacteria is an artificial situation because in an infected individual, all of the efflux pumps work in concert, depending upon which class of compounds they need to pump out. It will not be possible to mimic such a situation in in vitro assays by using either efflux inhibitors or KO mutants. However, the in vitro studies conducted here give us valuable information on the potential roles of specific efflux pumps in modulating the activities of different PSI.
It is interesting that although the MIC of meropenem is modulated by the efflux activities of various pumps, the bactericidal effect of the drug is not altered. This could be due to the fact that carbapenems are poor substrates for BlaC and therefore are more stable as a class (10, 11).
Multiple-drug resistance in M. tuberculosis has been shown to be associated with the intrinsic or inducible expression of efflux systems (5, 6, 7, 12). Our findings show that different β-lactams are effluxed to various extents by the three classes of pumps studied. Vancomycin and bacitracin, which also target the cell wall of bacteria, are effluxed by these pumps, as indicated by the increased potency of these drugs on the efflux pump KO strains than against the WT. Our findings suggest that efflux is one of the mechanisms that make β-lactams and other PSI ineffective against M. tuberculosis. Additional work in this direction will add significantly to our understanding of the mechanism of the intrinsic resistance of M. tuberculosis to this class of antibiotics.
ACKNOWLEDGMENTS
M.B. conceptualized the work and designed the experiments. N.D. and S.S. carried out all of the experiments. M.B. and N.D. wrote the manuscript.
Footnotes
Published ahead of print 18 January 2013
REFERENCES
- 1. Tremblay LW, Fan F, Blanchard JS. 2010. Biochemical and structural characterization of Mycobacterium tuberculosis β-lactamase with the carbapenems ertapenem and doripenem. Biochemistry 49:3766–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurz SG, Bonomo RA. 2012. Reappraising the use of β-lactams to treat tuberculosis. Expert Rev. Anti Infect. Ther. 10:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang F, Cassidy C, Sacchettini JC. 2006. Crystal structure and activity studies of the Mycobacterium tuberculosis beta-lactamase reveal its critical role in resistance to beta-lactam antibiotics. Antimicrob. Agents Chemother. 50:2762–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chambers HF, Turner J, Schecter GF, Kawamura M, Hopewell PC. 2005. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob. Agents Chemother. 49:2816–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Rossi E, Aínsa JA, Riccardi G. 2006. Role of mycobacterial efflux transporters in drug resistance: an unresolved question. FEMS Microbiol. Rev. 30:36–52 [DOI] [PubMed] [Google Scholar]
- 6. Balganesh M, Kuruppath S, Marcel N, Sharma S, Nair A, Sharma U. 2010. Rv1218c, an ABC transporter of Mycobacterium tuberculosis with implications in drug discovery. Antimicrob. Agents Chemother. 54:5167–5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balganesh M, Dinesh N, Sharma S, Kuruppath S, Nair AV, Sharma U. 2012. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob. Agents Chemother. 56:2643–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palomino J-C, Martin A, Camacho M, Guerra H, Swings J, Portaels F. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2720–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danilchanka O, Mailaender C, Niederweis M. 2008. Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 52:2503–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poole K. 2004. Resistance to β-lactam antibiotics. Cell. Mol. Life Sci. 61:2200–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta AK, Katoch VM, Chauhan DS, Sharma R, Singh M, Venkatesan K, Sharma VD. 2010. Microarray analysis of efflux pump genes in multi-drug resistant Mycobacterium tuberculosis during stress induced by common anti-tuberculosis drugs. Microb. Drug Resist. 16:21–28 [DOI] [PubMed] [Google Scholar]