Abstract
We investigated the in vitro activity of DS-8587, a novel fluoroquinolone, against Acinetobacter baumannii. The MICs of DS-8587 against clinical isolates and its inhibitory activity against target enzymes were superior to those of ciprofloxacin and levofloxacin. Furthermore, the antibacterial activity of DS-8587 was less affected by adeA/adeB/adeC or abeM efflux pumps than was that of ciprofloxacin and the frequency of single-step mutations with DS-8587 was lower than that with ciprofloxacin. DS-8587 might be an effective agent against A. baumannii infection.
TEXT
Acinetobacter baumannii, a Gram-negative coccobacillus, is an increasingly important nosocomial pathogen (1, 2). A. baumannii infection causes serious clinical problems worldwide, due to an increasing number of A. baumannii clinical isolates becoming resistant to the most commonly clinically utilized antibiotics (3–6). Colistin and tigecycline are the last-resort agents to treat multidrug-resistant (MDR) A. baumannii, but unfortunately, strains resistant to these agents have already appeared (7, 8). Therefore, development of a new treatment option for MDR A. baumannii is an urgent need.
DS-8587 is a novel fluoroquinolone which has potent activity against pathogens that cause community and nosocomial infections (9). In this study, we assessed the in vitro potency of DS-8587 against A. baumannii.
(Part of this work was presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA [10].)
DS-8587 (Fig. 1) and levofloxacin were synthesized at Daiichi Sankyo, Co., Ltd. Ciprofloxacin, tigecycline, and imipenem were purchased from LKT Laboratories, Inc. (St. Paul, MN). Norfloxacin, amikacin, gentamicin, and chloramphenicol were purchased from Sigma-Aldrich (St. Louis, MO). A. baumannii clinical isolates were collected by the LVFX Surveillance Group in 2007 in Japan (11). Isolates were confirmed to be A. baumannii by the presence of blaoxa-51-like (12) and sequencing of the 16S-23S rRNA intergenic spacer region (13). Susceptibility testing was performed by broth microdilution according to the recommended CLSI method (14).
Fig 1.
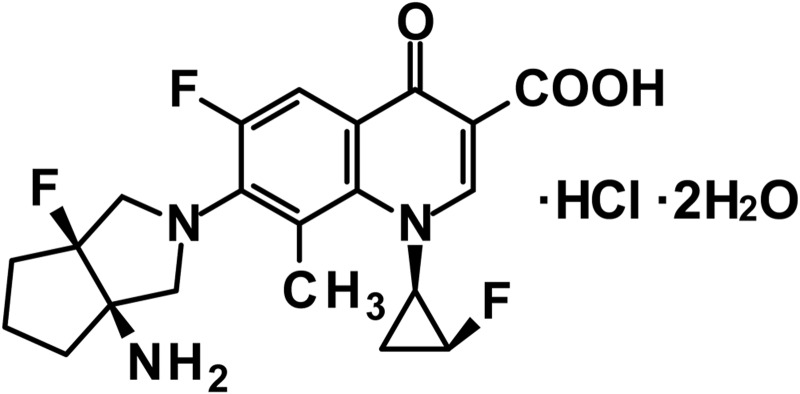
Chemical structure of DS-8587.
The antibacterial activities of DS-8587 and reference compounds against clinical isolates of A. baumannii, including quinolone-susceptible isolates and those with mutations in the quinolone resistance-determining regions (QRDRs) of gyrA and parC, are shown in Table 1. DS-8587 possessed excellent antibacterial activity against wild-type gyrA/parC strains, with MICs of ≤0.015 to 0.06 μg/ml. These MICs were 4- to 8-fold and 8- to 16-fold lower than those of levofloxacin and ciprofloxacin, respectively. For clinical isolates with gyrA/parC mutations, the MICs of DS-8587 were 0.5 to 1 μg/ml, which were 4- to 16-fold and 64- to 128-fold lower than those of levofloxacin and ciprofloxacin, respectively. The MIC ranges of tigecycline, imipenem, amikacin, and gentamicin for these clinical isolates were 0.12 to 2, 0.12 to 64, 2 to 16, and 1 to >128 μg/ml, respectively. Compared to the reference compounds, DS-8587 exhibited the most potent antibacterial activity against A. baumannii clinical isolates.
Table 1.
Antibacterial activity against clinical isolates of gyrA/parC wild-type, gyrA mutant, and gyrA/parC mutant strains of A. baumannii
| Strain | MIC (μg/ml) |
QRDR mutation |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| DS-8587 | Levofloxacin | Ciprofloxacin | Tigecycline | Imipenem | Amikacin | Gentamicin | gyrA | parC | |
| 19041 | ≤0.015 | 0.12 | 0.25 | 0.25 | 0.25 | 8 | 1 | ||
| 19247 | ≤0.015 | 0.12 | 0.25 | 0.5 | 0.25 | 4 | 1 | ||
| 19276 | ≤0.015 | 0.12 | 0.25 | 0.12 | 0.25 | 2 | 1 | ||
| 19192 | 0.03 | 0.12 | 0.25 | 0.12 | 0.25 | 4 | 1 | ||
| 19289 | 0.03 | 0.12 | 0.25 | 0.25 | 0.25 | 4 | 1 | ||
| 19066 | 0.06 | 0.25 | 0.5 | 1 | 0.25 | 16 | 4 | ||
| 19347 | 0.25 | 2 | 4 | 0.12 | 0.25 | 8 | 1 | Ser81Leu | |
| 19309 | 0.25 | 4 | 16 | 0.5 | 0.25 | 4 | 4 | Ser81Leu | |
| 19426 | 0.5 | 8 | 64 | 1 | 0.25 | 4 | 64 | Ser81Leu | Ser84Leu |
| 19477 | 1 | 8 | 64 | 2 | 2 | 8 | >128 | Ser81Leu | Ser84Leu |
| 19570 | 1 | 8 | 64 | 2 | 0.12 | 4 | 64 | Ser81Leu | Ser84Leu |
| 19424 | 1 | 8 | 64 | 2 | 2 | 8 | 64 | Ser81Leu | Ser84Leu |
| 19483 | 1 | 4 | 64 | 2 | 64 | 4 | 4 | Ser81Leu | Glu88Lys |
| 19485 | 1 | 8 | 64 | 2 | 64 | 4 | 4 | Ser81Leu | Glu88Lys |
To examine the influence of mutations in gyrA on the antibacterial activity of DS-8587, we selected an isogenic mutant of A. baumannii ATCC 19606 (CIP-4) under ciprofloxacin selection pressure. CIP-4 acquired a single mutation in the gyrA QRDR (Ser81Leu) and no mutation in parC. The MIC of DS-8587 against CIP-4 was 1 μg/ml, which was 8-fold higher than that for the parent strain (ATCC 19606). In contrast, the corresponding MICs of levofloxacin and ciprofloxacin were 8 and 32 μg/ml, which were 16- and 32-fold higher than those against the parent strain, respectively.
To evaluate the inhibitory activity of DS-8587 against wild-type A. baumannii DNA gyrase and topoisomerase IV and altered DNA gyrase and topoisomerase IV, we purified GyrA (wild type and Ser81Leu mutant; position equivalent to Ser83 in Escherichia coli) and GyrB for a supercoiling assay and ParC (wild type and Ser84Leu mutant; position equivalent to Ser80 in E. coli) and ParE for a decatenation assay, respectively (15, 16). The 50% inhibitory concentrations (IC50s) of DS-8587 for wild-type and altered (Ser81Leu in gyrA) DNA gyrase were 3.7- and 7.8-fold lower than those of levofloxacin and 4.9- and 8.4-fold lower than those of ciprofloxacin, respectively (Table 2). The IC50s of DS-8587 for wild-type and altered (Ser84Leu in parC) topoisomerase IV were 3.2- and 7.5-fold lower than those of levofloxacin and 2.1- and 5.6-fold lower than those of ciprofloxacin, respectively (Table 2).
Table 2.
Inhibitory activity against the wild-type and altered A. baumannii target enzymes
| Compound | IC50 (μg/ml) against enzyme: |
|||
|---|---|---|---|---|
| DNA gyrase |
Topoisomerase IV |
|||
| Wild type | Ser81Leu (GyrA) | Wild-type | Ser84Leu (ParC) | |
| DS-8587 | 1.06 | 10.02 | 1.70 | 5.81 |
| Levofloxacin | 3.95 | 78.45 | 5.51 | 43.59 |
| Ciprofloxacin | 5.16 | 84.14 | 3.51 | 32.34 |
Efflux-mediated resistance has been reported in A. baumannii (17). Among those efflux pumps, expression levels of adeA/adeB/adeC and abeM and corresponding antimicrobial susceptibilities in clinical isolates have been examined (18–21). To assess the influence of adeA/adeB/adeC and abeM on the antibacterial activity of DS-8587, we selected two isogenic mutants which overexpressed one of these efflux pumps. The adeA/adeB/adeC-overexpressing strain (19483 TGC-2) was obtained under the selection pressure of tigecycline (22). The abeM-overexpressing strain (NFLX-16-2) was obtained under the selection pressure of norfloxacin (21). In addition, we generated an abeM deletion mutant (2-1-12) from NFLX-16-2 by allelic exchange (23). As shown by quantitative real-time PCR analysis (primer pairs are shown in Table 3) (22), the expression levels of adeB in 19483 TGC-2 and abeM in NFLX-16-2 were 10.2- and 3.4-fold higher than those in the parental strains, respectively. MICs of levofloxacin and ciprofloxacin for the adeA/adeB/adeC-overexpressing strain were both 16-fold higher (Table 4) than those for the parent strain. In contrast, DS-8587 and tigecycline MICs were 8-fold higher than those for the parental strains. MICs of norfloxacin and ciprofloxacin for the abeM-overexpressing mutant compared to its abeM deletion mutant strain (2-1-12) showed 8- and 4-fold-higher MICs, respectively (Table 5). Meanwhile, MICs of both DS-8587 and levofloxacin showed only a 2-fold increase with the mutants.
Table 3.
Primers used in this study
| Target gene | Primer | Sequence (5′–3′) |
|---|---|---|
| adeB | qadeB-F | GCCTGCTTTATTGGCTGCTC |
| qadeB-R | GGCAACCCTTCATTCCAAAC | |
| abeM | qAbeM-F | TCAAGCAGGGTTCGGGTTA |
| qAbeM-R | TCGGCAACTAATGGTGTGGT | |
| rpoB | qrpoB-F | TGCGCGTTCAACTGGTTCT |
| qrpoB-R | TGCCCACACTTCCATCTCAC |
Table 4.
Antibacterial activity against laboratory-selected strain of adeA/adeB/adeC-overexpressing A. baumannii
| Strain | MIC (μg/ml) |
|||
|---|---|---|---|---|
| DS-8587 | Levofloxacin | Ciprofloxacin | Tigecycline | |
| 19483a | 1 | 4 | 64 | 2 |
| 19483 TGC-2b | 8 | 64 | 1,024 | 16 |
gyrA (Ser81Leu) and parC (Glu88Lys) mutant strain (same strain shown in Table 2).
Tigecycline-nonsusceptible strain derived from 19483, overexpressing adeABC.
Table 5.
Antibacterial activity against laboratory-selected abeM-overexpressing strain and deletion mutant of A. baumannii
| Strain | MIC (μg/ml) |
|||
|---|---|---|---|---|
| DS-8587 | Levofloxacin | Ciprofloxacin | Norfloxacin | |
| ATCC 19606 | 0.12 | 0.5 | 1 | 8 |
| NFLX-16-2a | 0.25 | 1 | 4 | 64 |
| 2-1-12b | 0.12 | 0.5 | 1 | 8 |
Norfloxacin-resistant strain derived from ATCC 19606, overexpressing abeM. No amino acid change in the QRDRs of gyrA and parC.
Derivative of NFLX-16-2 with deletion of abeM.
Frequencies of single-step resistance selection (16) with DS-8587 and ciprofloxacin at 4× MIC for two strains (19289 and 19347, the same strains shown in Table 1) were 4.2 × 10−8 and 7.3 × 10−8 with DS-8587 and 2.4 × 10−6 and 9.6 × 10−6 with ciprofloxacin, respectively. The mutant prevention concentration (MPC)/MIC ratios of DS-8587 and ciprofloxacin were 16 to 32 and both 32 for these two strains, respectively.
Our results demonstrate the potent in vitro antibacterial activity of DS-8587 against A. baumannii with excellent inhibitory activity against target enzymes and reduced efflux by adeA/adeB/adeC and abeM compared with other quinolones tested. These results may explain the lower frequency of single-step mutations with DS-8587 than with ciprofloxacin. These data, taken together with its reported antibacterial activity against MDR A. baumannii (MIC, 0.5 to 1 μg/ml) and in vitro bactericidal activity (24–26), support the clinical development of DS-8587 to treat infections caused by A. baumannii.
ACKNOWLEDGMENTS
We thank Eiko Namba and Miho Tanabe for their excellent technical assistance. We are grateful to Ryo Okumura, Dilip Jatashankar Upadhyay, and Ian Morrissey for helpful discussions and reviewing the manuscript before submission.
S.H., Y.O., M.C., and K.H. are employees of Daiichi Sankyo, Co., Ltd.
Footnotes
Published ahead of print 4 February 2013
REFERENCES
- 1. Bergogne-Berezin E, Towner KJ. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 3. Gootz TD, Marra A. 2008. Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev. Anti Infect. Ther. 6:309–325 [DOI] [PubMed] [Google Scholar]
- 4. Gordon NC, Wareham DW. 2010. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int. J. Antimicrob. Agents 35:219–226 [DOI] [PubMed] [Google Scholar]
- 5. Zavascki AP, Carvalhaes CG, Picao RC, Gales AC. 2010. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev. Anti Infect. Ther. 8:71–93 [DOI] [PubMed] [Google Scholar]
- 6. Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 67:1607–1615 [DOI] [PubMed] [Google Scholar]
- 8. Chen YH, Lu PL, Huang CH, Liao CH, Lu CT, Chuang YC, Tsao SM, Chen YS, Liu YC, Chen WY, Jang TN, Lin HC, Chen CM, Shi ZY, Pan SC, Yang JL, Kung HC, Liu CE, Cheng YJ, Liu JW, Sun W, Wang LS, Ko WC, Yu KW, Chiang PC, Lee MH, Lee CM, Hsu GJ, Hsueh PR. 2012. Trends in the susceptibility of clinically important resistant bacteria to tigecycline: results from the Tigecycline In Vitro Surveillance in Taiwan study, 2006 to 2010. Antimicrob. Agents Chemother. 56:1452–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiba M, Fujikawa K, Okumura R, Kurosaka Y, Hoshino K. 2012. Abstr. 52nd Intersci. Caonf. Antimicrob. Agents Chemother., abstr F-2037 [Google Scholar]
- 10. Higuchi S, Onodera Y, Chiba M, Hoshino K, Gotoh N. 2012. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., abstr F-2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamaguchi K, Ohno A, Ishii Y, Tateda K, Iwata M, Kanda M, Akizawa K, Shimizu C, Kon S, Nakamura K, Matsuda K, Tominaga M, Nakagawa T, Sugita A, Ito T, Kato J, Suwabe A, Yamahata K, Kawamura C, Tashiro H, Horiuchi H, Katayama Y, Kondou S, Misawa S, Murata M, Kobayashi Y, Okamoto H, Yamazaki K, Okada M, Haruki K, Kanno H, Aihara M, Maesaki S, Hashikita G, Miyajima E, Sumitomo M, Saito T, Yamane N, Kawashima C, Akiyama T, Ieiri T, Yamamoto Y, Okamoto Y, Okabe H, Moro K, Shigeta M, Yoshida H, Yamashita M, Hida Y, Takubo T, Kusakabe T, Masaki H, Heijyou H, Nakaya H, Kawahara K, Sano R, Matsuo S, Kono H, Yuzuki Y, Ikeda N, Idomuki M, Soma M, Yamamoto G, Kinoshita S, Kawano S, Oka M, Kusano N, Kang D, Ono J, Yasujima M, Miki M, Hayashi M, Okubo S, Toyoshima S, Kaku M, Sekine I, Shiotani J, Tazawa Y, Yoneyama A, Kumasaka K, Koike K, Taniguchi N, Ozaki Y, Uchida T, Murakami M, Inuzuka K, Gonda H, Yamaguchi I, Fujimoto Y, Iriyama J, Asano Y, Genma H, Maekawa M, Yoshimura H, Nakatani K, Baba H, Ichiyama S, Fujita S, Kuwabara M, Okazaki T, Fujiwara H, Ota H, Nagai A, Fujita J, Negayama K, Sugiura Y, Kamioka M, Murase M, Yamane N, Nakasone I, Okayama A, Aoki Y, Kusaba K, Nakashima Y, Miyanohara H, Hiramatsu K, Saikawa T, Yanagihara K, Matsuda J, Kohno S, Mashiba K. 2009. In vitro susceptibilities to levofloxacin and various antibacterial agents of 12,919 clinical isolates obtained from 72 centers in 2007. Jpn. J. Antibiot. 62:346–370 [PubMed] [Google Scholar]
- 12. Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 44:2974–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang HC, Wei YF, Dijkshoorn L, Vaneechoutte M, Tang CT, Chang TC. 2005. Species-level identification of isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex by sequence analysis of the 16S-23S rRNA gene spacer region. J. Clin. Microbiol. 43:1632–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinical and Laboratory Standards Institute 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15. Sekiguchi J, Disratthakit A, Maeda S, Doi N. 2011. Characteristic resistance mechanism of Mycobacterium tuberculosis to DC-159a, a new respiratory quinolone. Antimicrob. Agents Chemother. 55:3958–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okumura R, Hirata T, Onodera Y, Hoshino K, Otani T, Yamamoto T. 2008. Dual-targeting properties of the 3-aminopyrrolidyl quinolones, DC-159a and sitafloxacin, against DNA gyrase and topoisomerase IV: contribution to reducing in vitro emergence of quinolone-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 62:98–104 [DOI] [PubMed] [Google Scholar]
- 17. Coyne S, Courvalin P, Perichon B. 2011. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 55:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bratu S, Landman D, Martin DA, Georgescu C, Quale J. 2008. Correlation of antimicrobial resistance with beta-lactamases, the OmpA-like porin, and efflux pumps in clinical isolates of Acinetobacter baumannii endemic to New York City. Antimicrob. Agents Chemother. 52:2999–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Pi B, Zhou H, Yu Y, Li L. 2009. Triclosan resistance in clinical isolates of Acinetobacter baumannii. J. Med. Microbiol. 58:1086–1091 [DOI] [PubMed] [Google Scholar]
- 20. Magnet S, Courvalin P, Lambert T. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su XZ, Chen J, Mizushima T, Kuroda T, Tsuchiya T. 2005. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob. Agents Chemother. 49:4362–4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hornsey M, Ellington MJ, Doumith M, Thomas CP, Gordon NC, Wareham DW, Quinn J, Lolans K, Livermore DM, Woodford N. 2010. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1589–1593 [DOI] [PubMed] [Google Scholar]
- 23. Roca I, Marti S, Espinal P, Martinez P, Gibert I, Vila J. 2009. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:4013–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurosaka Y, Uoyama S, Ishii C, Hoshino K. 2012. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., abstr F-2035 [Google Scholar]
- 25. Okumura R, Fujikawa K, Onodera Y, Chiba M, Hoshino K. 2012. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., abstr F-2038 [Google Scholar]
- 26. Pandya S, Barman TK, Rao EPM, Kumar M, Singhal S, Upadhyay DJ, Kurosaka Y, Uoyama S, Ishii C, Hoshino K. 2012. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., abstr F-2043 [Google Scholar]


