Abstract
In this study, we aimed to evaluate the relationship between the rates of resistance of Pseudomonas aeruginosa to carbapenems and the levels and diversity of antibiotic consumption. Data were retrospectively collected from 20 acute care hospitals across 3 regions of Switzerland between 2006 and 2010. The main outcome of the present study was the rate of resistance to carbapenems among P. aeruginosa. Putative predictors included the total antibiotic consumption and carbapenem consumption in defined daily doses per 100 bed days, the proportion of very broad-spectrum antibiotics used, and the Peterson index. The present study confirmed a correlation between carbapenem use and carbapenem resistance rates at the hospital and regional levels. The impact of diversifying the range of antibiotics used against P. aeruginosa resistance was suggested by (i) a positive correlation in multivariate analysis between the above-mentioned resistance and the proportion of consumed antibiotics having a very broad spectrum of activity (coefficient = 1.77; 95% confidence interval, 0.58 to 2.96; P < 0.01) and (ii) a negative correlation between the resistance and diversity of antibiotic use as measured by the Peterson homogeneity index (coefficient = −0.52; P < 0.05). We conclude that promoting heterogeneity plus parsimony in the use of antibiotics appears to be a valuable strategy for minimizing the spread of carbapenem resistance in P. aeruginosa in hospitals.
INTRODUCTION
Pseudomonas aeruginosa is one of the many drug-resistant bacteria that cause most health care-associated infections (1). Among carbapenem antibiotics, imipenem-cilastatin and meropenem exhibit good efficiencies against P. aeruginosa (2). Unfortunately, concern has been raised since carbapenem-resistant strains, either endemic or epidemic, have been increasingly described globally (3, 4).
Antimicrobial use, especially of carbapenems, promotes P. aeruginosa resistance at either the individual or collective level (5–8). Both the frequency and antibiotic susceptibility profile of the Gram-negative bacilli, which cause health care-associated infections, constrain hospitals to increase their use of very broad-spectrum antibiotics. Hence, it is interesting to analyze the antibiotic resistance of P. aeruginosa in relation not only to carbapenem use but also to the diversity of antibiotics used in hospitals. The present study was aimed at determining if there is a relationship between the rates of resistance of P. aeruginosa to carbapenems and the level and diversity of antibiotic consumption in 20 acute care hospitals. Our goal was to examine whether a greater diversity of antibiotics can have a positive impact on resistance rates in P. aeruginosa.
MATERIALS AND METHODS
Design and setting.
This study was an observational, multicenter, and ecological study. It was based on the yearly data from a sentinel network of acute care hospitals (excluding outpatient, chronic psychiatry, and rehabilitation wards) located throughout Switzerland and participating in a national program for monitoring antibiotic resistance and antibiotic use (see www.anresis.ch). Twenty hospitals provided their data on antibiotic consumption and antibiotic susceptibility patterns in inpatient isolates over the period of 2006 to 2010. Furthermore, we were able to analyze multidrug-resistant (MDR) strains (see definition below) from 10 hospitals. We stratified the hospitals into three main regions: central, southern-western, and eastern Switzerland. The definition of regions was adapted from the one used in the above-mentioned monitoring program by merging the southern and the western regions (southern-western), as preliminary observations demonstrated similar patterns of resistance in these two regions.
Data on antimicrobial resistance.
The rates of nonsusceptibility to carbapenems (imipenem-cilastatin and meropenem) among P. aeruginosa isolates were obtained by dividing the number of isolates that either were resistant or had intermediate susceptibility by the total number of clinical isolates. MDR strains were defined as isolates that were resistant to two antibiotics (gentamicin, ciprofloxacin, piperacillin-tazobactam, and/or ceftazidime) in addition to carbapenems. To simplify, nonsusceptibility was referred to as resistance in the present study. The susceptibility breakpoints were determined according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (www.clsi.org). Duplicates (defined as the same microorganism with the same susceptibility profile in the same patient during the preceding year) were counted only once. We converted the resistance rates (R) in the logarithm of the odds (log odds) (ln{[R/(1 − R)] + 1}) to normalize the distribution of the above-mentioned dependent variable (9).
Data on antimicrobial consumption.
Data on the antibacterials were collected for systemic use (groups J01, J04AB, and P01AB of the Anatomical Therapeutic Chemical Classification [ATC]) (10). Antibiotic consumption (in grams or millions of international units) was converted to defined daily doses (DDD) and expressed as DDD/100 bed days, in accordance with the WHO Collaborating Centre for Drug Statistics Methodology (10). The total antibiotic consumption of each hospital was further characterized by measuring the consumption of carbapenems (imipenem-cilastatin and meropenem). We assessed the role of antibiotic diversity with two variables: (i) the proportion of consumed antibiotics having a very broad spectrum of activity (cefepime, ceftazidime, imipenem-cilastatin, meropenem, piperacillin-tazobactam) and (ii) the Peterson homogeneity index. Our interpretation is that a high proportion of very broad-spectrum antibiotics reflects low diversity, as less consideration is given, for instance, to streamlining or empirical use of other antibiotics in patients whose risk of being infected by multiresistant Gram-negative bacteria is low. The Peterson homogeneity index was described in a Sandiumenge et al. study (11) as 1 − {n/[2 × (n − 1)]} × ∑(ai − bi), where n is the number of antibiotics considered in the equation, ai is the proportion if all antibiotics considered were used in the same proportion, and bi is the proportion in the given study. It was observed that a high Peterson index corresponds to a high heterogeneity of use. Antibiotics whose consumption represented more than 10% of the total use over the study period were taken into consideration when calculating the index.
Statistical analysis.
Spearman's correlation was used to evaluate the relationship between resistance rates and the independent variables, namely, antibiotic consumption and diversity of use. Two dummy variables were created for the categorical variable “region” and were compared to the reference, which corresponded to the region of central Switzerland in the present study. We performed a multivariate linear regression to assess the relationship between resistance rates and the other variables. We also introduced the global antibiotic consumption, the proportion of very broad-spectrum antibiotics, and the Peterson index into the model. Carbapenem use was not included in the model in order to avoid multicollinearity. A P value of <0.05 was considered to indicate statistical significance. The statistical analyses were conducted using the Stata software 12.0 (Stata Corp., College Station, TX).
RESULTS
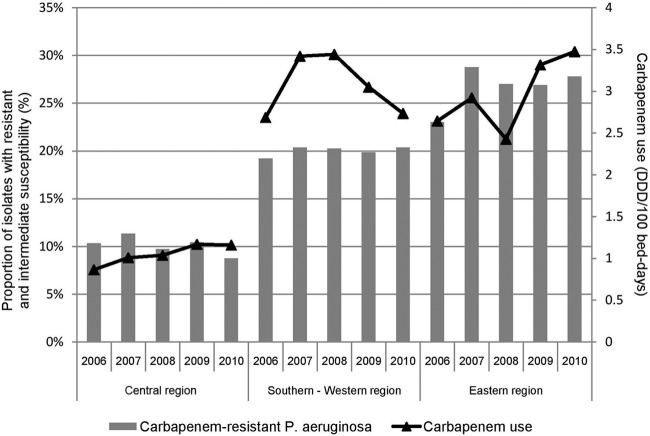
A total of 15,156 isolates of P. aeruginosa collected from January 2006 to December 2010 were analyzed in the present study. We observed that the resistance rate of P. aeruginosa to carbapenems was 19% (weighted mean) (range, 4% to 31%) for all hospitals. The lowest rate was observed in the hospitals of central Switzerland (10%) (range, 6% to 11%), followed by the ones of southern-western (20%) (range, 4% to 31%) and eastern Switzerland (26%) (range, 8% to 30%) (P = 0.27) (Table 1). Figure 1 illustrates the trends in rates of P. aeruginosa resistance to carbapenems versus the total consumption of antibiotics and carbapenems from 2006 to 2010.
Table 1.
Carbapenem-resistant Pseudomonas aeruginosa and antibiotic use pattern (weighted mean) for Switzerland and its 3 regions between 2006 and 2010
| Variable | Switzerland | Central regiona | Southern-Western region | Eastern region |
|---|---|---|---|---|
| No. of hospitals | 20 | 5 | 12 | 3 |
| No. of bed days | 11,371,144 | 4,249,179 | 4,740,146 | 2,381,819 |
| No. of P. aeruginosa isolates | 15,156 | 4,202 | 6,253 | 4,701 |
| Carbapenem-resistant P. aeruginosa (% [P]) | 19 | 10 | 20 (0.39) | 26 (0.11) |
| Global antibiotic consumption (DDD/100 bed-days [P]) | 54.4 | 53.7 | 54.3 (0.35) | 55.8 (0.10) |
| Consumption of carbapenems (DDD/100 bed-days [P]) | 2.3 | 1.0 | 3.1 (0.45) | 2.8 (0.41) |
| Proportion of very broad-spectrum antibioticsb (% [P]) | 10 | 8 | 11 (0.63) | 12 (0.03c) |
| Peterson index (P) | 0.401 | 0.451 | 0.420 (0.48) | 0.365 (0.17) |
The region of central Switzerland was considered the reference.
Cefepime, ceftazidime, imipenem-cilastatin, meropenem, and piperacillin-tazobactam were considered very broad-spectrum antibiotics.
Statistically significant result.
Fig 1.
Trends in resistance of Pseudomonas aeruginosa to carbapenems and carbapenem use in the 3 regions of Switzerland over the period of 2006 to 2010.
The total antibiotic consumption was 53.7 DDD per 100 bed days (weighted mean) (range, 32.3 to 62.8) in the hospitals of central Switzerland, 54.3 (37.1 to 62.1) in the hospitals of southern-western Switzerland, and 55.8 (40.6 to 63.5) in the hospitals of eastern Switzerland (Table 1). The consumption of carbapenems was 2.3 DDD/100 bed days (weighted mean) (range, 0.3 to 4.6) for all the hospitals, and the proportion of very broad-spectrum antibiotics was 10% (weighted mean) (range, 4% to 16%). The consumption of carbapenems and the proportion of very broad-spectrum antibiotics were lower in central Switzerland than those in southern-western and eastern Switzerland (Table 1). The mean Peterson index was 0.401 (range, 0.285 to 0.716). We observed a Peterson index in central Switzerland that was higher than that in southern-western and eastern Switzerland (Table 1).
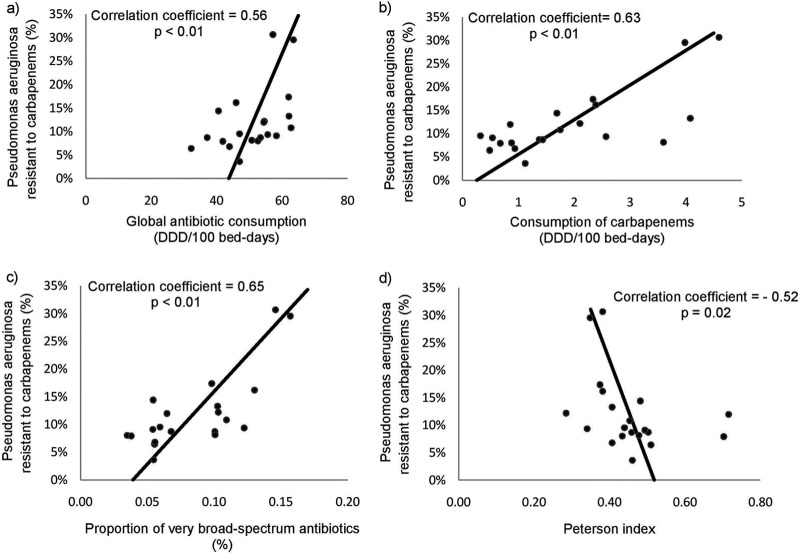
For all hospitals, a positive correlation was observed between the log odds of the resistance rates of P. aeruginosa and the total antibiotic consumption (Spearman's correlation coefficient = 0.56, P < 0.01), the consumption of carbapenems (coefficient = 0.63, P < 0.01), and the proportion of very broad-spectrum antibiotics (coefficient = 0.65, P < 0.01). We observed that the latter remained significant after the exclusion of carbapenems from the list (coefficient = 0.55, P = 0.01). Furthermore, we observed that the correlation with the Peterson index was negative (coefficient = −0.52, P = 0.02) (Table 2; Fig. 2). Moreover, we found a positive correlation between the resistance rates of the MDR strains and the consumption of carbapenems (coefficient = 0.81, P < 0.01) and the proportion of very broad-spectrum antibiotics (coefficient = 0.77, P < 0.01). The correlation with the Peterson index was negative (coefficient = −0.81, P < 0.01).
Table 2.
Relationship between the resistance rates of Pseudomonas aeruginosa to carbapenems and either the quantity or diversity of antibiotic consumption for 2006 to 2010
| Variable | Results for Spearman's correlation |
Results for multiple regression analysis |
|||
|---|---|---|---|---|---|
| coefa | P value | coefb | 95% CIc | P value | |
| Global antibiotic use | 0.56 | 0.009d | 6.8 × 10−4 | −0.003 to 0.005 | 0.735 |
| Use of carbapenemse | 0.63 | 0.003d | |||
| Proportion of very broad-spectrum antibiotics | 0.65 | 0.002d | 1.77 | 0.58 to 2.96 | 0.006d |
| Peterson index | −0.52 | 0.02d | 0.07 | −0.28 to 0.43 | 0.658 |
Spearman's correlation coefficient.
Regression coefficient.
CI, confidence interval.
Statistically significant result.
Carbapenems included imipenem and meropenem. This variable was not included in the model of the multiple regression analysis.
Fig 2.
Correlation between the resistance rates of Pseudomonas aeruginosa to carbapenems (%) and the global antibiotic consumption (a), the consumption of carbapenems (b), the proportion of very broad-spectrum antibiotics (c), and the Peterson index (d). Each dot represents one of the study hospitals.
In the multiple linear regression analysis performed at the hospital level, neither total antibiotic consumption nor the Peterson index influenced the rate of carbapenem-resistant P. aeruginosa. In contrast, the proportion of very broad-spectrum antibiotics was associated with increased resistance rates (P < 0.01) (Table 2). This increase was also observed when the MDR strains were included in the model (P < 0.05).
DISCUSSION
In the present study, we assessed the relationship between the carbapenem resistance in P. aeruginosa and the diversity and level of antibiotic consumption in 20 Swiss hospitals from 2006 to 2010. First, we confirmed a statistically significant correlation between carbapenem use and the rate of P. aeruginosa resistance to carbapenems at the hospital level. This was accurate at the regional level as well, as the region of central Switzerland had the lowest carbapenem use and the lowest rate of carbapenem-resistant P. aeruginosa in the country, although the differences were not statistically significant. In addition, the results show that the diversity of the antibiotics used in hospitals might have an impact on the rate of P. aeruginosa resistance to carbapenems only or to carbapenems and two other antipseudomonal agents. We observed that (i) a high Peterson index was correlated with a low resistance rate and (ii) the proportion of very broad-spectrum antibiotics was positively correlated with the rate of mono- or multiresistant P. aeruginosa. However, these findings were not unequivocal, since the Peterson index was not a significant predictor in the multivariate analysis. The proportion of very broad-spectrum antibiotics might reflect the impact of the use of carbapenems themselves. However, a role of diversity in antibiotics is suggested by the fact that the correlation between the proportion of very broad-spectrum antibiotics and carbapenem resistance remained significant when carbapenems were excluded from this group of antibiotics.
Previous studies described an association between carbapenem resistance in P. aeruginosa and the diversity of antibiotic use (12, 13). However, only a few such studies used a multicenter design. In 42 U.S. hospitals, Pakyz et al. found no association between diversity of use, as measured by Simpson's index and the Shannon-Weiner index, and the proportion of resistant pathogens (e.g., fluoroquinolone-, imipenem-, ceftazidime-, or cefepime-resistant P. aeruginosa) (14).
Antibiotic stewardship in hospitals is a key strategy for controlling antibiotic resistance. Guidelines for antibiotic stewardship, similar to the ones published by the IDSA-SHEA for U.S. hospitals (15), recommend formulary restriction and mandatory approval by infectious disease specialists for restricted antibiotics as core strategies, based on local antibiotic use and resistance problems. Restrictions to specific antibiotic families (carbapenems in the present case) might have a positive impact on carbapenem resistance. However, the use of alternatives might lead to a shift in resistance profiles (16). Therefore, it might be worthwhile to consider other strategies. Diversification in antibiotic use can help avoid the selection pressure that might result from the use of a restricted list of antibiotics (11). Antibiotic cycling (where the same antibiotic class is prescribed to all the patients in a ward for a given indication during a fixed period) can be a way to achieve diversity in antibiotic use. Mixing antibiotic use (i.e., allowing prescription of different antibiotics or antibiotic classes to consecutive patients for a given indication) is an alternative that might enable the cumbersome logistic difficulties of antibiotic cycling to be overcome. Hence, the latter alternative might be more effective (17). Our results confirm previous publications which demonstrated that diversity of use might minimize the emergence of resistance, especially in P. aeruginosa (18, 19).
However, the present study has several limitations. First, its ecologic design focused on the comparison of aggregated data rather than patient-level data, thereby leading to inferences from individual factors (20). Second, we did not include the consumption of carbapenems in the multivariate model. However, this was done intentionally to prioritize the impact of antibiotic diversity in the multivariate analysis while avoiding collinearity of carbapenem use with either the use of all the antibiotics or the proportion of very broad-spectrum antibiotics.
In conclusion, the present multicenter study shows that lower rates of P. aeruginosa resistance to carbapenems only or to carbapenems and other antipseudomonal agents were correlated with a lower consumption of antibiotics in general, and particularly of carbapenems, and with a lower proportion of very broad-spectrum antibiotics. The study also suggests a beneficial impact of diversifying the use of antibiotics. Promoting heterogeneity in antibiotic use, in addition to parsimony, might be a valuable strategy for minimizing the spread of resistance in hospitals.
ACKNOWLEDGMENTS
We recognize the important contribution of the sentinel network participants in providing the data. We thank B. Testa for careful reading of the manuscript.
We declare that there is no conflict of interest.
Footnotes
Published ahead of print 28 January 2013
REFERENCES
- 1. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 2. El Solh AA, Alhajhusain A. 2009. Update on the treatment of Pseudomonas aeruginosa pneumonia. J. Antimicrob. Chemother. 64:229–238 [DOI] [PubMed] [Google Scholar]
- 3. Driscoll JA, Brody SL, Kollef MH. 2007. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67:351–368 [DOI] [PubMed] [Google Scholar]
- 4. Navon-Venezia S, Ben-Ami R, Carmeli Y. 2005. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr. Opin. Infect. Dis. 18:306–313 [DOI] [PubMed] [Google Scholar]
- 5. El Amari EB, Chamot E, Auckenthaler R, Pechere JC, Van Delden C. 2001. Influence of previous exposure to antibiotic therapy on the susceptibility pattern of Pseudomonas aeruginosa bacteremic isolates. Clin. Infect. Dis. 33:1859–1864 [DOI] [PubMed] [Google Scholar]
- 6. Lepper PM, Grusa E, Reichl H, Hogel J, Trautmann M. 2002. Consumption of imipenem correlates with beta-lactam resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:2920–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogues AM, Dumartin C, Amadeo B, Venier AG, Marty N, Parneix P, Gachie JP. 2007. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infect. Control Hosp. Epidemiol. 28:1389–1395 [DOI] [PubMed] [Google Scholar]
- 8. Rossolini GM, Mantengoli E. 2005. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 11(Suppl 4):17–32 [DOI] [PubMed] [Google Scholar]
- 9. Bronzwaer S, Cars O, Buchholz U, Molstad S, Goettsch W, Veldhuijzen IK, Kool JL, Sprenger MJ, Degener JE. 2002. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg. Infect. Dis. 8:278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization Collaborating Centre for Drug Statistics Methodology 2011. Guidelines for ATC classification and DDD assignment, 14th ed. World Health Organization, Oslo, Norway [Google Scholar]
- 11. Sandiumenge A, Lisboa T, Gomez F, Hernandez P, Canadell L, Rello J. 2011. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE organisms. Chest 140:643–651 [DOI] [PubMed] [Google Scholar]
- 12. Goel N, Wattal C, Oberoi JK, Raveendran R, Datta S, Prasad KJ. 2011. Trend analysis of antimicrobial consumption and development of resistance in non-fermenters in a tertiary care hospital in Delhi, India. J. Antimicrob. Chemother. 66:1625–1630 [DOI] [PubMed] [Google Scholar]
- 13. Hsueh PR, Chen WH, Luh KT. 2005. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991-2003 at a university hospital in Taiwan. Int. J. Antimicrob. Agents 26:463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pakyz A, Powell JP, Harpe SE, Johnson C, Edmond M, Polk RE. 2008. Diversity of antimicrobial use and resistance in 42 hospitals in the United States. Pharmacotherapy 28:906–912 [DOI] [PubMed] [Google Scholar]
- 15. Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 44:159–177 [DOI] [PubMed] [Google Scholar]
- 16. Van Gastel E, Costers M, Peetermans WE, Struelens MJ. 2010. Nationwide implementation of antibiotic management teams in Belgian hospitals: a self-reporting survey. J. Antimicrob. Chemother. 65:576–580 [DOI] [PubMed] [Google Scholar]
- 17. Bal AM, Kumar A, Gould IM. 2010. Antibiotic heterogeneity: from concept to practice. Ann. N. Y. Acad. Sci. 1213:81–91 [DOI] [PubMed] [Google Scholar]
- 18. Bennett KM, Scarborough JE, Sharpe M, Dodds-Ashley E, Kaye KS, Hayward TZ, III, Vaslef SN. 2007. Implementation of antibiotic rotation protocol improves antibiotic susceptibility profile in a surgical intensive care unit. J. Trauma 63:307–311 [DOI] [PubMed] [Google Scholar]
- 19. Brown EM, Nathwani D. 2005. Antibiotic cycling or rotation: a systematic review of the evidence of efficacy. J. Antimicrob. Chemother. 55:6–9 [DOI] [PubMed] [Google Scholar]
- 20. Morgenstern H. 1995. Ecologic studies in epidemiology: concepts, principles, and methods. Annu. Rev. Public Health 16:61–81 [DOI] [PubMed] [Google Scholar]