Abstract
Biofilm formation by Pseudomonas aeruginosa has been implicated in the pathology of chronic wounds. Both the d and l isoforms of tryptophan inhibited P. aeruginosa biofilm formation on tissue culture plates, with an equimolar ratio of d and l isoforms producing the greatest inhibitory effect. Addition of d-/l-tryptophan to existing biofilms inhibited further biofilm growth and caused partial biofilm disassembly. Tryptophan significantly increased swimming motility, which may be responsible in part for diminished biofilm formation by P. aeruginosa.
TEXT
Biofilms are composed of bacterial cells enmeshed within an extracellular matrix of polysaccharides, DNA, and proteins (1–3). Biofilms form in a variety of clinical situations, including chronic skin wounds (4–6). Bacterial cells within biofilms are ∼1,000 times more resistant to antibiotic treatment than their planktonic counterparts (7, 8). Pseudomonas aeruginosa is an opportunistic pathogen that can cause infections involving biofilms (9) that protect bacterial cells from host defenses (10) and impair healing (11, 12). There is a need for topical treatments to prevent biofilm formation and induce disassembly of bacterial biofilms in chronic wounds. Hochbaum et al. and Kolodkin-Gal et al. reported that d amino acids inhibited biofilm formation and caused disassembly of existing biofilms formed by Bacillus subtilis and Staphylococcus aureus (13, 14). Supplementary data in one report suggested a similar effect on biofilm formation by P. aeruginosa (14). In this study we document the in vitro effects of tryptophan on P. aeruginosa biofilm formation and motility. These findings support our long-term objective to develop novel wound treatments to prevent biofilm formation in chronic wounds.
Pseudomonas aeruginosa ATCC 27853 was cultured in tryptic soy broth for 24 h at 37°C; in some experiments additional strains of P. aeruginosa were tested. P. aeruginosa biofilm quantification was adapted from a study by O'Toole and Kolter (15). Briefly, the bacterial suspension was diluted 1:2,500 (vol/vol) in M63 medium [2.0 g (NH4)SO4, 13.6 g KH2PO4, 0.5 mg FeSO4 · 7H2O, 10 ml 20% glycerol, and 1 ml 1 M MgSO4 in 1.0 liter of distilled H2O (diH2O)] to which one or more amino acids (tryptophan, tyrosine, methionine, or leucine) were added (0.5 to 10.0 mM). The bacterial suspensions were added to individual wells (0.2 ml) in tissue culture microtiter plates and incubated up to 72 h at 30°C. After incubation the biofilms were stained with 0.35% filtered crystal violet. Acetic acid (30% [vol/vol]) was added to each well, and absorbance was measured at 595 nm using a Beckman Coulter DTX880 multimode detector. In some experiments samples were also serially diluted in phosphate-buffered saline (PBS) and plated on Trypticase soy agar with 5% sheep blood to determine CFU. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test in Prism 5 (GraphPad Software, La Jolla, CA), with significance set at a P of <0.05. Data are presented as the means ± standard errors of the means. Photographs of representative wells were taken using an inverted microscope with attached camera.
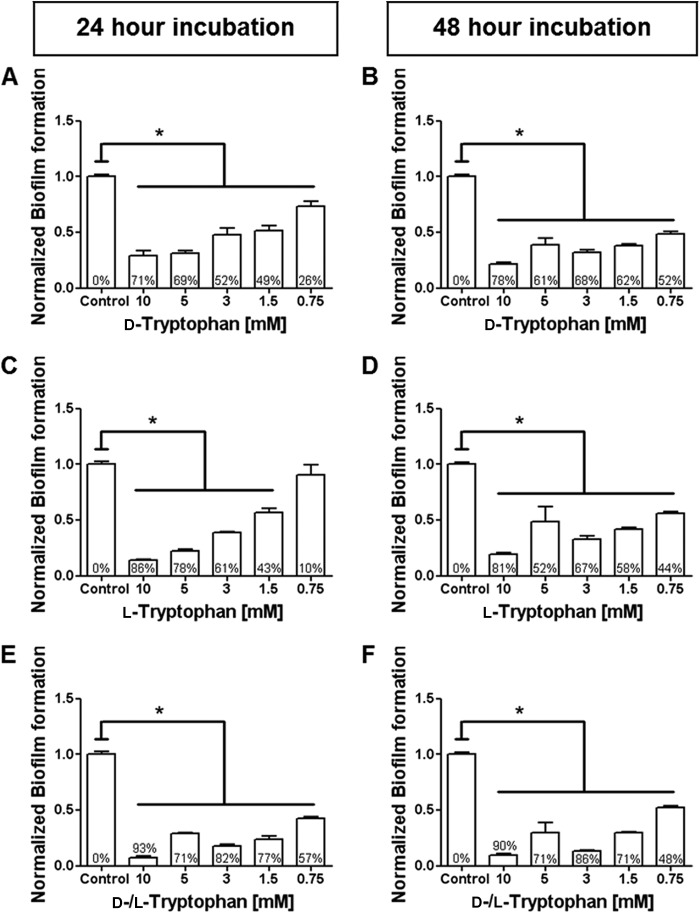
Our initial experiments showed that tryptophan and tyrosine inhibited P. aeruginosa biofilm formation, while methionine and leucine did not (see Fig. S1 in the supplemental material). Because tryptophan was most effective, it was selected for further investigation. At 10.0 mM, d-tryptophan inhibited P. aeruginosa biofilms by 71% at 24 h and 78% at 48 h (Fig. 1A and B). Similarly, at 10.0 mM, l-tryptophan inhibited P. aeruginosa biofilm formation by 86% at 24 h and 81% at 48 h (Fig. 1C and D). When both d- and l-tryptophan isoforms were mixed at an equimolar ratio (total tryptophan concentration = 10.0 mM), P. aeruginosa biofilm formation was inhibited by 93% at 24 h and 90% at 48 h (Fig. 1E and F). Because the equimolar combination of d- and l-tryptophan had the greatest effect, it was used in all subsequent experiments. Tryptophan (10.0 mM) reduced bacterial growth beyond 32 h, as assessed by measuring absorbance (Fig. 2A) and beyond 48 h, as assessed by measuring CFU (Fig. 2B). At 72 h a lesser concentration of tryptophan (5 mM) had no significant effect on CFU but significantly inhibited biofilm formation (Fig. 2C). These data suggest that inhibition of bacterial growth is not the sole reason for biofilm inhibition by tryptophan. Figure 3 shows microscopic images of biofilm inhibition by tryptophan. These effects were not restricted to strain ATCC 27853, as 10.0 mM tryptophan inhibited biofilm formation by two clinical isolates of P. aeruginosa (strains 2547 and 3170) (see Fig. S2 in the supplemental material). However, biofilm formation by a third strain (strain 1829), which was resistant to both carbapenems and aminoglycosides, was not inhibited by tryptophan (see Fig. S2 in the supplemental material).
Fig 1.
Both d- and l-tryptophan inhibit P. aeruginosa biofilm formation. P. aeruginosa strain ATCC 27853 was incubated at 30°C in M63 medium with the indicated concentrations of d- or l-tryptophan. Biofilm formation was quantified by crystal violet staining at the indicated time points, as described in materials and methods in the supplemental material. Results are normalized to biofilm formation in the absence of tryptophan (control). The percent inhibition for each tryptophan concentration is indicated within each bar. Inhibition of P. aeruginosa biofilm formation by d-tryptophan at 24 h ranged from 26 to 71% (A) and at 48 h ranged from 52 to 78% (B) over the concentrations tested. l-Tryptophan inhibition of P. aeruginosa biofilm formation at 24 h ranged from 10 to 86% (C) and at 48 h ranged from 44 to 81% (D). Equal molar combinations of d- and l-tryptophan inhibited biofilm formation at 24 h from 57 to 93% (E) and at 48 h from 48 to 90% (E). *, P < 0.05 versus control.
Fig 2.
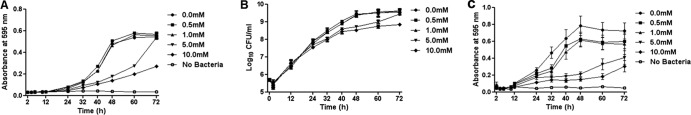
Effect of d-/l-tryptophan on growth of P. aeruginosa and biofilms. (A) Bacterial growth in M63 media with the indicated concentrations of tryptophan was assessed by measuring absorbance at 595 nm. Significant reductions (P < 0.05) were observed at 40 to 60 h for the 5.0 mM treatment group and at 32 to 72 h for the 10.0 mM treatment group. (B) Reduction in CFU/ml was also observed with 5.0 mM tryptophan at 48 to 60 h and with 10.0 mM tryptophan at 48 to 72 h (P < 0.05). (C) Dose-dependent inhibition of biofilm formation (crystal violet stain). Each data point is plotted as the mean ± standard error of 3 independent experiments.
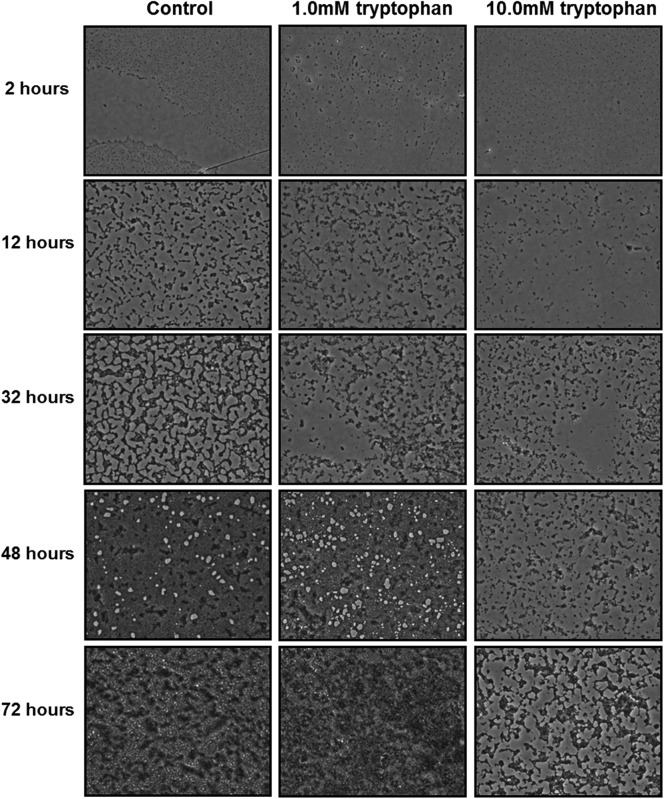
Fig 3.
Inhibition of biofilm growth by tryptophan. Representative images (magnification, ×20) of P. aeruginosa biofilms grown on microtiter plates in M63 medium with the indicated concentrations of d-/l-tryptophan at 30°C for 2 to 72 h.
We next examined whether tryptophan caused disassembly of existing P. aeruginosa biofilms. After determining the initial level of biofilm at 48 h or 72 h of incubation, the medium was removed and replaced with fresh M63 medium with various amounts of tryptophan (0.0 to 10.0 mM). The plate was then incubated for an additional 24 h at 30°C (see Fig. S3 and S4 in the supplemental material). Treatment with 10.0 mM tryptophan resulted in modest disassembly of 48-h biofilms (Fig. 4A) and significant disassembly (P < 0.05) of 72-h biofilms (Fig. 4B). Biofilm treatment with less tryptophan (1.0 mM) prevented further biofilm growth but did not cause biofilm disassembly. These findings are consistent with a recent report that similar concentrations of d amino acids caused disassembly of established S. aureus biofilms (13).
Fig 4.
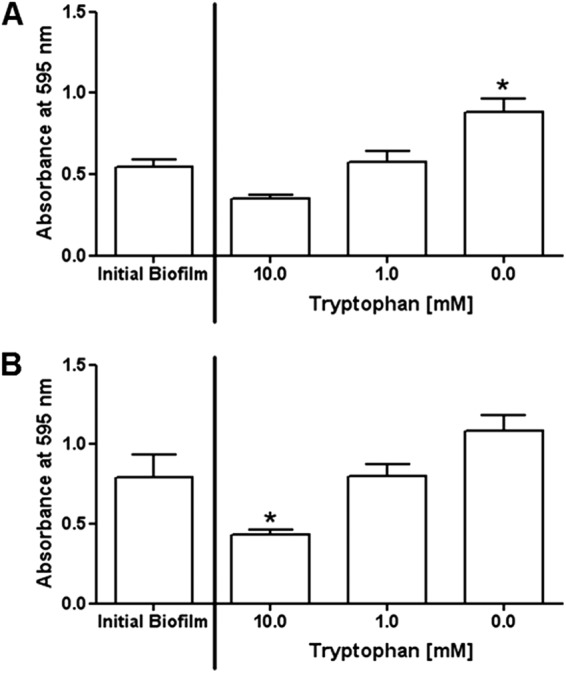
Addition of tryptophan causes disassembly of existing P. aeruginosa biofilms. P. aeruginosa biofilms were allowed to form in wells with M63 medium incubated at 30°C for 48 (A) or 72 h (B). The medium was removed, fresh medium with the indicated amounts of d-/l-tryptophan (0.0 to 10.0 mM) was added, and the plate was incubated for an additional 24 h at 30°C. Biofilm formation (crystal violet stain) was evaluated for the initial biofilm and after 24 h of incubation with or without tryptophan. *, P < 0.05 compared to the initial biofilm.
Because there is an inverse relationship between bacterial motility and biofilm formation (16–19), we investigated tryptophan's effect on P. aeruginosa motility. Swimming and twitching motilities were assessed by adding 0.3% or 1.0% agar (Difco) (15, 20–22), respectively, to M63 medium with tryptophan (0.0 to 10.0 mM). Tryptophan dose dependently increased swimming motility by approximately 40% at 10.0 mM (Fig. 5A). In contrast tryptophan induced a biphasic response in P. aeruginosa twitching motility (Fig. 5B).
Fig 5.
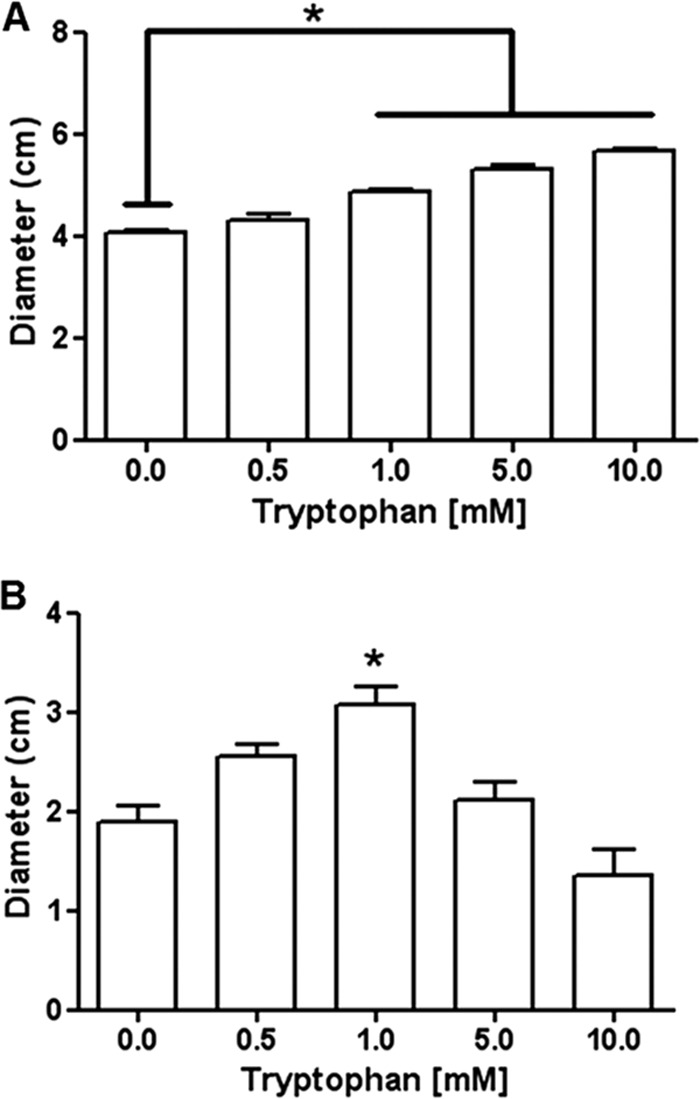
Tryptophan increases swimming motility and has a biphasic effect on twitching motility by P. aeruginosa. (A) Swimming motility increased in a dose-dependent manner (6 to 40%) with addition of tryptophan to the agar medium containing agar. (B) Twitching motility increased (12 to 63%) at low concentrations (0.5 and 1.0 mM) of tryptophan and was slightly less than the control at 10.0 mM. *, P < 0.05 compared to no tryptophan.
In contrast to the report by Kolodkin-Gal et al. (14), we find d and l isoforms of tryptophan to be equally effective at inhibiting P. aeruginosa biofilm formation and the combination of d and l isoforms to be more effective than either isoform alone. l-Tryptophan recently was reported to inhibit biofilm formation by Escherichia coli by increasing catalysis of l-tryptophan into indole (23). However, this is not likely the mechanism for P. aeruginosa, because it does not convert tryptophan into indole (data not shown). Instead, we propose that tryptophan inhibits biofilm formation by P. aeruginosa in part by modulating bacterial cell motility. Flagellar arrest is required for biofilm formation by P. aeruginosa (17–20). Based on our observation of enhanced swimming motility, we infer that tryptophan increases P. aeruginosa flagellar activity. Biofilms contain vast numbers of nonmotile bacterial cells (24, 25). If tryptophan increases bacterial cell motility, it may favor detachment of cells from the biofilm, as reported for the natural life cycle of a biofilm (26). Together these events will reduce biofilm formation and favor biofilm disassembly. Ongoing investigations indicate that tryptophan inhibits P. aeruginosa biofilm formation on biological wound dressings (Biobrane) (data not shown). We also find that tryptophan at the concentrations used to inhibit biofilm formation (1 to 10 mM) is not cytotoxic for human cells (HaCaT cell line) (data not shown). Future studies will explore the potential use of tryptophan to inhibit biofilm formation on wound dressings and in experimental wounds.
ACKNOWLEDGMENTS
This project was supported in part by NIH grants RC2 AR058971-01 and R21 EB014594, by Wisconsin Agricultural Experiment Station grant WIS01607, through the TL1 fellowship (K.S.B.), supported by the Clinical and Translational Science Award program, and through the NIH National Center for Advancing Translational Sciences, grant UL1TR000427.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print 14 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00007-13.
REFERENCES
- 1. Branda SS, Vik S, Friedman L, Kolter R. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20–26 [DOI] [PubMed] [Google Scholar]
- 2. Flemming HC, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8:623–633 [DOI] [PubMed] [Google Scholar]
- 3. López D, Vlamakis H, Kolter R. 2010. Biofilms. Cold Spring Harb. Perspect. Biol. 2:a000398 doi:10.1101/cshperspect.a000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. 2003. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Invest. 112:1466–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dass CL, Walsh MF, Seo S, Shiratsuchi H, Craig DH, Basson MD. 2009. Irrigant divalent cation concentrations influence bacterial adhesion. J. Surg. Res. 156:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ. 2006. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 188:8213–8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34–40 [DOI] [PubMed] [Google Scholar]
- 8. Hengzhuang W, Wu H, Ciofu O, Song Z, Høiby N. 2011. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 55:4469–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjarnsholt T, Kirketerp-Møller, Jensen PØ K, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. 2008. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 16:2–10 [DOI] [PubMed] [Google Scholar]
- 10. Harmsen M, Yang L, Pamp SJ, Tolker-Nielsen T. 2010. An update on Pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Immunol. Med. Microbiol. 59:253–268 [DOI] [PubMed] [Google Scholar]
- 11. Nithya C, Begum MF, Pandian SK. 2010. Marine bacterial isolates inhibit biofilm formation and disrupt mature biofilms of Pseudomonas aeruginosa PAO1. Appl. Microbiol. Biotechnol. 88:341–358 [DOI] [PubMed] [Google Scholar]
- 12. Sambanthamoorthy K, Gokhale AA, Lao W, Parashar V, Neiditch MB, Semmelhack MF, Lee I, Waters CM. 2011. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 55:4369–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. 2011. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J. Bacteriol. 193:5616–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. 2010. D-amino acids trigger biofilm disassembly. Science 328:627–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449–461 [DOI] [PubMed] [Google Scholar]
- 16. Bernier SP, Ha DG, Khan W, Merritt JH, O'Toole GA. 2011. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res. Microbiol. 162:680–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conrad JC, Gibiansky ML, Jin F, Gordon VD, Motto DA, Mathewson MA, Stopka WG, Zelasko DC, Shrout JD, Wong GCL. 2011. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys. J. 100:1608–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295–304 [DOI] [PubMed] [Google Scholar]
- 19. Ruer S, Stender S, Filloux A, de Bentzmann S. 2007. Assembly of fimbrial structures in Pseudomonas aeruginosa: functionality and specificity of chaperone-usher machineries. J. Bacteriol. 189:3547–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bala A, Kumar R, Harjai K. 2011. Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. J. Med. Microbiol. 60:300–306 [DOI] [PubMed] [Google Scholar]
- 21. Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K. 2008. Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J. Bacteriol. 190:662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rashid MH, Kornberg A. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 97:4885–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimazaki J, Furukawa S, Ogihara H, Morinaga Y. 2012. l-Tryptophan prevents Escherichia coli biofilm formation and triggers biofilm degradation. Biochem. Biophys. Res. Commun. 419:715–718 [DOI] [PubMed] [Google Scholar]
- 24. Karatan E, Watnick P. 2009. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73:310–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mikkelsen H, Sivaneson M, Filloux A. 2011. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 13:1666–1681 [DOI] [PubMed] [Google Scholar]
- 26. Boles BR, Thoendel M, Singh PK. 2005. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 57:1210–1223 [DOI] [PubMed] [Google Scholar]