Abstract
As the risk of tenofovir-associated renal toxicity has been found to be proportional to the drug plasma concentration, our aim was to measure the determinants of tenofovir plasma exposure in HIV-positive patients with normal renal function. A cross-sectional analysis was conducted in HIV-positive patients chronically receiving tenofovir-containing highly active antiretroviral therapies (HAARTs). Patients on tenofovir-containing antiretroviral regimens, presenting 22 to 26 h after drug intake, having estimated glomerular filtration rates above 60 ml/min, reporting high adherence to antiretroviral medications (above 95% of the doses), and signing a written informed consent were included. Plasma tenofovir concentrations were measured through a validated high-performance liquid chromatography–mass spectrometry (HPLC/LC-MS) method. The tenofovir trough concentrations in 195 patients (median, 50 ng/ml, and interquartile range, 35 to 77 ng/ml) were significantly associated with the estimated glomerular filtration rate, body mass index, and third-drug class (protease-containing versus protease-sparing regimens) (with the highest exposure in unboosted-atazanavir recipients). The results of multivariate analysis showed that the third-drug class and the weight/creatinine ratio were independent predictors of tenofovir trough concentrations. This cross-sectional study shows that tenofovir trough concentrations are predicted by the weight/creatinine ratio and by the coadministered antiretrovirals, with protease inhibitors (whether boosted or unboosted) being associated with the highest plasma exposure. These data, previously available in healthy subjects or for some drugs only, could be useful for designing strategies to manage tenofovir-associated toxicity, since this toxicity has been reported to be dose dependent.
INTRODUCTION
Although the majority of successful highly active antiretroviral therapy (HAART)-treated HIV-positive patients are taking tenofovir disoproxil fumarate (TDF) as part of their nucleoside/nucleotide reverse transcriptase inhibitor [N(t)RTI] backbone, the drug is being increasingly associated with renal tubular dysfunction (1–3). While in clinical reports, the impact of TDF on renal function is mostly described in terms of decrease of estimated glomerular filtration rate (GFR), TDF appears unlikely to directly harm any glomerular structure and its effect on GFR estimation depends upon the decreased creatinine secretion that is secondary to tubular dysfunction (3). In four independent clinical studies, TDF pharmacokinetic (PK) exposure was found to be associated with alterations in a series of renal function markers, both glomerular and tubular (4–7). According to drug-drug interaction studies of healthy volunteers, the PK exposure of tenofovir seems to be rather sensitive to the choice of companion drugs, which suggests that, depending on the specific HAART regimen, its impact on tubular function may also vary. In agreement with this assumption, in large-scale cohort studies, the likelihood of TDF-associated renal dysfunction was found to vary depending upon the antiretrovirals being concurrently administered, with ritonavir-boosted protease inhibitors (PIs/r) being associated with the highest risk (3). Since only limited data on tenofovir exposure in patients are available (8) and in order to better understand the relationship between specific antiretrovirals and the drug pharmacokinetic profile, we carried out a cross-sectional pharmacokinetic survey in patients under successful and renally safe (estimated creatinine clearance [eCLCR], >60 ml/min) chronic TDF administration.
(Some of the data in the manuscript were presented as a poster at the 18th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 5 to 8 March 2012 [9].)
MATERIALS AND METHODS
Patients on TDF-containing antiretroviral regimens were consecutively enrolled at the Department of Infectious Diseases of the University of Torino and at the Amedeo di Savoia Hospital, Torino, Italy, between September 2010 and January 2011. The protocol was approved by the local ethics committee. Patients were included if they had taken TDF 22 to 26 h before, reported high adherence to antiretroviral medications (above 95% of the doses), presented no concomitant renal disease, and signed a written informed consent. Patients diagnosed with chronic kidney disease (defined by estimated creatinine clearance below 60 ml/min), on hemodialysis, or affected by diabetes mellitus were excluded from this study. Tenofovir trough concentrations were measured through a validated high-performance liquid chromatography–mass spectrometry (HPLC/LC-MS) method with a limit of detection of 2 ng/ml (10). eCLCR was calculated using the Cockroft-Gault formula. The results are expressed as median values and interquartile ranges; nonparametric tests (Spearman, Mann-Whitney, and Kruskal-Wallis) were used for all the analyses (comparisons and correlations), while a multivariate linear regression analysis was used to evaluate the effects of several covariates (with a P value of <0.20 at bivariate analysis) on tenofovir plasma exposure.
RESULTS
One hundred ninety-five adult HIV-positive patients (68.2% male) were enrolled in this study; they were mainly of Caucasian ethnicity (166 [85.1%]). The median values and interquartile ranges for age, body mass index (BMI), plasma creatinine, and eCLCR were 45 years (39 to 50), 23.1 kg/m2 (21.1 to 25.8), 0.96 mg/dl (0.84 to 1.08), and 84.4 ml/min (70.2 to 102.9). Patients were cotreated with unboosted atazanavir (ATV) (34 patients [17.4%]), with a boosted protease inhibitor (118 patients [60.5%]; 54 patients on ATV/ritonavir [ATV/r], 30 on lopinavir/ritonavir [LPV/r], and 23 on darunavir/ritonavir [DRV/r]), with nonnucleoside transcriptase inhibitors (NNRTIs) (32 patients [16.4%];16 on nevirapine [NVP] and 13 on efavirenz [EFV]), and with raltegravir (11 patients [5.6%]). The demographic and treatment characteristics of the study participants are summarized in Table 1, stratified by third-drug class.
Table 1.
Demographic and clinical characteristics
| Characteristic | No. (%) or median value (interquartile range) for group receiving: |
P valuea | |||
|---|---|---|---|---|---|
| Unboosted atazanavir | Boosted PIs | NNRTIs | Raltegravir | ||
| No. of patients (n = 195) | 34 | 118 | 32 | 11 | |
| Gender (male) | 20 (58.8) | 84 (71.2) | 23 (71.9) | 6 (54.5) | 0.56 |
| Ethnicity (Caucasian) | 27 (79.4) | 106 (89.8) | 24 (75) | 9 (81.8) | 0.38 |
| Age (yr) | 44.5 (37.8–50) | 44 (39–49.5) | 47 (39–54) | 43 (41–47) | 0.74 |
| BMI (kg/m2) | 24.4 (22.7–27.5) | 23 (20.5–25.3) | 22.7 (21.1–26.5) | 23.7 (20.5–29.6) | 0.18 |
| Creatinine (mg/dl) | 0.9 (0.84–1.09) | 0.96 (0.84–1.07) | 1.02 (0.86–1.09) | 1.03 (0.94–1.15) | 0.24 |
| eCLCR (ml/min) | 89.5 (75.6–104) | 83.1 (69.8–102.7) | 81 (69–109.2) | 83.5 (71–93.7) | 0.97 |
| Duration of tenofovir treatment (mo) | 29.1 (18.3–56.5) | 17.5 (7–46) | 33.9 (7.4–68) | 11.2 (1.8–51.4) | 0.09 |
Variables were compared with Chi-square and Kruskall-Wallis tests.
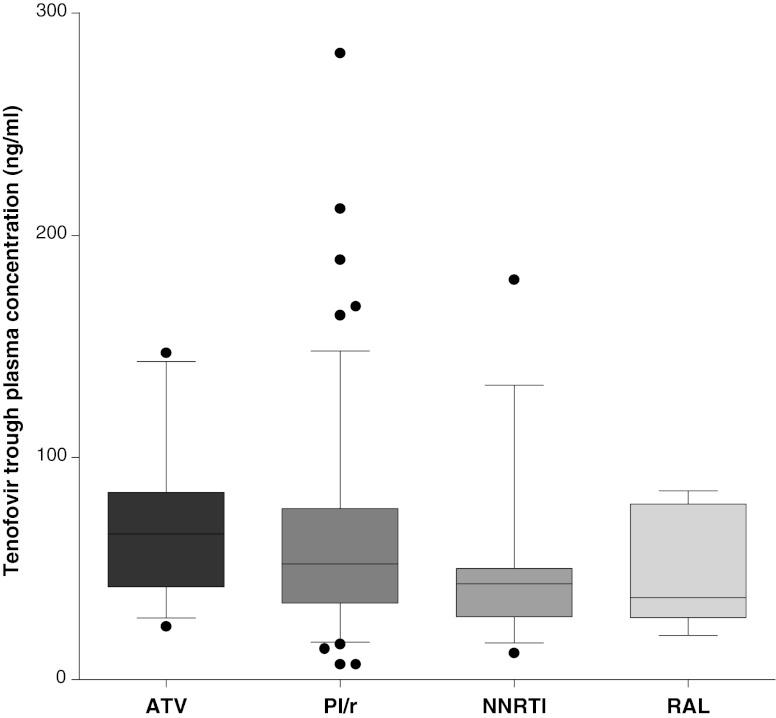
Samples were collected at steady state and after a median uninterrupted time of TDF intake of 22.2 months (interquartile range, 8.9 to 56.2). The median tenofovir trough plasma concentration was 50 ng/ml (35 to 77), with a coefficient of variation of 64.6%. Tenofovir trough concentrations were significantly associated with eCLCR (rho, 0.24; P = 0.012), BMI (rho, 0.16; P = 0.025), and weight/creatinine ratio (rho, 0.24; P = 0.001); a borderline association with age emerged (rho, 0.13; P = 0.07). The tenofovir concentrations according to the third-drug class were higher in unboosted atazanavir recipients (median value and interquartile range, 65 ng/ml [41.7 to 84.2]) than in patients under treatment with boosted PIs (51.5 ng/ml [34 to 77]), NNRTIs (43 ng/ml [28.2 to 50]), and raltegravir (37 ng/ml [28 to 79]) (Kruskal-Wallis test, P = 0.02) (Fig. 1). Dunn's multiple comparison test identifies as statistically significant the pairwise comparison (with post hoc correction) between atazanavir and NNRTI recipients. Receiving a protease inhibitor (whether with or without ritonavir) was associated with higher tenofovir plasma trough concentrations (54.5 ng/ml [37 to 80] versus 43 ng/ml [28 to 50] in the NNRTI and raltegravir group; P = 0.007). No significant differences were found in BMI, plasma creatinine, and eCLCR among these groups; nevertheless, patients on boosted PIs and raltegravir had shorter durations of tenofovir exposure (Table 1). No statistically significant intraclass differences in tenofovir exposure were found among patients on boosted PIs (ATV/r 54 ng/ml [35 to 76], LPV/r 55 ng/ml [40 to 78], and DRV/r 43 ng/ml [29 to 77]; P = 0.27) or NNRTIs (NVP 41 ng/ml [29 to 53] and EFV 46 ng/ml [28 to 50]; P = 0.67). A multivariate linear regression analysis was performed using the backward exclusion procedure and including age, BMI, eCLCR, weight/creatinine ratio, and third-drug class; the third-drug class (P = 0.004; beta, 0.22; 95% inhibitory concentration [IC95], 6.8 to 35.3) and the weight/creatinine ratio (P = 0.04; beta, −2.02; IC95, −0.73 to 0.009) were independently associated with tenofovir trough concentrations.
Fig 1.
Tenofovir plasma trough concentrations (ng/ml) according to different third-drug classes. Boxplots and whiskers represent median values, interquartile ranges, and 95% confidence intervals, respectively; black dots are single outliers. Median values and 95% confidence intervals are 65.5 ng/ml (27.7–143.2) in the ATV (atazanavir), 51.5 ng/ml (16.9–146.9) in the PI/r (boosted protease inhibitor), 43 ng/ml (16.5–132.5) in the NNRTI (nonnucleoside reverse transcriptase inhibitor), and 37 ng/ml (20–84.8) in the RAL (raltegravir) groups.
DISCUSSION
In this cross-sectional study in HIV-positive patients, we report new clinical data on tenofovir plasma PK exposure according to different concomitant drugs. Information on this was mainly derived from data for healthy volunteers, with only a few clinical studies performed on specific tenofovir-antiretroviral combinations in HIV-infected patients (such as lopinavir/ritonavir) (11). Our results further support the hypothesis of a tenofovir dose/concentration-dependent toxicity, since TDF PK exposure was found to vary significantly according to the third antiretroviral being concurrently taken, with a magnitude that reproduces the relative risk of TDF-associated nephrotoxicity according to concurrently administered drugs seen in cohort studies. In looking at TDF concentrations, it must be considered that in the three studies associating higher tenofovir exposures to kidney tubular dysfunction, the mid-dose value (approximately 12 h after drug intake) (4–7) was used, while our plasma samples were taken at 24 h.
While other factors were found to influence tenofovir plasma trough concentrations (age, BMI, and eCLCR, including the previously suggested combined parameter weight/plasma creatinine) (12, 13), the choice of the third-drug class seems to be the only readily modifiable factor. As was found in EUROSIDA, D:A:D:, and two additional retrospective surveys, (3, 14–16), the use of boosted PIs together with TDF is associated with the highest risk of renal toxicity, with reduced risk in the case of NNRTI or raltegravir coadministration. Recent data report that tenofovir with either boosted protease inhibitor leads to a greater initial decline in eCLCR than tenofovir with efavirenz and that this decline may be worse with ATV/r than with LPV/r (17). The mechanism by which boosted PIs increase TDF exposure is thought to be dependent upon the inhibition of multiple resistance protein (MRP) transporters at the apical side of renal tubular cells, with consequent TDF intratubular accumulation and possible chronic harm to mitochondria and with intestinal P glycoprotein inhibition (and related increased absorption) as a possible complementary mechanism. It is worth noting that the highest exposure was found in unboosted ATV recipients: MRP-2 inhibition both by boosted PIs and atazanavir could contribute to explaining this effect. It is unclear, however, why unboosted atazanavir intake was associated with higher tenofovir concentrations than boosted atazanavir, and we are not able to find a causative reason for this observed effect. In any case, since in the absence of ritonavir, ATV exposure is also lower, such a difference might be attributable to an intrinsically greater inhibition of TDF extrusion from tubular cells by ATV (than by ritonavir), which would thus interact with MRPs without competing with ritonavir for drug transporter binding. The only available data suggest that the addition of unboosted ATV to TDF in healthy volunteers was associated with increased tenofovir plasma concentrations (the geometric mean ratios for area under the concentration-time curve [AUC], maximum concentration of drug in serum [Cmax], and Cmin, respectively, were 1.24, 1.14, and 1.22) (18).
Some limitations of this study should be highlighted: the cross-sectional design implies that patients with severe tubular and renal toxicities have not been included due to selection bias and inclusion criteria. However, such a selection does limit the risk of confounding the cause with the effect, since no patients with altered creatinine clearance values were studied. Fewer patients were included in the efavirenz and the raltegravir groups due to their habit of taking antiretrovirals at nighttime. Finally, the measurement of adherence to treatment by self-report is not precise, and it is possible that some patients did not take the drug as they had reported.
These data warrant further investigation in order to evaluate the clinical impact in terms of risk of tubular toxicity. Apart from the implicit opportunity to switch tenofovir and/or the companion drug (to an antiretroviral associated with lower TDF PK exposure), the identification of patients with increased tenofovir exposure should drive attention toward the possibility of reducing the TDF dose or the frequency of administration. This dose reduction (one pill every other day) has already been suggested in patients with significant renal impairment and could be justified by the long intracellular half-life of tenofovir diphosphate. As an alternative (or complementary) approach, the use of probenecid might also be considered, since in a small case series, the drug was found to actually decrease the development of tenofovir-associated tubular damage in patients with hepatitis B virus infection. This effect is likely to be attributable to a significant reduction of TDF uptake by proximal tubular renal cells due to probenecid interference with apical organic anion transporters (OATs), although the data in this report cannot support such a strategy (19).
Considering the prior demonstration of a dose-concentration relationship between tenofovir and renal toxicity, these data confirm on real-life clinical grounds that TDF pharmacokinetics also depends on the antiretrovirals being administered concurrently. As a consequence, based on this knowledge, different strategies to reduce the impact of TDF on renal function can be suggested and warrant proper clinical investigation.
ACKNOWLEDGMENTS
A.C. received travel grants and speaker honoraria from Abbott, Janssen-Cilag, Bristol-Meyer-Squibb, ViiV, and Merck Sharp & Dome. S.B. and G.D.P, received travel grants and speaker's honoraria from Gilead, Abbott, Janssen-Cilag, Bristol-Meyer-Squibb, ViiV, and Merck Sharp & Dome. All other authors declare no potential conflict of interest. This study was funded internally.
Footnotes
Published ahead of print 4 February 2013
REFERENCES
- 1. Hall AM, Hendry BM, Nitsch D, Connolly JO. 2011. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am. J. Kidney Dis. 57:773–780 [DOI] [PubMed] [Google Scholar]
- 2. Woodward CLN, Hall AM, Williams IG, Madge S, Copas A, Nair D, Edwards SG, Johnson MA, Connolly JO. 2009. Tenofovir-associated renal and bone toxicity. HIV Med. 10:482–487 [DOI] [PubMed] [Google Scholar]
- 3. Mocroft A, Kirk O, Reiss P, De Wit S, Sedlacek D, Beniowski M, Gatell J, Phillips AN, Ledergerber B, Lundgren JD. 2010. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. EuroSIDA Study Group. AIDS 24:1667–1678 [DOI] [PubMed] [Google Scholar]
- 4. Rodríguez-Nóvoa S, Labarga P, D'avolio A, Barreiro P, Albalate M, Vispo E, Solera C, Siccardi M, Bonora S, Di Perri G, Soriano V. 2010. Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. AIDS 24:1064–1066 [DOI] [PubMed] [Google Scholar]
- 5. Poizot-Martin I, Solas C, Allemand J, Obry-Roguet V, Pradel V, Bregigeon S, Faucher O, Lacarelle B. 28 November2012. Renal impairment in patients receiving a tenofovir-cART regimen: impact of tenofovir trough concentration. J. Acquir. Immune Defic. Syndr. [Epub ahead of print.] doi:10.1097/QAI.0b013e31827ce4ee [DOI] [PubMed] [Google Scholar]
- 6. Ezinga M, Wetzels J, van der Ven A, Burger D. 2012. Kidney tubular dysfunction is related to tenofovir plasma concentration, poster 603. 19th Conf. Retrovir. Oppor. Infect., Seattle, WA [Google Scholar]
- 7. Avihingsanon A, Praditpornsilpa K, Ramautarsing R, Wongsabut J, Clarke A, Mek-Anantawat W, Ubolyam S, Avihingsanon Y, Hiransuthikul N, Ruxrungtham K. 2012. Increased risk of subclinical kidney tubular abnormalities in HIV+ individuals on long-term ART: Asian Cohort perspective, poster 871. 19th Conf. Retrovir. Oppor. Infect., Seattle, WA [Google Scholar]
- 8. Anderson PL. 2008. Recent developments in the clinical pharmacology of anti-HIV nucleoside analogs. Curr. Opin. HIV AIDS 3:258–265 [DOI] [PubMed] [Google Scholar]
- 9. Calcagno A, Gonzalez de Requena D, Simiele M, D'Avolio A, Tettoni MC, Salassa B, Orofino G, Libanore V, Di Perri G, Bonora S. 2012. Determinants of tenofovir plasma trough concentrations: a cross-sectional analysis in the clinical setting, poster 604. 18th Conf. Retrovir. Oppor. Infect., Seattle, WA [Google Scholar]
- 10. D'Avolio A, Sciandra M, Siccardi M, Baietto L, Gonzalez de Requena D, Bonora S, Di Perri G. 2008. A new assay based on solid-phase extraction procedure with LC-MS to measure plasmatic concentrations of tenofovir and emtricitabine in HIV infected patients. J. Chromatogr. Sci. 46:524–528 [DOI] [PubMed] [Google Scholar]
- 11. Kiser JJ, Carten ML, Aquilante CL, Anderson PL, Wolfe P, King TM, Delahunty T, Bushman LR, Fletcher CV. 2008. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin. Pharmacol. Ther. 83:265–272 [DOI] [PubMed] [Google Scholar]
- 12. Jullien V, Tréluyer J, Rey E, Jaffray P, Krivine A, Moachon L, Lillo-Le Louet A, Lescoat A, Dupin N, Salmon D, Pons G, Urien S. 2005. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrob. Agents Chemother. 49:3361–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cevik M, Dickinson L, Ahmed A, Jones R, Back D, Ward B, Asboe D, Pozniak A, Boffito M. 2011. Tenofovir (TFV) pharmacokinetics (PK) in HIV infected individuals over 40 years of age, abstr PS6/1. 13th Eur. AIDS Clin. Soc. Conf., Belgrade, Serbia [Google Scholar]
- 14. Ryom L, the D:A:D Study Group 2012. Exposure to ARV and the risk of renal impairment among HIV+ persons with normal baseline renal function: the D:A:D study, poster 865. 19th Conf. Retrovir. Oppor. Infect., Seattle, WA [Google Scholar]
- 15. Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, Witt M, Diamond C, Haubrich R, Louie S. 2008. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. California Collaborative Treatment Group 578 Team. J. Infect. Dis. 197:102–108 [DOI] [PubMed] [Google Scholar]
- 16. Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG. 2012. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 26:867–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young J, Schäfer J, Fux CA, Furrer H, Bernasconi E, Vernazza P, Calmy A, Cavassini M, Weber R, Battegay M, Bucher HC, the Swiss HIV Cohort Study 2012. Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. AIDS 26:567–575 [DOI] [PubMed] [Google Scholar]
- 18. Kaul S, Bassi K, Damle B, Xie J, Gale J, Kearney B, Hanna G. 2003. Pharmacokinetic (PK) evaluation of the combination of atazanavir (ATV), enteric coated didanosine (ddI-EC), and tenofovir disoproxil fumarate (TDF) for a once-daily antiretroviral regimen, abstr A-1616. 43rd Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 19. Izzedine H, Thibault V, Valantin MA, Peytavin G, Schneider L, Benhamou Y. 2010. Tenofovir/probenecid combination in HIV/HBV-coinfected patients: how to escape Fanconi syndrome recurrence. AIDS 24:1078–1079 [DOI] [PubMed] [Google Scholar]