Abstract
Fusobacterium nucleatum is one of the most common anaerobic bacteria in periodontitis and is responsible for several extraoral infections, including respiratory tract diseases. In this study, we examined whether F. nucleatum induces mucin secretion in airway epithelial cells. We also examined the effects of macrolides on F. nucleatum-induced mucus production compared with the effects of other antibiotics that exert anti-anaerobic activities. The production of MUC5AC, the major core protein of mucin secreted from the airway surface epithelium, in bronchial epithelial cells after stimulation with culture supernatants (Sup) of F. nucleatum was analyzed by performing enzyme-linked immunosorbent assay and quantitative RT-PCR. The cell-signaling pathway of F. nucleatum Sup stimulation was also analyzed by Western blotting. For inhibition studies, cells were treated with azithromycin, clarithromycin, clindamycin (CLDM), and metronidazole (MTZ). The F. nucleatum Sup induced NCI-H292 cells to express MUC5AC at both the protein level and the mRNA level in both a time- and dose-dependent manner. Macrolides inhibited F. nucleatum Sup-induced MUC5AC production, while CLDM and MTZ were less effective. F. nucleatum Sup induced the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2), and this induction was suppressed by macrolides. F. nucleatum Sup-induced MUC5AC production was blocked by the ERK pathway inhibitor U0126. F. nucleatum is likely to contribute to excessive mucin production, which suggests that periodontitis may correlate with the pathogenesis of chronic respiratory tract infection. Macrolides seem to reduce this mucin production and might represent an additional means of therapeutic intervention for F. nucleatum respiratory tract infections other than CLDM and MTZ.
INTRODUCTION
Although mucus secretion is useful for host protection against pathogens and irritants, mucus hypersecretion causes airway obstruction and impairment of gas exchange in chronic inflammatory lung diseases, including asthma, cystic fibrosis, diffuse panbronchiolitis (DPB), and chronic obstructive pulmonary disease (COPD). Therefore, preventing mucus overproduction is beneficial for individuals with these diseases. Many factors, including bacterial infection, that contribute to mucus hypersecretion have been described (1, 2). However, there are few studies that focus on the relationship between oral bacterium infection and mucus hypersecretion.
Poor oral hygiene has been suggested to be a risk factor for respiratory disease (3), and several studies indicate that oral care reduces the incidence and mortality of pneumonia (4–7). However, the detailed mechanisms of the relationship between poor oral hygiene and respiratory tract disease are not fully understood.
Fusobacterium nucleatum is a common anaerobic bacterium of periodontitis, which is also found as etiologic pathogen of respiratory anaerobic infection (8). As the important virulence factor, F. nucleatum produces large amounts of butyric acid during anaerobic glycolysis. Recently, several reports indicated that butyric acid plays a critical role in a variety of diseases, including HIV infection (9, 10) and ulcerative colitis (11). The aspiration of products originating from periodontal tissues has been suggested as a possible mechanism of the effects of oral bacteria on the respiratory tract (12). With respect to the frequency of aspiration, Marik and Kaplan reported that approximately half of all healthy adults aspirate small amounts of oropharyngeal secretions during sleep (13). On the basis of these reports, we expect that F. nucleatum, a major periodontal bacterium, might have a pathogenic effect on airway epithelium cells via aspiration of its products.
In this study, we examined the effects of F. nucleatum culture supernatant (Sup) on airway epithelium cell mucus secretion. The major macromolecular constituents of mucus are the mucin glycoproteins. Among mucin proteins, we focused on MUC5AC, the major core protein of mucin secreted from the airway surface epithelium.
We also examined the effects of the macrolides azithromycin (AZM) and clarithromycin (CAM) on the F. nucleatum Sup-induced mucus production and compared their effects to those of other antibiotics which have anti-anaerobic activities (e.g., clindamycin [CLDM] and metronidazole [MTZ]). Macrolide antibiotics have been shown to be effective for the treatment of chronic airway diseases (14, 15). The beneficial effects of macrolide therapy not only are related to its bactericidal properties but also extend to its immune-modulating and anti-inflammatory effects (16). We previously reported that macrolides inhibit MUC5AC production induced by several factors (Pseudomonas aeruginosa autoinducer [17], lipopolysaccharide [18], nontypeable Haemophilus influenzae [19], and Chlamydophila pneumoniae [20]) in human lung epithelial cells and found that mucin reduction by macrolides is related to several intracellular signal transduction, including extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation (17, 18), NF-κB activation (17, 20), or AP-1 activation (19). As macrolides have no bactericidal activities against F. nucleatum, the effect of macrolides on mucin production compared with that of CLDM and MTZ would provide insight concerning the treatment of F. nucleatum respiratory tract infections.
The aims of this study were to determine whether F. nucleatum Sup exerts a stimulatory effect on the production of MUC5AC and to clarify whether macrolides have a different effect on F. nucleatum Sup-induced MUC5AC production than CLDM and MTZ.
MATERIALS AND METHODS
F. nucleatum strain and culture conditions to obtain F. nucleatum Sup.
A clinical isolate of F. nucleatum (strain FNU-191), maintained as a stock culture in the Department of Laboratory Medicine, Nagasaki University Hospital, Nagasaki, Japan, was used in this study. We identified the strain by PCR amplification and sequencing analysis of the 16S rRNA gene. The supernatant was obtained as described previously (10). Briefly, the F. nucleatum strain was cultured on PV Brucella HK agar (Kyokuto Pharmaceutical Industrial Co., Tokyo, Japan) for 48 h under anaerobic conditions, scraped and suspended in modified GAM (Gifu anaerobic medium) broth (Nissui Pharmaceutical Industrial Co., Tokyo, Japan), and cultured in an anaerobic chamber for 48 h. The supernatant was then collected by centrifugation at 10,000 rpm for 50 min at 4°C to remove the bacteria and filter sterilized through a 0.22-μm-pore-size membrane filter (Millipore, Bedford. MA). In the preliminary experiments, we examined MUC5AC induction of 6 Fusobacterium strains, including the reference strain (ATCC 10953). We found that all the supernatants of the Fusobacterium strain similarly induced MUC5AC production at a 1:39 to 1:79 dilution and inhibited MUC5AC production at a 1:4 to 1:19 dilution. In order to test for clinical relevancy, we selected the clinically isolated strain of F. nucleatum for the further experiments. Therefore, all the experiments in this study were performed using the FNU-191 strain.
Cell culture.
The NCI-H292 (human airway epithelial) cell line was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The cells were grown at 37°C with 5% CO2 in fully humidified air. For the MUC5AC production studies, cells were exposed to F. nucleatum Sup for reverse transcriptase PCR (RT-PCR), enzyme-linked immunosorbent assay (ELISA), or Western blotting. For controls, the cells were incubated with GAM broth.
Preparations of antibiotic dilutions.
AZM and CAM were provided by Pfizer (Tokyo, Japan) and Taisho-Toyama (Tokyo, Japan), respectively. Clindamycin and metronidazole were obtained from Nacalai Tesque (Kyoto, Japan). Each drug, except MTZ, was diluted in dimethyl sulfoxide (DMSO) at final concentrations of 1 to 100 μg/ml for the following experiments. Only MTZ was diluted in acetic acid.
ELISA.
The NCI-H292 cells were plated in a 24-well plate, and MUC5AC protein was measured by performing ELISA as previously described (17–20). After F. nucleatum Sup stimulation, the culture medium was collected as the cell supernatant. This supernatant was then incubated at 40°C in a 96-well plate until dry. The plated cultures were blocked with 2% bovine serum albumin for 1 h at room temperature and were then incubated with the anti-MUC5AC antibody diluted in phosphate-buffered saline (PBS) containing 0.05% Tween 20 for 1 h. Horseradish peroxidase (HRP)-conjugated anti-goat IgG was then dispensed into each well. After 1 h, the plates were washed three times with PBS. Color was developed using a 3,3′,5,5′-tetramethylbenzene peroxidase solution, and the reaction was stopped by the addition of 2 N H2SO4. Absorbance was read at 450 nm.
Inhibition of cell signaling activity.
The ERK inhibitor U0126, the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580, and the specific NF-κB inhibitor caffeic acid phenethyl ester (CAPE) were used at concentrations of 10 μM (in DMSO stock solution). Cells were treated with these inhibitors 30 min before F. nucleatum Sup stimulation. Control cultures were treated with an equal volume of DMSO. All the inhibitors were purchased from Calbiochem (San Diego, CA).
RT-PCR.
We evaluated MUC5AC mRNA expression by RT-PCR as described previously (17–20). Total RNA was extracted from NCI-H292 cells cultured in 6-well plates using QuickGene-Mini80 and QuickGene RNA cultured-cell kits (Fujifilm Co., Tokyo, Japan), according to the manufacturer's instructions. Total RNA (1 μg) was reverse transcribed into cDNA using oligo(dT) primers and SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) and was then treated with RNase H. To quantify the expression of the MUC5AC gene, PCR primers and TaqMan probes were designed and used as reported previously (forward primer, 5′-CAGCCACGTCCCCTTCAATA-3′; reverse primer, 5′-ACCGCATTTGGGCATCC-3′; TaqMan probe, 5′ 6-carboxyfluorescein [FAM]-CCACCTCCGAGCCCGTCACTGAG-TAMRA [6-carboxytetramethylrhodamine] 3′) (21). MUC5AC was amplified for 40 cycles (15 s at 95°C and 30 s at 60°C) using a LightCycler system. To normalize MUC5AC expression, human porphobilinogen deaminase (hPBGD) was also measured using an hPBGD primer set (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. Data are presented as the ratio of hPBGD to MUC5AC gene.
Western blot analysis.
Proteins were separated by performing reducing sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ) in a solution of 20% methanol, 25 mM Tris-HCl, 0.2 M glycine. Nonspecific binding was blocked by incubating the membranes with 10% fetal bovine serum in Tris-buffered saline with 0.1% Tween 20 for 1 h at room temperature. Immunoreactive proteins were detected by incubating the membrane with rabbit anti-human ERK1/2, anti-phospho-ERK1/2, anti-human p38, anti-phospho-p38, anti-human I-κB, or anti-phospho-I-κB antibody (each at 1:1,000) overnight at 4°C. Between steps, the membrane were washed three times for 15 min each with Tris-buffered saline that contained 0.1% Tween 20. Subsequently, the membranes were incubated for 1 h with anti-rabbit immunoglobulin G conjugated to HRP (1:10,000), rewashed, and developed with enhanced chemiluminescence reagents (Amersham Pharmacia Biotech).
Statistical analysis.
All data were expressed as means and standard errors of the means (SEM). Differences were examined for statistical significance by using the one-way analysis of variance for comparisons involving more than two groups and the Student t test for comparisons between two groups. P values less than 0.05 were considered statistically significant.
RESULTS
F. nucleatum Sup upregulates MUC5AC gene and protein expression.
To determine whether F. nucleatum Sup can induce mucin production in NCI-H292 cells, we evaluated MUC5AC expression at both the mRNA and the protein level after the addition of F. nucleatum Sup. Stimulation of the NCI-H292 cells with GAM broth (1:9 dilution) had a small effect on MUC5AC production compared to stimulation with RPMI medium alone. However, the amounts of MUC5AC were significantly larger in F. nucleatum Sup-stimulated cultures at the 1:79 to 1:319 dilution than in those with GAM broth stimulation. The protein level (Fig. 1A) and mRNA expression (Fig. 1B) were maximal at the 1:79 dilution. The upregulation of MUC5AC by the addition of F. nucleatum Sup at a 1:79 dilution occurred in a time-dependent manner, and the protein level (Fig. 2A) was maximal at 24 h after stimulation. The mRNA expression level (Fig. 2B) increased until 12 h and decreased at 18 h after stimulation. The maximal mRNA expression was obtained at 10 h after stimulation (data not shown); thus, we analyzed all other experiments concerning MUC5AC mRNA at 10 h after stimulation.
Fig 1.
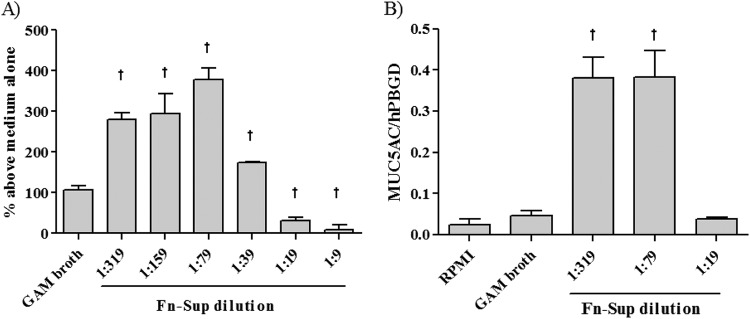
Dose-dependent effect of F. nucleatum culture supernatant (Fn-Sup) on MUC5AC expression. Confluent NCI-H292 cells were stimulated using modified GAM medium (1:9 dilution) or various concentrations of F. nucleatum Sup (dilution ratio, 1:319 to 1:9). (A) MUC5AC protein was measured by performing enzyme-linked immunosorbent assay (ELISA) at 24 h after the addition of F. nucleatum Sup (n = 3). (B) The mRNA level of MUC5AC expression at 10 h after the addition of F. nucleatum Sup was analyzed by RT-PCR (n = 3). Data are expressed as means and SEM from three experiments. Daggers indicate P values of <0.01 for comparisons with the value obtained with modified GAM stimulation.
Fig 2.
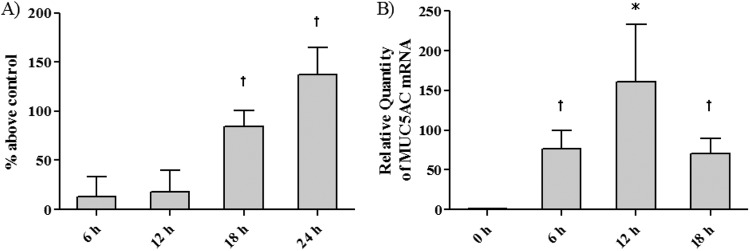
Time-dependent effect of F. nucleatum culture supernatant on MUC5AC synthesis. NCI-H292 cells were stimulated with modified GAM medium (1:64 dilution) or F. nucleatum Sup (dilution ratio,1:64). (A) MUC5AC protein was measured by an enzyme-linked immunosorbent assay (ELISA) (n = 4). (B) The mRNA level of MUC5AC expression after the addition of F. nucleatum Sup was analyzed by RT-PCR (n = 3). Data are means and SEM. An asterisk and a dagger indicate P values of <0.05 and <0.01, respectively, for comparisons with the 0-h data.
F. nucleatum Sup phosphorylates ERK.
MAPKs are important signals related to MUC5AC production. To examine the cell-signaling pathway of F. nucleatum Sup stimulation in NCI-H292 cells, we examined the phosphorylation of kinase by Western blotting (Fig. 3). We analyzed kinase phosphorylation in both GAM broth- and F. nucleatum Sup-activated cells at 0 to 720 min after stimulation. Compared to GAM broth (Fig. 3A), maximal ERK phosphorylation of F. nucleatum Sup-activated cells was observed 240 min after stimulation (Fig. 3B), while ERK phosphorylation of GAM broth-activated cells was observed mainly 480 min after stimulation. We also performed an inhibition assay of the cell-signaling pathway (Fig. 4). The ERK inhibitor U0126 effectively suppressed MUC5AC protein production compared to no treatment. Although the NF-κB inhibitor CAPE and the p38 MAP kinase inhibitor SB203580 also suppressed MUC5AC production, phosphorylation of IκB or p38 was not detected by Western blotting.
Fig 3.
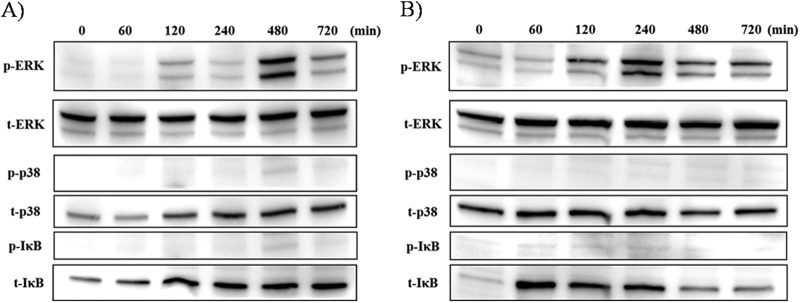
Time-dependent phosphorylation of ERK 1/2, p38, and IκB after modified GAM broth stimulation (control) (A) and F. nucleatum culture supernatant stimulation (B). Cells were treated with control and with F. nucleatum Sup for each time and evaluated by Western blotting. ERK1/2 phosphorylation was induced 120 min after stimulation with F. nucleatum Sup and reached it maximum at 240 min after stimulation. The ERK1/2 phosphorylation induced by the control was maximal at 480 min after stimulation. p38 and IκB phosphorylation was not evident in both stimulation. Data are representative of three separate experiments.
Fig 4.
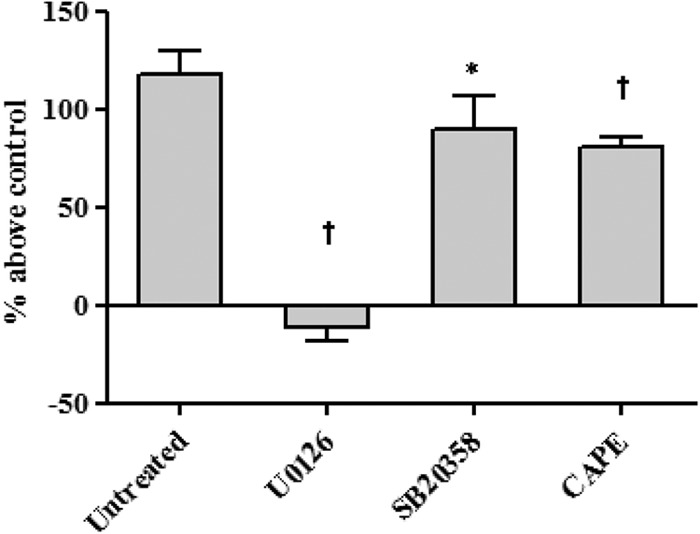
Effect of MAP kinase inhibitor on MUC5AC production in cells activated by F. nucleatum culture supernatant. Cells were pretreated with U0126 (ERK), SB203580 (p38 MAP kinase), and CAPE (NF-κB) 30 min before F. nucleatum Sup stimulation. All the inhibitors effectively suppressed MUC5AC protein production compared with F. nucleatum Sup stimulation alone. Data are means and SEM from four experiments. An asterisk and a dagger indicate P values of <0.05 and <0.01, respectively, for comparisons with the control (modified GAM stimulation).
Macrolides inhibit MUC5AC production by F. nucleatum Sup-activated cells.
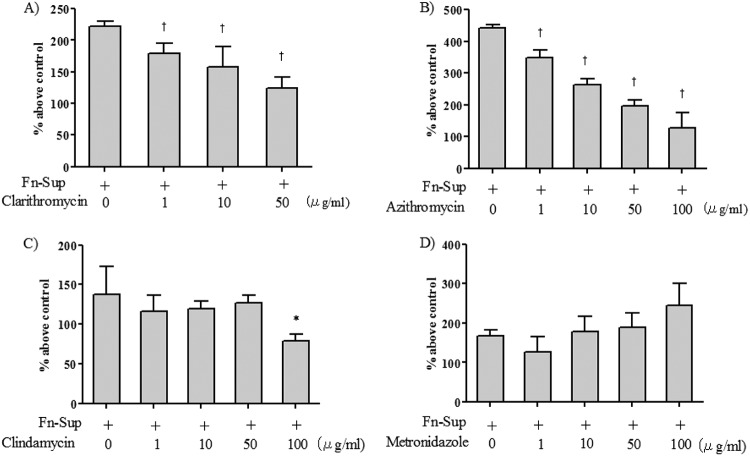
To evaluate the effect of the macrolides CLDM and MTZ on F. nucleatum Sup-induced MUC5AC production, we treated cells with a 1- to 100-μg/ml concentration of each drug. Since CAM could not be dissolved at 100 μg/ml, we examined its effects at a 1- to 50-μg/ml concentration. As shown in Fig. 5A and B, the macrolides significantly reduced MUC5AC protein level at the 1- to 100-μg/ml concentrations in a dose-dependent manner. CLDM significantly reduced MUC5AC protein levels at the 100-μg/ml concentration, and MTZ did not reduce MUC5AC protein at any concentration. At the maximum dosage of each drug, we also examined the effect on MUC5AC mRNA expression. For controls and untreated groups, the cells were also stimulated with amount of DMSO or acetic acid contained in the drug dilutions. Since only MTZ needed to be dissolved with acetic acid, the evaluation of MTZ was examined separately.
Fig 5.
Effects of azithromycin (AZM), clarithromycin (CAM), clindamycin (CLDM), and metronidazole (MTZ) on MUC5AC production induced by F. nucleatum culture supernatant (Fn-Sup). Cells were treated with 1 to 100 μg/ml of each drug (CAM was used at 1 to 50 μg/ml, as the maximal dose of CAM that could be diluted in DMSO was 50 μg/ml). CAM and AZM dose-dependently suppressed F. nucleatum Sup-induced MUC5AC production. CLDM significantly suppressed F. nucleatum Sup-induced MUC5AC production only at 100 μg/ml, while MTZ resulted in no reduction of MUC5AC at any concentration. Data are means and SEM from four experiments. An asterisk and a dagger indicate P values of <0.05 and <0.01, respectively, for comparisons with data from F. nucleatum Sup stimulation alone.
As shown in Fig. 6, the macrolides significantly reduced the mRNA expression level of MUC5AC, while no significant reduction was found with CLDM and MTZ.
Fig 6.
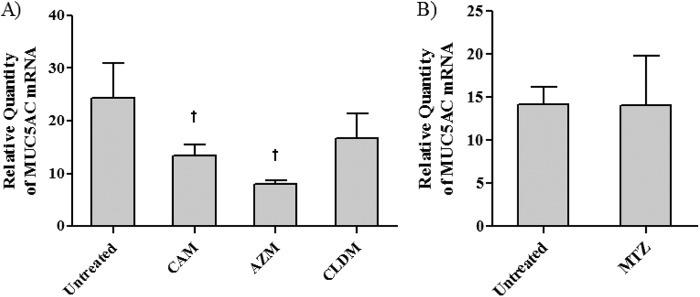
Effects of azithromycin (AZM), clarithromycin (CAM), clindamycin (CLDM), and metronidazole (MTZ) on MUC5AC mRNA expression induced by F. nucleatum culture supernatant. Cells were treated with 100 μg/ml of each drug (CAM was at 50 μg/ml). CAM and AZM significantly suppressed F. nucleatum Sup-induced MUC5AC mRNA expression. Data are means and SEM from five experiments (n = 3 for the control). An asterisk and a dagger indicate P values of <0.05 and <0.01, respectively, for comparisons with data from F. nucleatum Sup stimulation alone.
Macrolides downregulate the phosphorylation of ERK in F. nucleatum Sup-activated NCI-H292 cells.
In order to investigate a potential role of the F. nucleatum Sup-activated cell-signaling pathway of the macrolides CLDM and MTZ, we examined the phosphorylation of ERK, the most significantly upregulated signaling pathway during F. nucleatum Sup-induced activation. As shown in Fig. 7A, the macrolides suppressed the phosphorylation of ERK compared to F. nucleatum Sup alone and with CLDM. MTZ did not affect the detection level compared to stimulation alone (Fig. 7B).
Fig 7.
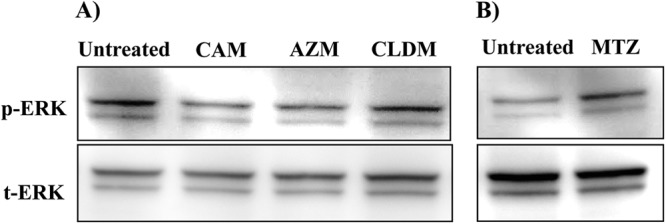
Effects of the macrolides clindamycin (A) and metronidazole (B) on ERK phosphorylation. Cells were stimulated with F. nucleatum culture supernatant concurrently with each drug at the maximal concentration (50 μg/ml for CAM, 100 μg/ml for AZM, CLDM, and MTZ) and evaluated 360 min after stimulation. Equal amounts of protein were analyzed. Macrolides inhibited the levels of phosphorylation of ERK compared to stimulation alone. Data are representative of three separate experiments.
DISCUSSION
The present study is the first to demonstrate that the product of F. nucleatum induces MUC5AC via phosphorylation of ERK1/2. We also found that macrolides inhibited MUCAC production induced by the products of F. nucleatum, while CLDM and MTZ were less effective.
F. nucleatum is a Gram-negative anaerobic species of the phylum Fusobacteria that is numerically dominant in dental plaque biofilms and important in biofilm ecology and human infectious diseases (8). F. nucleatum is one of the most common oral species isolated from extraoral infections of the blood, brain, chest, lung, liver, joints, and abdomen, from obstetrical and gynecological infections, and from abscesses. In addition, the products of F. nucleatum have been recently reported to reactivate HIV-1 in latent infections (10). Among the components of its supernatant, butyric acid is thought to inhibit the catalytic action of histone deacetylases and induce transcription of silenced genes, including the HIV-1 provirus (9). Interestingly, butyric acid in Fusobacterium species has been reported to be involved in the pathogenesis of ulcerative colitis by inducing cell toxicity (11). Considering these discoveries, F. nucleatum is an increasingly significant pathogen with the potential to have a societal impact on human infections. However, there are few descriptive data of F. nucleatum concerning its relationship with respiratory tract diseases.
In the present study, we demonstrated that the products of F. nucleatum have additive effects on mucin production in airway epithelial cells. Interestingly, high concentrations of F. nucleatum Sup inhibited MUC5AC production, while relatively low concentrations of F. nucleatum Sup increased MUC5AC production. This suggests that aspiration of saliva containing even low concentrations of F. nucleatum products may cause hypersecretion in the associated disease. The reason why high concentrations of F. nucleatum Sup inhibit MUC5AC production is not clear; however, as low concentrations of F. nucleatum products may be found more frequently in oral contents, F. nucleatum may play a negative role in the pathogenesis of chronic respiratory tract infections via aspiration of its products.
In this study, we also demonstrated that macrolides reduce MUC5AC production induced by F. nucleatum Sup. Long-term treatment with macrolide antibiotics is considered to be effective in DPB and CF due to their anti-inflammatory effects rather than antimicrobial effects (14, 15). In addition, a multicenter, double-blind, randomized clinical trial conducted in Greece showed that intravenous CAM administration for 3 consecutive days improves the length of illness and mortality of VAP (22), which indicate the macrolide might also be beneficial for acute infections with short-term treatment. However, although CLDM and MTZ are both known to reduce cytokine production induced by certain bacterial component or products (23–25), the existence of anti-inflammatory effects of CLDM and MTZ, similar to those of macrolides, remains uncertain. In the present study, CLDM and MTZ did not exhibit a concentration-dependent reduction of F. nucleatum Sup-induced MUC5AC production compared to macrolides. To investigate the reason for this discrepancy in the effects of CLDM and MTZ against F. nucleatum Sup-induced MUC5AC production, we examined the MAPK signal transduction pathway. Among a variety of signal transduction molecules, MAPK has been shown to play an important role in mucin production (26). In this study, F. nucleatum Sup induced the phosphorylation of ERK1/2. Enhanced MUC5AC protein production was also strongly reduced by an inhibitor of MEK (U0126). This result indicates that F. nucleatum Sup mainly upregulates MU5AC production through MAPK transduction. However, AZM and CAM inhibited phosphorylation of ERK1/2 induced by F. nucleatum Sup, while CLDM and MTZ did not. Together, these data indicate that macrolides are effective in preventing MU5AC production by mechanisms different from those of CLDM or MTZ. Thus, stimulation with F. nucleatum Sup would be affected by AZM and CAM upstream of ERK1/2.
The main limitation of our study is that modified GAM broth also had a positive effect on MUC5AC production and ERK1/2 upregulation. However, the effect of GAM broth alone on MUC5AC production was significantly small compared to F. nucleatum Sup stimulation. ERK1/2 phosphorylation showed a unique pattern independent of F. nucleatum Sup treatment. Although F. nucleatum Sup induced phosphorylation of ERK1/2 at 4 h after stimulation, GAM broth did so at 8 h. Although we were able to verify the potential of F. nucleatum for mucin production in this study, further study is needed to identify a more detailed mechanism of F. nucleatum-induced MUC5AC production that focuses on particular components of F. nucleatum products, such as butyric acid.
Our results provide novel evidence that F. nucleatum may induce mucus hypersecretion, which suggests that periodontitis may have a connection to the pathogenesis of chronic respiratory tract infection. Our study also shows that macrolides reduce this mucin production and may act as an additional therapeutic intervention distinct from CLDM and MTZ.
ACKNOWLEDGMENTS
This research was funded by the Department of Laboratory Medicine and Second Department of Internal Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan. This study was not sponsored by any grants, gifts, or fellowships.
All authors contributed to the design, data collection, analysis, preparation, and critical revision of the manuscript.
We have no potential conflicts of interest with any companies or organizations whose products or services are discussed in this article.
Footnotes
Published ahead of print 4 February 2013
REFERENCES
- 1. Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. 1998. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta 1406:251–259 [DOI] [PubMed] [Google Scholar]
- 2. Shimizu T, Shimizu S, Hattori R, Gabazza EC, Majima Y. 2003. In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. Am. J. Respir. Crit. Care Med. 168:581–587 [DOI] [PubMed] [Google Scholar]
- 3. Sjögren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. 2008. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J. Am. Geriatr. Soc. 56:2124–2130 [DOI] [PubMed] [Google Scholar]
- 4. Adachi M, Ishihara K, Abe S, Okuda K, Ishikawa T. 2002. Effect of professional oral health care on the elderly living in nursing homes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 94:191–195 [DOI] [PubMed] [Google Scholar]
- 5. DeRiso AJ, II, Ladowski JS, Dillon TA, Justice JW, Peterson AC. 1996. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 109:1556–1561 [DOI] [PubMed] [Google Scholar]
- 6. Yoneyama T, Yoshida M, Matsui T, Sasaki H. 1999. Oral care and pneumonia. Lancet 354:515. [DOI] [PubMed] [Google Scholar]
- 7. Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, Ihara S, Yanagisawa S, Ariumi S, Morita T, Mizuno Y, Ohsawa T, Akagawa Y, Hashimoto K, Sasaki H; Oral Care Working Group. 2002. Oral care reduces pneumonia in older patients in nursing homes. J. Am. Geriatr. Soc. 50:430–433 [DOI] [PubMed] [Google Scholar]
- 8. Signat B, Roques C, Poulet P, Duffaut D. 2011. Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 13:25–36 [PubMed] [Google Scholar]
- 9. Imai K, Ochiai K. 2011. Role of histone modification on transcriptional regulation and HIV-1 gene expression: possible mechanisms of periodontal diseases in AIDS progression. J. Oral Sci. 53:1–13 [DOI] [PubMed] [Google Scholar]
- 10. Imai K, Yamada K, Tamura M, Ochiai K, Okamoto T. 2012. Reactivation of latent HIV-1 by a wide variety of butyric acid-producing bacteria. Cell. Mol. Life Sci. 69:2583–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. 2003. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut 52:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azarpazhooh A, Leake JL. 2006. Systematic review of the association between respiratory diseases and oral health. J. Periodontol. 77:1465–1482 [DOI] [PubMed] [Google Scholar]
- 13. Marik PE, Kaplan D. 2003. Aspiration pneumonia and dysphagia in the elderly. Chest 124:328–336 [DOI] [PubMed] [Google Scholar]
- 14. Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. 1998. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157:1829–1832 [DOI] [PubMed] [Google Scholar]
- 15. Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW., III 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749–1756 [DOI] [PubMed] [Google Scholar]
- 16. Giamarellos-Bourboulis EJ. 2008. Immunomodulatory therapies for sepsis: unexpected effects with macrolides. Int. J. Antimicrob. Agents 32(Suppl 1):39–43 [DOI] [PubMed] [Google Scholar]
- 17. Imamura Y, Yanagihara K, Mizuta Y, Seki M, Ohno H, Higashiyama Y, Miyazaki Y, Tsukamoto K, Hirakata Y, Tomono K, Kadota J, Kohno S. 2004. Azithromycin inhibits MUC5AC production induced by the Pseudomonas aeruginosa autoinducer N-(3-oxododecanoyl) homoserine lactone in NCI-H292 cells. Antimicrob. Agents Chemother. 48:3457–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishimoto H, Mukae H, Sakamoto N, Amenomori M, Kitazaki T, Imamura Y, Fujita H, Ishii H, Nakayama S, Yanagihara K, Kohno S. 2009. Different effects of telithromycin on MUC5AC production induced by human neutrophil peptide-1 or lipopolysaccharide in NCI-H292 cells compared with azithromycin and clarithromycin. J. Antimicrob. Chemother. 63:109–114 [DOI] [PubMed] [Google Scholar]
- 19. Araki N, Yanagihara K, Morinaga Y, Yamada K, Nakamura S, Yamada Y, Kohno S, Kamihira S. 2010. Azithromycin inhibits nontypeable Haemophilus influenzae-induced MUC5AC expression and secretion via inhibition of activator protein-1 in human airway epithelial cells. Eur. J. Pharmacol. 644:209–214 [DOI] [PubMed] [Google Scholar]
- 20. Morinaga Y, Yanagihara K, Miyashita N, Seki M, Izumikawa K, Kakeya H, Yamamoto Y, Mukae H, Yamada Y, Kohno S, Kamihira S. 2009. Azithromycin, clarithromycin and telithromycin inhibit MUC5AC induction by Chlamydophila pneumoniae in airway epithelial cells. Pulm. Pharmacol. Ther. 22:580–586 [DOI] [PubMed] [Google Scholar]
- 21. Inoue D, Yamaya M, Kubo H, Sasaki T, Hosoda M, Numasaki M, Tomioka Y, Yasuda H, Sekizawa K, Nishimura H, Sasaki H. 2006. Mechanisms of mucin production by rhinovirus infection in cultured human airway epithelial cells. Respir. Physiol. Neurobiol. 154:484–499 [DOI] [PubMed] [Google Scholar]
- 22. Giamarellos-Bourboulis EJ, Pechère JC, Routsi C, Plachouras D, Kollias S, Raftogiannis M, Zervakis D, Baziaka F, Koronaios A, Antonopoulou A, Markaki V, Koutoukas P, Papadomichelakis E, Tsaganos T, Armaganidis A, Koussoulas V, Kotanidou A, Roussos C, Giamarellou H. 2008. Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin. Infect. Dis. 46:1157–1164 [DOI] [PubMed] [Google Scholar]
- 23. Lappin E, Ferguson AJ. 2009. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 9:281–290 [DOI] [PubMed] [Google Scholar]
- 24. Pichereau S, Moran JJ, Hayney MS, Shukla SK, Sakoulas G, Rose WE. 2012. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J. Antimicrob. Chemother. 67:123–129 [DOI] [PubMed] [Google Scholar]
- 25. Rizzo A, Paolillo R, Guida L, Annunziata M, Bevilacqua N, Tufano MA. 2010. Effect of metronidazole and modulation of cytokine production on human periodontal ligament cells. Int. Immunopharmacol. 10:744–750 [DOI] [PubMed] [Google Scholar]
- 26. Selsted ME, Ouellette AJ. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551–557 [DOI] [PubMed] [Google Scholar]