Abstract
The continuing increase in antibiotic-resistant microorganisms is driving the search for new antibiotic targets and improved antimicrobial agents. Ketolides are semisynthetic derivatives of macrolide antibiotics, which are effective against certain resistant organisms. Solithromycin (CEM-101) is a novel fluoroketolide with improved antimicrobial effectiveness. This compound binds to the large 50S subunit of the ribosome and inhibits protein biosynthesis. Like other ketolides, it should impair bacterial ribosomal subunit formation. This mechanism of action was examined in strains of Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae. The mean 50% inhibitory concentrations (IC50s) for solithromycin inhibition of cell viability, protein synthesis, and growth rate were 7.5, 40, and 125 ng/ml for Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae, respectively. The net formation of the 50S subunit was reduced in all three organisms, with IC50s similar to those given above. The rates of 50S subunit formation measured by a pulse-chase labeling procedure were reduced by 75% in cells growing at the IC50 of solithromycin. Turnover of 23S rRNA was stimulated by solithromycin as well. Solithromycin was found to be a particularly effective antimicrobial agent, with IC50s comparable to those of telithromycin and significantly better than those of azithromycin and clarithromycin in these three microorganisms.
INTRODUCTION
Antimicrobial drug resistance is developing at an alarming rate worldwide (1). The identification of novel antimicrobial targets is an important research endeavor (2). In addition, the development of resistance is leading to an increase in novel drug development efforts (3). A very significant bacterial cellular target is the ribosome (4), and a number of new antimicrobials are in development to inhibit ribosomal functions in translation (5). The bacterial ribosome consists of a smaller 30S subunit and a larger 50S particle. The large subunit is the target for a number of different antimicrobial agents (6).
Macrolide antibiotics are well-characterized antimicrobials with excellent activity against many Gram-positive microorganisms (4, 6). However, significant resistance against the most commonly used compounds, i.e., erythromycin, clarithromycin, and azithromycin, has developed in many organisms (1). Ketolides are semisynthetic derivatives of macrolide antibiotics which are effective against certain resistant organisms (7). They lack the cladinose sugar of macrolides and contain a bridged 11,12-aryl side chain. Ketolides exhibit significantly tighter binding to the 50S ribosomal subunits of different microorganisms. Both telithromycin and cethromycin have shown improved activity against macrolide-resistant organisms (6). Solithromycin (CEM-101), shown in Fig. S1 in the supplemental material, is a novel fluoroketolide with improved antimicrobial effectiveness (reviewed in reference 5). As a fluoroketolide, its activity against macrolide-resistant organisms is enhanced by the addition of an alkyl-aryl side chain allowing tighter binding to the ribosome, thus overcoming acquired and cross-resistance obtained through posttranscriptional methylation of the 23S rRNA in the 50S ribosomal subunit. In addition, the fluorine at the C-2 position of the 14-membered macrocyclic lactone allows for tighter binding, resulting in lower MICs. Its site of binding to the Escherichia coli 50S subunit has been characterized (8). This interaction inhibits protein biosynthesis and should impair bacterial ribosomal subunit formation, much like with other ketolides (9–11). In this study, the mechanism of action of solithromycin was tested in three important human pathogens, Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae. Inhibitory effects on 50S subunit synthesis rates and stimulation of 23S rRNA turnover were observed in all three microorganisms.
MATERIALS AND METHODS
Bacterial strains and reagents.
Methicillin-susceptible Staphylococcus aureus (MSSA) strain RN1786 was obtained from Richard Novick (New York University), and a clinical isolate of methicillin-resistant S. aureus (MRSA) (strain A1024) was provided by Joyce Sutcliffe (Tetraphase Pharmaceuticals) (12). H. influenzae strain CDC G-79 (13) and S. pneumoniae strain ATCC 49619 (9, 10) were kindly provided by Donald Ferguson of our institution. [3H]uridine (45 mCi/mmol) and [35S]methionine (Tran35S-label; 1.175 Ci/mmol) were purchased from American Radiochemicals (St. Louis, MO) and MP Biomedical (Solon, OH), respectively. Solithromycin was provided by Cempra Pharmaceuticals (Chapel Hill, NC) and was dissolved at 1 mg/ml in acetic acid at a pH of 5.5.
Measurements of cell growth, viability, subunit assembly, and translation rates.
The 4-fold assay to examine growth rate, CFU, protein synthesis rates, and ribosomal subunit formation was conducted as described previously (14). Briefly, S. aureus cells were grown in tryptic soy broth (TSB) at 37°C for a period of two doublings in the presence of solithromycin at five different concentrations. Cell growth in sidearm flasks was monitored using a Klett-Summerson colorimeter. H. influenzae cells were grown in TSB supplemented with hematin (1 μg/ml) and NAD (10 μg/ml). S. pneumoniae cells were grown in 13- by 100-mm screw-cap tubes in TSB supplemented with bovine lipoprotein (MP Biomedical) at a final concentration of 0.4%. Cell viability was measured by colony counting on TSB or blood agar base (BAB) plates after serial dilution in 1× A salts (14).
RNA was labeled by incubating cells with 1 μCi/ml [3H]uridine (1 μg/ml) and allowing the cultures to grow for two doublings in the presence or absence of solithromycin. Isotope incorporation was halted by adding uridine to a final concentration of 50 μg/ml, followed by a 30-min chase period. Cells were collected by centrifugation at 6,000 rpm for 10 min and held at −70°C until lysis. H. influenzae and S. pneumoniae cells were lysed with a lysozyme freeze-thaw method as described previously (14). Lysostaphin was used in place of lysozyme for preparing S. aureus lysates.
Lysates were centrifuged through 5 to 20% sucrose gradients in S buffer (10 mM Tris-HCl [pH 8.0], 0.5 mM MgCl2, 50 mM NH4Cl) to separate ribosomal subunits. Centrifugation was performed in an SW41 rotor for 5 h at 187,813 × g. Fractions were collected from the gradients and mixed with 3 ml of Scintisafe gel (Fisher Scientific), and the incorporation of [3H]uridine was measured by liquid scintillation counting.
Protein synthesis rates were determined by adding [35S]methionine (1 μCi/ml) to the cultures before the uridine chase period. Samples of 0.4 ml were collected at 5-min intervals for 15 min. Samples were precipitated with 10% trichloroacetic acid (TCA). The TCA-precipitated material was collected on Whatman GF/A glass fiber filters, washed with 10% TCA, and measured by liquid scintillation counting.
Uridine pulse-chase labeling.
S. aureus and H. influenzae cells were grown in 12 ml of TSB in the presence and absence of the antibiotic. Solithromycin at the 50% inhibitory concentration (IC50) for each organism was added to the cultures at a Klett reading of 10. After growth for one cell doubling, the RNA was pulse-labeled with [3H]uridine at 1 μCi/ml for 90 s and then chased with uridine at 50 μg/ml. Samples of 2 ml were taken at six time intervals. Cells were collected by centrifugation at 6,000 rpm for 10 min, washed in S buffer, and stored at −70°C before lysis and sucrose gradient centrifugation. For S. pneumoniae, 12 9-ml cultures of cells comprised of one control set and one set with solithromycin at 7.5 ng/ml were grown to a Klett reading of 15. The cells were pulse-labeled with [3H]uridine (1 μCi/ml) for 3 min and then chased with uridine at 45 μg/ml. At 15-min intervals, one culture was removed, and the cells were collected by centrifugation, washed, and stored frozen until lysis for sucrose gradient centrifugation as described above.
Analysis of total RNA via Agilent Bioanalyzer.
Microbial cells were grown in 5 ml of TSB with and without solithromycin at the indicated concentrations. Total RNA was isolated from S. aureus and S. pneumoniae by phenol and CHCl3 extraction (15). RNA was isolated from H. influenzae cells using the total RNA isolation kit from Norgen (Thorold, Canada). RNA quality was examined using an Agilent Bioanalyzer 2100 and the RNA 6000 lab on a chip; 0.5 to 1 μg of total RNA was loaded onto each well of the RNA 6000 chip. Sample preparation and analysis were carried out according to manufacturer's recommendations for total RNA analysis.
RESULTS
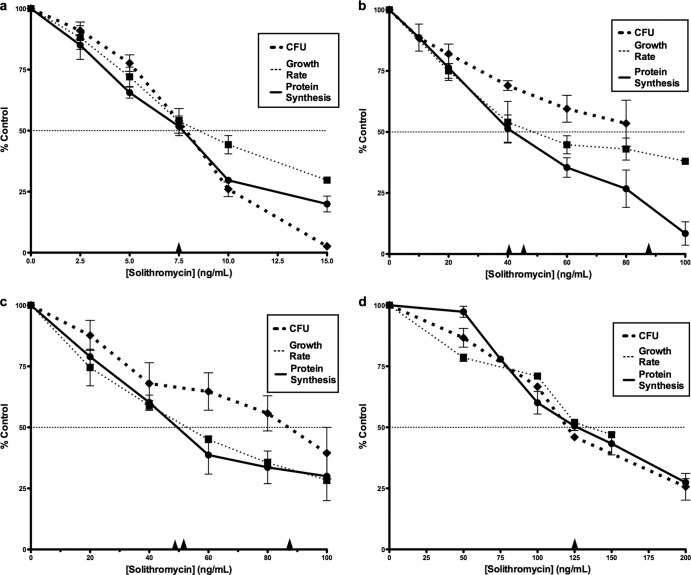
Solithromycin was initially examined for inhibitory effects on growth rates, viable-cell numbers, and protein synthesis rates in four microorganisms, including methicillin-susceptible and methicillin-resistant strains of S. aureus. The fluoroketolide was an effective inhibitor of each process examined. The concentration-dependent reductions in growth rate, cell viability, and translation rate are shown in Fig. 1 for the four strains tested. A summary of the IC50s for inhibition by solithromycin is given in Table 1. The inhibition was calculated as a percentage of the control growth rate, CFU, or rate of radiolabeled methionine incorporation as shown in Fig. S2 in the supplemental material.
Fig 1.
Inhibition of protein synthesis rate, growth rate, and CFU by solithromycin for S. pneumoniae (a), methicillin-susceptible S. aureus (MSSA) (b), methicillin-resistant S. aureus (MRSA) (c), and H. influenzae (d). Percentages of the control growth rate, cell number, and protein synthesis rate are shown. Arrowheads indicate IC50s. Standard errors are indicated (n = 2).
Table 1.
IC50s for solithromycin inhibition in three organisms
| Parameter | IC50 (ng/ml)a in: |
|||
|---|---|---|---|---|
| S. pneumoniae ATCC 49619 | S. aureus RN1786 (MSSA) | S. aureus A1024 (MRSA) | H. influenzae G-79 | |
| Growth rate | 7.5 | 40 | 52 | 125 |
| CFU | 7.5 | 80 | 85 | 125 |
| Protein synthesis | 7.5 | 40 | 48 | 125 |
| 50S subunit assembly | 14.5 | 65 | 93 | 230 |
| 30S subunit assembly | >20 | 85 | >100 | >250 |
S. pneumoniae was the most sensitive to inhibition by solithromycin of the four strains examined. In S. pneumoniae, the mean IC50 for inhibition of each of the three processes was 7.5 ng/ml (Fig. 1a and Table 1). For S. aureus the antibiotic had similar effects in both a methicillin-susceptible strain and a methicillin-resistant derivative, with mean IC50s for all three processes of 40 ng/ml and 55 ng/ml for MSSA and MRSA, respectively (Fig. 1b and c). Figure 1d is a composite showing inhibition of growth rate, cell viability, and protein synthesis by solithromycin in H. influenzae cells, where a mean IC50 of 125 ng/ml was found.
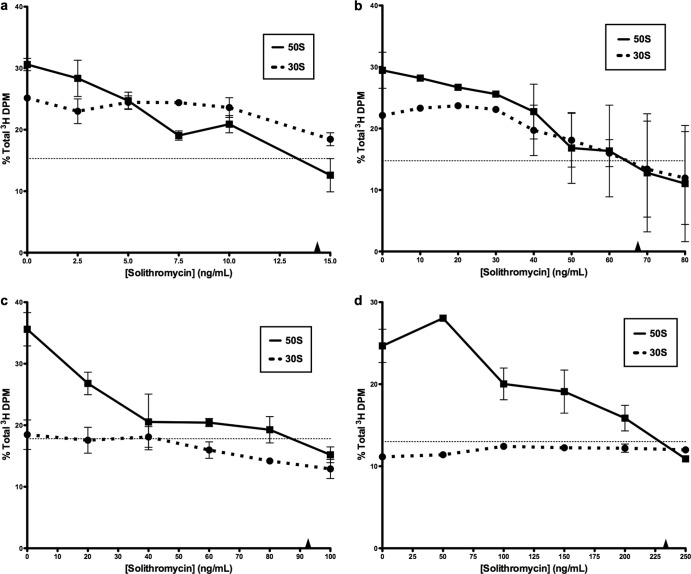
Solithromycin inhibition of ribosome assembly was examined over a range of antibiotic concentrations. The reduction in 50S subunit amounts was significant in each organism examined. These were calculated as a percentage of the total gradient radioactivity found in the 30S or 50S subunit area of the gradient (see Fig. S3 in the supplemental material). The IC50s for 50S subunit synthesis inhibition are given in Table 1. For 50S subunit formation in S. pneumoniae, the IC50 for assembly inhibition was 15 ng/ml (Fig. 2a; Table 1). Solithromycin was found to interfere with 50S ribosomal subunit formation in both methicillin-sensitive and methicillin-resistant strains of Staphylococcus aureus, with IC50s of 65 and 93 ng/ml, respectively (Fig. 2b and c). In addition, 30S subunit formation was affected in the MSSA strain (IC50, 85 ng/ml). A specific inhibitory effect on 50S formation was also seen in H. influenzae cells (IC50, 230 ng/ml) (Fig. 2d). No effect on 30S subunit formation was observed in this organism.
Fig 2.
Solithromycin inhibition of ribosomal subunit formation in S. pneumoniae (a), MSSA (b), MRSA (c), and H. influenzae (d). Percentages of the total gradient radioactivity in the 30S and 50S subunits are shown. The horizontal line indicates the 50% value. Arrowheads indicate IC50s. Standard errors are indicated (n = 2).
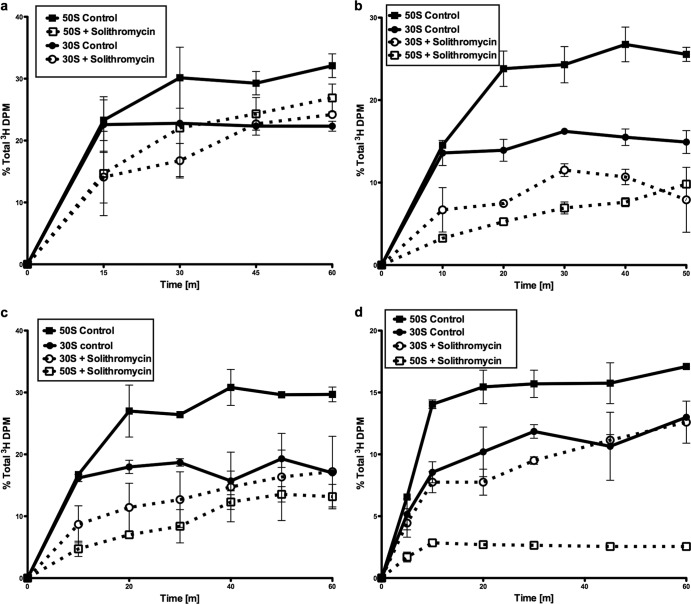
The ketolides telithromycin and cethromycin have been shown to inhibit the biogenesis of the 50S subunit in each of these three organisms (11). Solithromycin was examined for inhibitory effects on subunit formation by a pulse-chase labeling procedure. The rates of 30S and 50S synthesis in S. pneumoniae cells in the absence and presence of solithromycin are shown in Fig. 3a. The rate of formation of the 50S subunit was reduced to 50% of the control rate using solithromycin at the IC50. As previously seen, the fluoroketolide had a very small effect on 30S subunit assembly. The rate of 50S synthesis was also reduced by this antimicrobial in both S. aureus strains examined, with a lesser effect on the rate of 30S subunit formation (Fig. 3b and c). The antibiotic lowered the subunit synthesis rates by more than 50% in both S. aureus strains. A very significant reduction in the 50S subunit synthesis rate was also seen in H. influenzae, with this rate being reduced by about 80% (Fig. 3d).
Fig 3.
Ribosomal subunit synthesis rates for S. pneumoniae (a), MSSA (b), MRSA (c), and H. influenzae (d). 30S and 50S subunit synthesis rates in the presence or absence of solithromycin at the IC50 are shown. The mean 30S subunit assembly rate with and without antibiotic treatment is shown. The percentage of the total gradient radioactivity in the subunit at each time point is indicated. Standard errors are indicated (n = 2).
A common observation for subunit assembly inhibition by antibiotics is the turnover of rRNA from the stalled assembly intermediate (11, 16). The Agilent gel-on-a-chip procedure was used to examine the status of rRNA in solithromycin-inhibited cells. The electropherogram for each rRNA sample is shown in Fig. 4. Significant degradation of the 23S rRNA was seen in each case, with a reduced effect on the 16S rRNA of the 30S subunit (Table 2). rRNA turnover was most extensive in the antibiotic-treated MRSA strain (Table 2). The gel pattern for H. influenzae cells is unique because these cells do not contain intact 23S rRNA (17). Like 23S rRNA in other microorganisms (18, 19), the rRNA is fragmented by RNase III removal of an intervening sequence (20). The fragmented RNA pattern in Fig. 4 is characteristic of this organism (17). However, antibiotic treatment enhanced the degradation of RNA in solithromycin-treated cells (Table 2).
Fig 4.
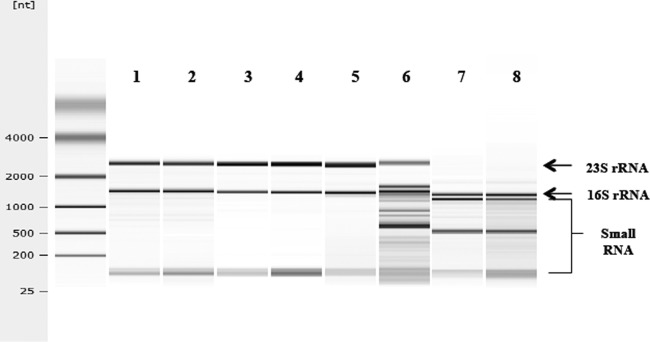
Agilent gel electropherogram of total RNA samples from control and solithromycin-treated cells. Lane 1, S. pneumoniae, no antibiotic; lane 2, S. pneumoniae plus solithromycin; lane 3, MSSA, no antibiotic; lane 4, MSSA plus solithromycin; lane 5, MRSA, no antibiotic; lane 6, MRSA plus solithromycin; lane 7, H. influenzae, no antibiotic; lane 8, H. influenzae plus solithromycin. The positions of the 23S and 16S rRNAs are indicated. Enhanced degradation of the rRNAs can be seen as smaller RNA species in the antibiotic-treated samples.
Table 2.
Summary of Agilent gel-on-a-chip assays for rRNAsa
| Organism | Solithromycin concn (ng/ml) | RNA integrity no. | % of: |
||
|---|---|---|---|---|---|
| 16S rRNA | 23S rRNA | Small RNA | |||
| S. pneumoniae | 0 | 8.6 | 23.5 | 23.1 | 18.4 |
| 10 | 8.7 | 22.1 | 21.4 | 22.5 | |
| MSSA | 0 | 10 | 23.4 | 40.3 | 24.6 |
| 65 | 9.8 | 17.3 | 31.2 | 39.4 | |
| MRSA | 0 | 9.3 | 23.2 | 30.7 | 10.7 |
| 65 | 5.8 | 9.7 | 6.1 | 4.1 | |
| H. influenzae | 0 | (5.8)b | 16.1 | 1.1 | 10.1 |
| 150 | (4.8)b | 10.2 | 0.0 | 16.0 | |
Total RNA was isolated from cells grown at the indicated concentrations of solithromycin. Percentages of total RNA values were computed by the Agilent software from the electropherogram shown in Fig. 4.
The RNA integrity number could not be calculated because of the lack of 23S rRNA.
DISCUSSION
The results of this study show that solithromycin is a particularly effective antimicrobial agent inhibiting ribosome formation and function in two Gram-positive organisms and in a Gram-negative strain. This novel ketolide is comparable to both telithromycin and cethromycin in its inhibitory properties against a number of different microorganisms (5).
Of the three organisms examined in this study, S. pneumoniae was the most susceptible to inhibition by solithromycin. The IC50 of 7.5 ng/ml found for inhibition of translation and 50S assembly is comparable to the IC50s of 5 ng/ml observed for cethromycin (9) and 15 ng/ml measured for telithromycin (10). The solithromycin IC50 is consistent with MIC50 values of 15 to 60 ng/ml reported in various studies of S. pneumoniae strains (8, 21–25). This compound was as effective as telithromycin against S. pneumoniae and significantly better than azithromycin in in vivo studies (21, 22, 24). The drug also showed impressive activity against S. pneumoniae strains carrying several resistance determinants (8, 22, 25).
Solithromycin was similarly effective against MSSA and MRSA strains, with IC50s of 40 and 55 ng/ml, respectively (Table 1). Its activity compares favorably with the IC50s of 35 and 80 ng/ml measured for cethromycin and telithromycin in this organism (26). MIC50 values of 60 to 120 ng/ml have been reported for S. aureus treated with solithromycin (21, 25), with the MRSA strains slightly less susceptible to the drug (21). The MRSA strain used in this study also showed a reduced susceptibility to retapamulin compared with the control MSSA organism (27). The compound was also effective in treating S. aureus-infected macrophage cells (28).
Importantly, a relatively low IC50 of 125 ng/ml against H. influenzae was observed in this study (Table 1). This antibiotic was a substantially better inhibitor of 50S subunit synthesis than cethromycin and telithromycin, which have IC50s of 1.25 μg/ml (13).
Solithromycin was significantly more effective than azithromycin or clarithromycin against this Gram-negative organism (21, 22, 25). MIC50 values of 0.5 to 2 μg/ml have been reported for solithromycin in H. influenzae (21, 22, 25).
The compound has been recently examined for its inhibitory effects on translation in a cell-free system (8). Comparisons with telithromycin and azithromycin showed its superior inhibitory effects on protein synthesis. The binding properties of solithromycin were also measured, and a crystal structure revealed its binding interactions with 23S RNA nucleotides in domains II and V in the 50S ribosomal subunit. The binding sites are similar to those found for the binding of telithromycin to the 50S subunit (8). However, the fluorine at the 2 position of solithromycin interacts with an additional ribosomal site compared with the sites of interaction of other ketolides, and thus solithromycin has activity against many strains that are resistant to telithromycin.
As seen with other macrolide and ketolide compounds, solithromycin was an effective inhibitor of 50S ribosomal subunit formation in all three organisms. Rates of subunit synthesis were reduced in every case by at least 50% at the IC50. The IC50 for subunit assembly inhibition was equivalent to the value for protein synthesis inhibition, as determined previously for telithromycin and cethromycin. At the IC50 for subunit assembly inhibition, cells have only one-half the number of control ribosomes. Of those, one-half are inhibited in protein synthesis activity, giving an apparent 2-fold difference in IC50, as expected.
As seen previously with azithromycin (11, 16), inhibition of 50S subunit biogenesis by solithromycin led to turnover of 23S rRNA in these cells. RNA analysis by the Agilent gel procedure revealed the extent of RNA turnover in these organisms. Impairment of 30S subunit formation by solithromycin was seen at elevated antibiotic concentrations and could result in enhanced 16S rRNA turnover in addition, as seen previously (11, 16). The enhanced sensitivity of the MRSA strain to rRNA degradation in the presence of solithromycin is similar to the enhanced sensitivity of this strain to retapamulin seen previously (27).
This novel antimicrobial agent is an important new compound for the treatment of infectious diseases. It is superior to other related antibiotics against specific Gram-positive organisms and is especially promising as an antibiotic for the treatment of respiratory tract infections.
ACKNOWLEDGMENT
We thank Prabha Fernandes at Cempra Pharmaceuticals for support of this research.
Footnotes
Published ahead of print 14 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02316-12.
REFERENCES
- 1. Chen LF, Chopra T, Kaye KS. 2009. Pathogens resistant to antibacterial agents. Infect. Dis. Clin. North Am. 23:817–845 [DOI] [PubMed] [Google Scholar]
- 2. Champney WS. (ed). 2008. New antibiotic targets. Methods Mol. Med. 142:1–27418437301 [Google Scholar]
- 3. Theuretzbacher U. 2011. Resistance drives antibacterial drug development. Curr. Opin. Pharmacol. 11:433–438 [DOI] [PubMed] [Google Scholar]
- 4. McCoy LS, Xie Y, Tor Y. 2011. Antibiotics that target protein synthesis. Wiley Interdiscip. Rev. RNA 2:209–232 [DOI] [PubMed] [Google Scholar]
- 5. Sutcliffe JA. 2011. Antibiotics in development targeting protein synthesis. Ann. N. Y. Acad. Sci. 1241:122–152 [DOI] [PubMed] [Google Scholar]
- 6. Wilson DN. 2011. On the specificity of antibiotics targeting the large ribosomal subunit. Ann. N. Y. Acad. Sci. 1241:1–16 [DOI] [PubMed] [Google Scholar]
- 7. Bryskier A, Agourides C, Chantot JF. 1997. Ketolides: new semisynthetic 14-membered macrolides, p 39–50 In Zinner SH, Young LS, Acar JF, Neu HC. (ed), Expanding indications for the new macrolides, azalides and streptogramins. Marcel Dekker, New York, NY [Google Scholar]
- 8. Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, Mankin AS. 2010. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54:4961–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Champney WS, Pelt J. 2002. The ketolide antibiotic ABT773 is an inhibitor of protein synthesis and 50S ribosomal subunit formation in Streptococcus pneumoniae cells. Curr. Microbiol. 45:155–160 [DOI] [PubMed] [Google Scholar]
- 10. Champney WS, Pelt J. 2002. Telithromycin inhibition of protein synthesis and 50S ribosomal subunit formation in Streptococcus pneumoniae cells. Curr. Microbiol. 45:328–333 [DOI] [PubMed] [Google Scholar]
- 11. Champney WS. 2006. The other target for ribosomal antibiotics: inhibition of bacterial ribosomal subunit formation. Infect. Disord. Drug Targets 6:377–390 [DOI] [PubMed] [Google Scholar]
- 12. Champney WS, Burdine R. 1998. Macrolide antibiotic inhibition of translation and 50S ribosomal subunit assembly in methicillin-resistant Staphylococcus aureus cells. Microb. Drug Resist. 4:169–174 [DOI] [PubMed] [Google Scholar]
- 13. Champney WS, Tober CL. 2003. Preferential inhibition of protein synthesis by ketolide antibiotics in Haemophilus influenzae cells. Curr. Microbiol. 46:103–108 [DOI] [PubMed] [Google Scholar]
- 14. Champney WS. 2008. Three methods to assay inhibitors of ribosomal subunit assembly. Methods Mol. Med. 142:63–73 [DOI] [PubMed] [Google Scholar]
- 15. Rio DC, Ares M, Jr, Hannon GJ, Nilsen TW. 2011. RNA: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 16. Silvers JA, Champney WS. 2005. Accumulation and turnover of 23S ribosomal RNA in azithromycin-inhibited ribonuclease mutant strains of Escherichia coli. Arch. Microbiol. 184:66–77 [DOI] [PubMed] [Google Scholar]
- 17. Song X-M, Forsgren A, Janson H. 1999. Fragmentation heterogeneity of 23S ribosomal RNA in Haemophilus species. Gene 230:287–293 [DOI] [PubMed] [Google Scholar]
- 18. Miller WL, Pabbaraju K, Sanderson KE. 2000. Fragmentation of 23S rRNA in strains of Proteus and Providencia results from intervening sequences in the rrn (rRNA) genes. J. Bacteriol. 182:1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pronk LM, Sanderson KE. 2001. Intervening sequences in rrl genes and fragmentation of 23S rRNA in genera of the family enterobacteriacae. J. Bacteriol. 183:5782–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evguenieva-Hackenberg E, Klug G. 2000. RNase III processing of intervening sequences found in helix 9 of 23S rRNA in the alpha class of Proteobacteria. J. Bacteriol. 182:4719–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farrell DJ, Castanheira M, Sader HS, Jones RN. 2010. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J. Infect. 61:476–483 [DOI] [PubMed] [Google Scholar]
- 22. Farrell DJ, Sader HS, Castanheira M, Biedenbach DJ, Rhomberg PR, Jones RN. 2010. Antimicrobial characterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents 35:537–543 [DOI] [PubMed] [Google Scholar]
- 23. McGhee P, Clark C, Kosowska-Shick KM, Nagai K, Dewasse B, Beachel L, Appelbaum PC. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob. Agents Chemother. 54:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Putnam SD, Castanheira M, Moet GJ, Farrell DJ, Jones RN. 2010. CEM-101, a novel fluoroketolide: antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria. Diagn. Microbiol. Infect. Dis. 66:393–401 [DOI] [PubMed] [Google Scholar]
- 25. Woosley LN, Castanheira M, Jones RN. 2010. CEM-101 activity against Gram-positive organisms. Antimicrob. Agents Chemother. 54:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Champney WS, Tober CL. 2001. Structure-activity relationships for six ketolide antibiotics. Curr. Microbiol. 42:203–210 [DOI] [PubMed] [Google Scholar]
- 27. Champney WS, Rodgers WK. 2007. Retapamulin inhibition of translation and 50S ribosomal subunit formation in Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:3385–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemaire S, Van Bambeke F, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53:3734–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]