Abstract
We evaluated the efficacy of tenofovir disoproxil fumarate (TDF) in patients with lamivudine failure (LAM-F) in comparison with that in nucleoside/nucleotide analogue (NA)-naïve patients with chronic hepatitis B (CHB). The criteria for inclusion were being NA naïve or having previous LAM-F and receiving TDF therapy for at least 6 months. Biochemical and virological tests were performed at the baseline, at 3-month intervals in the first year, and every 6 months thereafter. The primary outcome measure for efficacy was a complete virological response (CVR), defined as an HBV DNA level of <20 IU/ml. CVR rates were calculated by Kaplan-Meier analysis, and a multivariate Cox proportional-hazard model was generated in order to find predictive factors independently associated with the time to a CVR. We included 197 patients in the study (136 males; mean age, 43 ± 12 years; 105 patients were NA naïve). Sixty-five patients had hepatitis B e antigen (HBeAg)-positive CHB. The median duration of TDF treatment was 29 (range, 6 to 52) months. Seventy-one patients (77%) in the LAM-F group were treated with TDF add-on therapy. The CVR rates of the NA-naïve and LAM-F groups were comparable in HBeAg-negative (94% versus 96% at month 36, P = 0.10) and HBeAg-positive patients (67% versus 83% at month 36, P = 0.48). According to the multivariate Cox regression model, only HBeAg positivity (hazard ratio [HR], 0.39; 95% confidence interval [CI], 0.26 to 0.59; P < 0.001) and a high baseline HBV DNA level (HR, 0.44; 95% CI, 0.29 to 0.67; P < 0.001) had a significant influence on the time to a CVR. The similar cumulative CVR rates during the follow-up show that TDF has comparable efficacy in lamivudine-experienced and NA-naïve patients, and the presence of resistance mutations did not alter the response rates.
INTRODUCTION
The emergence of antiviral resistance has been an important challenge in the treatment of chronic hepatitis B (CHB) for years, until the era of nucleoside/nucleotide analogues (NA) with a high genetic barrier to resistance. Current guidelines suggest that entecavir and tenofovir, which are the most potent drugs, should be used as first-line NAs to prevent resistance in the long term (1, 2). Tenofovir is an acyclic NA with activity against both HIV and hepatitis B virus (HBV). It is a structural congener of adefovir and has potent and selective inhibitor activity against HBV DNA polymerase-reverse transcriptase in vitro (3). Tenofovir inhibits viral polymerases by direct binding and termination of DNA chain elongation (4).
Tenofovir disoproxil fumarate (TDF), the oral prodrug of tenofovir, was licensed in 2008 for the treatment of CHB in many countries. Its approval was based on two prospective ongoing randomized trials that showed the superiority of TDF at 300 mg/day over adefovir at 10 mg/day at week 48 (5). TDF demonstrated potent antiviral activity against wild-type HBV in both hepatitis B e antigen (HBeAg)-negative (study 102) and -positive (study 103) CHB patients. Five years of experience with TDF therapy of CHB showed sustained viral suppression and regression of fibrosis and even cirrhosis (6). Only a subset of patients were lamivudine experienced in these studies, and TDF demonstrated similar antiviral efficacy in both lamivudine-experienced (≥12 weeks) and -inexperienced patients (<12 weeks). However, the authors did not report on the influence of baseline genotypic resistance on the response to TDF therapy in the treatment-experienced patients. Although there is cumulative clinical and published evidence that indicates that TDF has substantial antiviral efficacy against resistant strains of HBV, especially in lamivudine resistance, none of these studies tested the efficacy of TDF against wild-type HBV in NA-naïve patients (7–10). Several in vitro studies showed almost unaltered or slightly decreased efficacy of TDF against lamivudine-resistant strains of HBV in comparison with that against the wild-type virus (11–14). Nevertheless, the clinical implications of lamivudine resistance in CHB patients treated with TDF have not been completely elucidated yet. We therefore conducted a retrospective cohort study to evaluate and compare the efficacy of TDF therapy in lamivudine-experienced and NA-naïve patients with CHB.
MATERIALS AND METHODS
Patients.
This study was designed as a single-center, retrospective cohort study in the Department of Gastroenterohepatology, Istanbul Faculty of Medicine, Istanbul University. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local institutional review board. Data reported here were collected retrospectively from outpatient visit charts. Patients with CHB who had received TDF at 300 mg/day between June 2008 and December 2012 were consecutively included in the study. Treatment indication was in accordance with current European Association for the Study of the Liver (EASL) guidelines (1). All patients had detectable hepatitis B surface antigen (HBsAg) for at least 6 months, histologic evidence of chronic hepatitis, and detectable HBV DNA before initiation of TDF treatment. The study inclusion criteria were (i) being NA naïve or having previous lamivudine failure (LAM-F) due to the emergence of resistance or a suboptimal virological response to lamivudine (an HBV DNA level of >50 IU/ml after at least 6 months of treatment), (ii) treatment with TDF for at least 6 months, (iii) a serum creatinine level of <1.5 mg/dl, and (iv) serologic HIV and hepatitis C and D virus negativity. Lamivudine resistance was defined as the presence of mutations that confer decreased susceptibility to lamivudine and/or a virological breakthrough (defined as an increase in the serum HBV DNA level by ≥1 log10 above the nadir) during treatment (2).
Clinical, laboratory, and histological assessments.
All of the patients included had a baseline physical examination and results of serum biochemistry tests, including alanine aminotransferase (ALT), serum creatinine, albumin, and total bilirubin levels. Patients were routinely assessed by the investigators after starting TDF treatment at 3-month intervals in the first year and every 6 months thereafter. At each visit, adverse events were recorded and serum samples were taken for serum biochemistry, viral marker, and HBV DNA testing. Baseline viral markers (HBsAg, anti-HBsAg antibody, HBeAg, and anti-HBeAg antibody levels) were measured by standard commercial immunoassays, and HBV DNA was quantitated by a real-time PCR technique (COBAS AmpliPre/COBAS TaqMan HBV Test, v2.0) with a lower detection limit of 20 IU/ml. Determination of HBV drug resistance was performed by INNO-LiPA HBV DR v2/v3 (Innogenetics NV, Ghent, Belgium) before the initiation of TDF therapy in lamivudine-experienced patients. The HBV DNA level at each visit was available, the HBsAg and anti-HBsAg antibody levels of every patient were tested at the baseline and annually, and the HBeAg and anti-HBeAg antibody levels of patients with HBeAg-positive CHB were measured at 6-month intervals. Child-Pugh scores were measured at the baseline in cirrhotic patients as previously described (15). All noncirrhotic patients had a baseline liver biopsy performed either before the initiation of TDF or before previous lamivudine therapy. Fibrosis and the histological activity index (HAI) were scored according to the Ishak scoring system (16). Surveillance for hepatocellular carcinoma (HCC) was performed by ultrasonography and the measurement of α-fetoprotein at 6-month intervals.
Treatment and endpoints.
All NA-naïve patients with CHB received TDF monotherapy; while most of the lamivudine-experienced patients were administered an add-on combination with TDF. The primary endpoint of this study was the proportion of patients achieving a complete virological response (CVR), which was defined as an undetectable HBV DNA level (<20 IU/ml) during the follow-up period. Univariate and multivariate analyses were performed to find independent factors that influence the time to a CVR. Secondary endpoints were ALT normalization, HBeAg and HBsAg loss or seroconversion, determination of the frequency and causes of virological breakthrough during TDF treatment, and assessment of adverse events. After stratification of the patients according to HBeAg status, primary and secondary endpoints were compared between patients with LAM-F and NA-naïve patients.
Statistical analysis.
Statistical analyses were performed by using IBM SPSS v20 (IBM SPSS Inc., Chicago, IL). Continuous variables are presented as means ± standard deviations or medians (ranges), while categorical variables are expressed as frequencies (percentages). Comparisons of continuous variables were performed by independent-sample Student t test or Mann-Whitney U test when appropriate, depending on the results of the Kolmogorov-Smirnoff and Shapiro-Wilk normality tests. A paired-sample t test or a Wilcoxon test was used for comparisons of variables in paired samples. Differences between categorical variables were evaluated by using the Pearson chi-square test or Fisher's exact test when necessary. A z test of column proportions with Bonferroni adjustment was used for multiple comparisons in contingency tables larger than two by two. Kaplan-Meier analyses with time-to-event subgroup comparisons were performed by using the log-rank test. A Cox proportional-hazard model including variables with P values of ≤0.10 was used to identify predictive factors independently associated with the time to a CVR. The results of the model were presented as a hazard ratio (HR) with the 95% confidence interval (CI). A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Baseline patient characteristics.
A total of 320 patients were initiated on TDF therapy during the study period. One hundred twenty-three patients did not fulfill the inclusion criteria and were excluded from the analysis; 54 patients had been treated with TDF for less than 6 months or were lost to follow-up, 52 patients had adefovir failure, 9 patients had a partial response to entecavir, 5 patients had chronic renal failure, and 3 patients were serologically positive for hepatitis D virus. The remaining 197 patients (136 males; mean age, 43 ± 12 years) were eligible to be included in the study and were divided into two groups according to previous treatment experience. The baseline demographic, clinical, and laboratory characteristics of 197 patients are summarized in Table 1. There were 105 patients (53%) who were NA naïve, and 92 patients (47%) started TDF therapy after LAM-F. The median duration of failed lamivudine therapy was 48 (range, 6 to 120) months. Forty-three patients had a history of previous alpha interferon (IFN-α) experience before any NA therapy. HBV drug resistance analyses revealed that 74 patients had mutations associated with lamivudine resistance. Wild-type HBV was detected in 15 patients with suboptimal responses to lamivudine (after a median of 24 [range, 6 to 84] months of therapy) and 3 patients with virological breakthrough during lamivudine therapy. None of the patients were found to be nonadherent to lamivudine therapy. The most frequently detected point mutations were rtM204I/V (69 patients), rtL180M (49 patients), and rtL80I/V (42 patients). The most common mutation, rtM204I/V, was found in conjunction with a variety of compensatory resistance mutations, particularly rtL80I/V and/or rtL180M. The presence of baseline polymerase gene mutations conferring lamivudine resistance is detailed in Table 2.
Table 1.
Baseline demographic, clinical, and laboratory characteristics of the patients in this study
| Characteristic | Overall | NA naïve | LAM-F | P value |
|---|---|---|---|---|
| No. (%) of patients | 197 (100) | 105 (53) | 92 (47) | |
| Mean age (yr) ± SD | 43 ± 12 | 40 ± 12 | 45 ± 12 | 0.014 |
| No. (%) of: | ||||
| Males | 136 (69) | 73 (70) | 63 (68) | 0.87 |
| Females | 61 (31) | 32 (30) | 29 (32) | |
| No. (%) with cirrhosis | 45 (23) | 15 (14) | 30 (33) | 0.002 |
| Child-Pugh class A | 32 (71) | 13 (87) | 19 (63) | >0.05a |
| Child-Pugh class B | 11 (24) | 2 (13) | 9 (30) | >0.05a |
| Child-Pugh class C | 2 (4) | 0 (0) | 2 (7) | >0.05a |
| Baseline ALT level (IU/liter) | ||||
| Mean ± SD | 98 ± 120 | 119 ± 123 | 74 ± 113 | <0.001 |
| Median (range) | 56 (9–706) | 77 (19–706) | 40 (9–681) | |
| No. (%) with baseline ALT level above ULN | 119 (60) | 82 (78) | 37 (40) | <0.001 |
| No. (%) with HBeAg status of: | ||||
| Negative | 132 (67) | 65 (62) | 67 (73) | 0.10 |
| Positive | 65 (33) | 40 (38) | 25 (27) | |
| Baseline HBV DNA level (log10 IU/ml) | ||||
| Mean ± SD | 7.48 ± 8.02 | 7.66 ± 8.14 | 7.11 ± 7.49 | <0.001 |
| Median (range) | 6.46 (1.73–9.13) | 7.12 (2.09–9.13) | 5.42 (1.73–8.14) | |
| No. (%) with genotypic resistance | 74 (38) | 74 (80) | ||
| Median liver histology (range) | ||||
| Ishak stage | 2 (2–6) | 2 (2–6) | 2 (2–6) | 0.19 |
| HAI | 6 (5–15) | 7 (5–14) | 6 (6–15) | 0.11 |
| No. (%) treated with: | ||||
| TDF monotherapy | 126 (64) | 105 (100) | 21 (23) | <0.001 |
| Lamivudine-TDF combination | 71 (36) | 0 (0) | 71 (77) | |
| Treatment duration (mo) | ||||
| Mean ± SD | 28 ± 14 | 28 ± 14 | 29 ± 14 | 0.48 |
| Median (range) | 29 (6–52) | 28 (6–52) | 30 (6–51) |
Comparison of column proportions, χ2 test with Bonferroni adjustment.
Table 2.
Baseline polymerase sequence mutations in 74 patients with LAM-F
| Characteristic | No. (%) of patients |
|---|---|
| Resistance mutations | |
| rtL80I/V | 42 (56.7) |
| rtV173L | 5 (6.7) |
| rtL180M | 49 (66) |
| rtA181T | 1 (1.3) |
| rtM204I/V | 69 (93) |
| Most common combinations of resistance mutations | |
| rtL80I/V, rtL180M, and rtM204I/V | 24 (32) |
| rtL180M and rtM204I/V | 22 (30) |
| rtL80I/V and rtM204I/V | 15 (20) |
There were 45 patients (23%) with cirrhosis, and 13 (6.6%) of them had decompensated liver disease (Child-Pugh class B or C). The liver histology results, including Ishak stage and HAI, of the two groups were similar. The number of patients with HBeAg-negative and HBeAg-positive CHB were 132 (67%) and 65 (33%), respectively. NA-naïve patients had significantly higher baseline ALT (119 ± 123 versus 74 ± 113 IU/liter, P < 0.001, respectively) and HBV DNA (7.66 ± 8.14 versus 7.11 ± 7.49 log10 IU/ml, P < 0.001, respectively) levels than patients with LAM-F. Pretreatment HBV DNA levels were higher in both HBeAg-negative (7.13 versus 6.99, P < 0.001) and HBeAg-positive patients in the NA-naïve group; however, the difference was more pronounced in HBeAg-positive patients (7.98 versus 7.32, P < 0.001).
Response to TDF therapy.
The mean duration of treatment with TDF was 29 (range, 6 to 52) months, and the durations were similar in the two groups (Table 1). Of the patients with LAM-F, 71 (77%) were treated with a combination of lamivudine and TDF and 21 (23%) patients were treated with TDF monotherapy. The proportion of HBeAg-negative patients with a CVR during the follow-up was 85% (55/65) in the NA-naïve group and 91% (61/67) in the LAM-F group (P = 0.26). The CVR rate in HBeAg-positive patients was also similar in the NA-naïve group and the LAM-F group (60% [24/40] versus 64% [16/25], P = 0.75). The cumulative CVR rates of HBeAg-negative patients in the NA-naïve and LAM-F groups were 52% versus 67% at 6 months, 82% versus 81% at 12 months, 88% versus 93% at 24 months, and 94% versus 96% at 36 months, respectively (Fig. 1a; log-rank test, P = 0.10). The cumulative CVR rates in the NA-naïve and LAM-F groups of HBeAg-positive patients were 15% versus 12% at 6 months, 39% versus 43% at 12 months, 61% versus 74% at 24 months, and 67% versus 83% at 36 months, respectively (Fig. 1b; log-rank test, P = 0.48). Despite a higher baseline HBV DNA level in the NA-naïve group, HBV DNA suppression curves during TDF therapy were similar in the two groups, especially after 6 months of treatment (Fig. 2). The times to a CVR were comparable in the monotherapy and add-on combination therapy arms of patients with LAM-F (89% versus 88% at 24 months; log-rank test, P = 0.23). Univariate Cox regression analyses of the total group revealed that age at the initiation of TDF therapy (HR, 1.016; 95% CI, 1.002 to 1.030; P = 0.02), HBeAg positivity (HR, 0.35; 95% CI, 0.24 to 0.51; P < 0.001), combination therapy (HR, 1.44; 95% CI, 1.04 to 1.99; P = 0.027), a high baseline ALT level (above the upper limit of the normal range [ULN]) (HR, 0.56; 95% CI, 0.41 to 0.77; P < 0.001), and a high baseline HBV DNA level (>2 × 106 IU/ml) (HR, 0.38; 95% CI, 0.28 to 0.53; P < 0.001) were associated with the achievement of a CVR (Table 3). However, gender, cirrhosis, a history of prior IFN-α therapy, LAM-F, the presence of any resistance mutation, and duration of TDF therapy did not achieve significance in the prediction of a CVR. In a multivariate Cox proportional-hazard model, only HBeAg positivity (HR, 0.39; 95% CI, 0.26 to 0.59; P < 0.001) and a high baseline HBV DNA level (HR, 0.44; 95% CI, 0.29 to 0.67; P < 0.001) were independent factors predictive of a CVR (Table 4). The addition of individual mutations to the equation did not improve the multivariate model.
Fig 1.
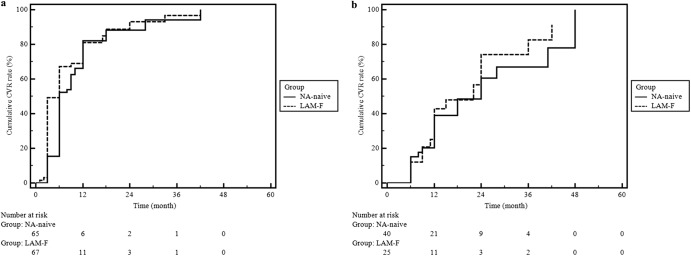
(a) CVR curves of HBeAg-negative patients stratified by lamivudine experience. Analysis was done by the Kaplan-Meier method; log-rank test, P = 0.10. (b) CVR curves of HBeAg-positive patients stratified by lamivudine experience. Analysis was done by the Kaplan-Meier method; log-rank test, P = 0.48.
Fig 2.
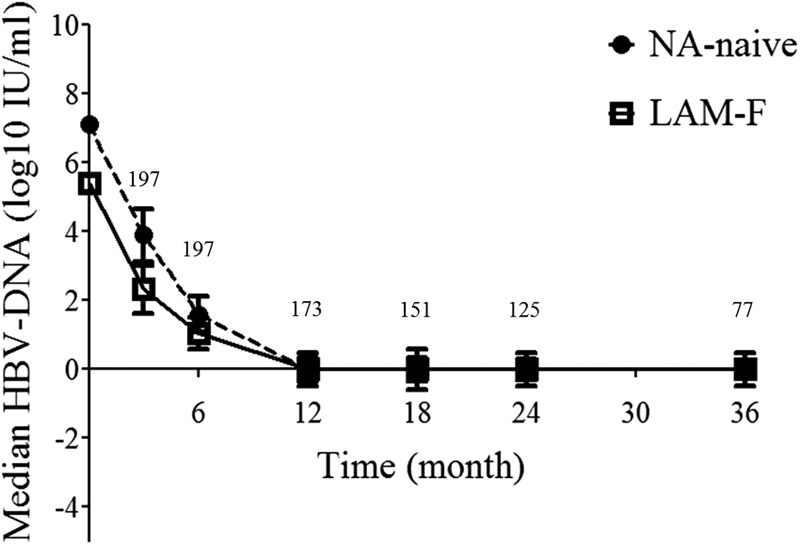
Changes in the median HBV DNA level were similar in the two groups. Error bars represent the standard errors of the means. The number of patients is shown for each time point above the curve.
Table 3.
Factors predictive of a CVRa according to univariate analyses
| Factor | Bb | SEc | P value | HRd | 95% CI for HR |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.016 | 0.007 | 0.020 | 1.016 | 1.002 | 1.030 |
| Gender (male) | −0.120 | 0.171 | 0.485 | 0.887 | 0.634 | 1.242 |
| Cirrhosis | 0.179 | 0.187 | 0.339 | 1.196 | 0.829 | 1.725 |
| Previous IFN-α therapy | 0.281 | 0.188 | 0.136 | 1.324 | 0.915 | 1.915 |
| LAM-F | 0.315 | 0.161 | 0.051 | 1.370 | 0.999 | 1.879 |
| HBeAg positivity | −1.047 | 0.190 | <0.001 | 0.351 | 0.242 | 0.510 |
| Lamivudine-TDF combination therapy | 0.365 | 0.164 | 0.027 | 1.440 | 1.043 | 1.988 |
| Any resistance mutation | 0.318 | 0.163 | 0.051 | 1.375 | 0.998 | 1.893 |
| High baseline ALT level (above ULN) | −0.579 | 0.162 | <0.001 | 0.560 | 0.408 | 0.770 |
| High baseline HBV DNAe | −0.963 | 0.166 | <0.001 | 0.382 | 0.276 | 0.529 |
| Treatment duration | 0.004 | 0.006 | 0.495 | 1.004 | 0.992 | 1.017 |
An HBV DNA level of <20 IU/liter. HBeAg-negative CHB was the indicator variable for HBeAg status in the analysis.
B, regression coefficient.
SE, standard error.
HR, Exp(B).
>2 × 106 IU/liter.
Table 4.
Multivariate Cox proportional-hazard model to identify factors independently associated with a CVRa
| Factor | Bb | SEc | P value | HRd | 95% CI for Exp(B) |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.003 | 0.008 | 0.683 | 1.003 | 0.988 | 1.018 |
| LAM-F | 0.086 | 0.326 | 0.791 | 1.090 | 0.576 | 2.064 |
| HBeAg positivity | −0.932 | 0.206 | <0.001 | 0.394 | 0.263 | 0.589 |
| Lamivudine-TDF combination therapy | 0.125 | 0.355 | 0.724 | 1.134 | 0.565 | 2.272 |
| Any resistance mutation | −0.265 | 0.374 | 0.479 | 0.767 | 0.369 | 1.597 |
| High baseline ALT level (above ULN) | −0.077 | 0.200 | 0.701 | 0.926 | −0.625 | 1.371 |
| High baseline HBV DNA levele | −0.810 | 0.210 | <0.001 | 0.445 | 0.295 | 0.671 |
An HBV DNA level of <20 IU/liter. HBeAg-negative CHB was the indicator variable for HBeAg status in the analysis.
B, regression coefficient.
SE, standard error.
HR, Exp(B).
>2 × 106 IU/liter.
Secondary endpoints.
ALT normalization rates (NA-naïve group [85%, 70/82] versus LAM-F group [89%, 33/37], P = 0.77) and times to ALT normalization did not differ between the two groups during the follow-up period (Fig. 3; log-rank test, P = 0.93). The estimated mean time to ALT normalization was 10 (95% CI, 8 to 13) months. HBeAg loss or seroconversion occurred in 30% (12/40) and 32% (8/25) of the patients in the NA-naïve and LAM-F groups, respectively (P = 0.87). The times to HBeAg loss or seroconversion were similar in the two groups (Fig. 4; log-rank test, P = 0.76). An NA-naïve patient decided to withdraw from TDF therapy at 12 months, after she became aware of being pregnant. Fortunately, the patient gave birth to a healthy baby without any flare-up during pregnancy. She was lost to follow-up for 18 months after labor, and when she returned to follow-up, spontaneous HBsAg loss and seroconversion to anti-HBsAg antibody were detected. We did not identify any other patient with HBsAg loss during the treatment course.
Fig 3.
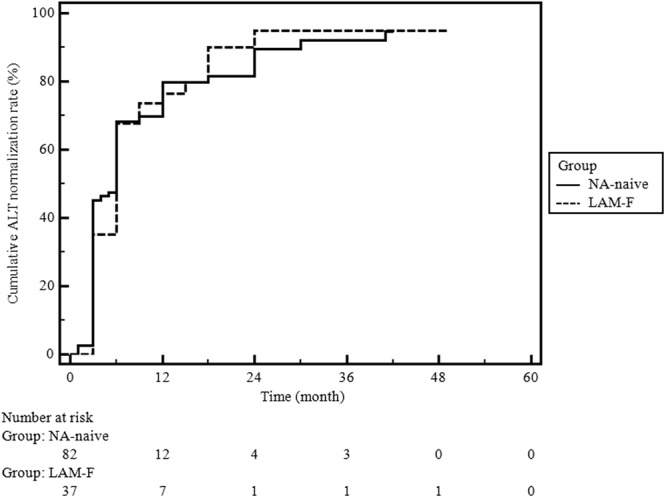
Cumulative ALT normalization rates in the NA-naïve and LAM-F groups. Analysis was done by the Kaplan-Meier analysis method; log-rank test, P = 0.93.
Fig 4.
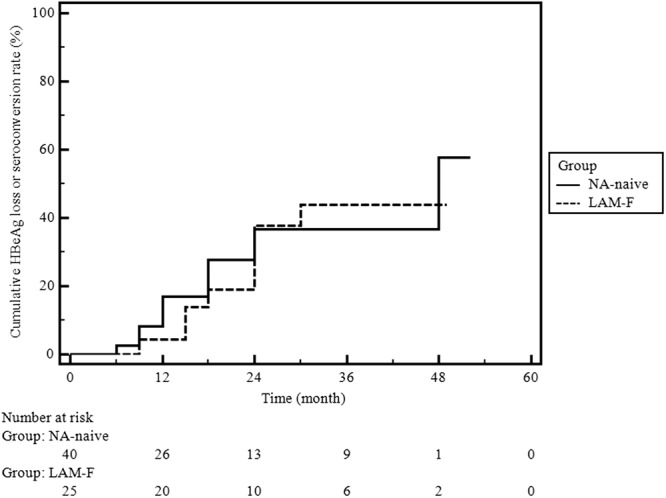
Cumulative HBeAg loss or seroconversion rates in the NA-naïve and LAM-F groups. Analysis was done by the Kaplan-Meier method; log-rank test, P = 0.76.
Adherence to therapy and adverse events.
During the follow-up visits, 18 patients (9%) were found to be nonadherent to TDF therapy. Nine patients developed virological breakthrough because of nonadherence; fortunately, none of them experienced an adverse clinical outcome. All of the patients with a virological breakthrough responded well to TDF therapy after continuation of treatment. A female patient with cirrhosis decompensated during TDF treatment because of persistent HBV replication without having a virological breakthrough. The patient had poor compliance with the medication and follow-up, and she was listed for orthotopic liver transplantation after clinical deterioration. Three patients with adverse gastrointestinal events such as nausea and diarrhea during the first 2 weeks of therapy withdrew from TDF treatment. After reinitiation of the medication, the same symptoms occurred and both patients were switched to other NAs. The serum creatinine level changed significantly over the course of treatment, from a mean of 0.89 (range, 0.5 to 1.4) mg/dl at the baseline to 0.93 (range, 0.5 to 2.2) mg/dl at the end of the follow-up period (P = 0.001). Eleven patients (5.6%) had an increase in serum creatinine of ≥0.5 mg/dl, and 4 (2%) patients had serum creatinine levels of ≥1.5 mg/dl with an increase of less than 0.5 mg/dl during treatment. Four (27%) of those patients with altered renal function during therapy were cirrhotic. All patients responded to the adjustment of the TDF dosage interval and could remain on treatment. Mild hypophosphatemia (a serum phosphorus level of <2.7 mg/dl) was detected in only five patients, without a change in serum creatinine. HCC occurrence was not detected in any patient during the follow-up period.
DISCUSSION
In the present study, with a follow-up duration of up to 4 years, we compared the efficacy of TDF therapy in NA-naïve and lamivudine-experienced patients in whom genotypic resistance is prevalent. Patients with LAM-F received add-on or monotherapy with TDF at 300 mg/day, while NA-naïve patients received monotherapy during the course of treatment. Because of the different number of HBeAg-positive patients in each group, the primary endpoint was analyzed separately according to HBeAg status. For HBeAg-negative patients, the CVR rates and the time-to-CVR curves (Fig. 1a) were similar in the two groups. The CVR rates and time-to-CVR curves of HBeAg-positive patients were slightly better in the LAM-F group (Fig. 1b), probably because of higher pretreatment HBV DNA levels in NA-naïve patients. Nonetheless, the difference did not reach significance. There were also differences other than the baseline HBV DNA levels between the groups, including age at TDF initiation, number of patients with cirrhosis, and ALT levels. Those baseline differences were not unexpected because patients with previous treatment experience tend to have a longer disease duration, more fibrosis, and less inflammation. We assume that those baseline features, except the HBV DNA level, should not confound the interpretation of the results, especially after the performance of regression analyses.
Univariate and multivariate Cox proportional-hazard models were built in order to find out if previous lamivudine experience or the presence of resistance mutations had any negative effect on the achievement of the primary endpoint. In multivariate analysis, only HBeAg status and baseline HBV DNA levels were found to be independent predictors of the time to a CVR. After adjustment for confounding factors, lamivudine experience, the presence of any resistance mutation, and receiving combination therapy were not found to have any influence on the time to a CVR. Biochemical and serological response rates, which were defined as ALT normalization and HBeAg loss or seroconversion, respectively, were also comparable in the two groups.
Large pivotal clinical trials demonstrated that TDF therapy produces potent antiviral activity among primarily treatment-naïve patients with CHB (5). However, the efficacy of TDF in patients with lamivudine resistance has not been directly compared to that in treatment-naïve patients. Only a small subset of patients from studies 102 and 103 had previously been treated with lamivudine for ≥12 weeks. The TDF responses of these lamivudine-experienced patients were compared to those of lamivudine-naïve subjects, who had less than 12 weeks of lamivudine experience, and similar efficacies were reported after 48 weeks of treatment (respectively, 88% versus 86% with an HBV DNA level of <400 copies/ml) (17). However, mutations associated with lamivudine resistance (rtM204I/V with or without rtL180M) were observed in only five patients in the TDF treatment arm (18). On the contrary, our study included mostly patients with genotypic resistance in the lamivudine-experienced group. The first study which primarily investigated the efficacy of TDF in lamivudine resistance included 20 patients with a virological breakthrough under lamivudine therapy and a subsequent suboptimal response to adefovir (8). In this uncontrolled study, 18 patients were HBeAg positive and 6 patients had HBV with genotypic resistance, 19 patients achieved an undetectable HBV DNA level (<400 copies/ml) within a median follow-up time of 3.5 months. A subsequent uncontrolled study by van Bömmel et al. included 131 patients (65% HBeAg positive) with previous NA failure 113 of whom had resistance analysis, and lamivudine or adefovir resistance was detected in 62% and 19% of them, respectively (9). Overall, 79% of the patients achieved an HBV DNA level of <400 copies/ml after a mean TDF treatment duration of 23 months. The authors reported substantial efficacy whether there was lamivudine resistance or not. However, the study did not include NA-naïve patients as a control group and the investigators used direct sequencing to search for resistance mutations instead of the HBV DR v2/v3 assay, which is more sensitive and can detect specific mutations earlier (19–22). A prospective study by Patterson et al. included 59 patients (39 HBeAg-positive patients) with lamivudine (20 patients)- or adefovir (17 patients)-resistant HBV infections. At the end of 96 weeks of follow-up, 64% of the patients achieved an undetectable viral load, which is <15 IU/ml. Another prospective randomized study by Berg et al. included 105 patients with suboptimal responses to adefovir, and 60% of them were lamivudine experienced (20). The investigators randomized patients to TDF-emtricitabine combination and TDF monotherapy arms. The authors reported similar efficacies in both the combination and monotherapy groups; and the response to TDF was not influenced by the presence of any resistance mutation. However, only a minority of the subjects in this study had HBV with genotypic resistance to lamivudine. In our study, it was confirmed that add-on combination therapy does not provide any additive suppressive effect on HBV replication, even in patients with previous LAM-F, which is in accordance with the study by Berg et al. (20) and current EASL guidelines (1).
TDF therapy demonstrated a very good safety and tolerability profile in the present study, which is concordant with previous studies. There were only two patients who could not tolerate the therapy because of adverse gastrointestinal effects and were switched to other NAs. Ten patients (6.1%) required dose interval adjustments because of an increase in serum creatinine levels. All patients responded to dose adjustment and remained on therapy without any further deterioration of renal function. The occurrence of hypophosphatemia during the follow-up period was uncommon in this cohort, and it was clinically insignificant in two patients who developed a mild decrease in serum phosphate levels. Virological breakthrough was encountered only in patients who were nonadherent to therapy, and none of them required any change in treatment.
Tenofovir susceptibility analyses of lamivudine-resistant strains of HBV revealed resistance values between 1.1- and 5.7-fold different from the susceptibility of the wild-type virus (11–14, 23, 24). The clinical implications of these in vitro susceptibility results have not been investigated in a controlled study design. In our study, we evaluated the efficacy of TDF in patients with lamivudine-resistant HBV infections, while NA-naïve patients were included in the control group. The results of the multivariate Cox proportional-hazard model suggest that lamivudine experience or the presence of any resistance mutation has no influence on the efficacy of TDF in vivo. Only a high baseline HBV DNA level and being HBeAg positive, which is also associated with the baseline viral load, determine the time required to achieve an undetectable HBV DNA level. Of note, none of the earlier studies evaluated the effect of individual resistance mutations on the time to a complete response while adjusting for confounding factors. In conclusion, TDF at 300 mg/day as monotherapy or add-on therapy is safe, well-tolerated, and equally effective in patients infected with wild-type HBV and those infected with lamivudine-resistant strains of HBV.
ACKNOWLEDGMENTS
Bulent Baran is the first author and primary investigator and was responsible for the acquisition, analysis, and interpretation of data, statistical analysis, and writing of the manuscript. Ozlem Mutluay Soyer was responsible for acquisition of data and patient recruitment. Asli Cifcibasi Ormeci, Suut Gokturk, Sami Evirgen, and Hamza Ugur Bozbey were responsible for acquisition of data and patient recruitment. Filiz Akyuz, Cetin Karaca, Kadir Demir, and Fatih Besisik were responsible for critical revision of the manuscript for important intellectual content. Derya Onel was responsible for serologic and virologic analyses. Mine Gulluoglu was responsible for evaluation and analysis of liver specimens. Selim Badur was responsible for serologic and virologic analyses. Sabahattin Kaymakoglu, mentor and primary investigator, was responsible for the study concept and design, critical revision of the manuscript for important intellectual content, and study supervision.
We have no financial or other relationships that might lead to a conflict of interest.
No grant or other financial support was received for this study.
Footnotes
Published ahead of print 4 February 2013
REFERENCES
- 1. European Association for the Study of the Liver 2012. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J. Hepatol 57:167–185 [DOI] [PubMed] [Google Scholar]
- 2. Lok AS, McMahon BJ. 2009. Chronic hepatitis B: update 2009. Hepatology 50:661–662 [DOI] [PubMed] [Google Scholar]
- 3. Heijtink RA, Kruining J, de Wilde GA, Balzarini J, de Clercq E, Schalm SW. 1994. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 38:2180–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cherrington JM, Allen SJW, Bischofberger N, Chen MS. 1995. Kinetic interaction of the diphosphates of 9-(2-phosphonylmethoxyethyl)adenine and other anti-HIV active purine congeners with HIV reverse-transcriptase and human DNA polymerase-alpha, polymerase-beta and polymerase-gamma. Antivir. Chem. Chemother. 6:217–221 [Google Scholar]
- 5. Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F. 2008. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 359:2442–2455 [DOI] [PubMed] [Google Scholar]
- 6. Marcellin P, Buti M, Gane E, Krastev Z, Flisiak R, Germanidis G, Washington MK, Barnes CN, Flaherty JF, Bornstein JD, McHutchison JG, Heathcote EJ. 2011. Five years of treatment with tenofovir df (TDF) for chronic hepatitis B (CHB) infection is associated with sustained viral suppression and significant regression of histological fibrosis and cirrhosis. Hepatology 54:1011A–1012A [Google Scholar]
- 7. van Bömmel F, Wunsche T, Mauss S, Reinke P, Bergk A, Schurmann D, Wiedenmann B, Berg T. 2004. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology 40:1421–1425 [DOI] [PubMed] [Google Scholar]
- 8. van Bömmel F, Zollner B, Sarrazin C, Spengler U, Huppe D, Moller B, Feucht HH, Wiedenmann B, Berg T. 2006. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology 44:318–325 [DOI] [PubMed] [Google Scholar]
- 9. van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Huppe D, Stein K, Trojan J, Sarrazin C, Bocher WO, Spengler U, Wasmuth HE, Reinders JG, Moller B, Rhode P, Feucht HH, Wiedenmann B, Berg T. 2010. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 51:73–80 [DOI] [PubMed] [Google Scholar]
- 10. Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, Desmond PV, Roberts SK, Locarnini S, Bowden S, Angus PW. 2011. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut 60:247–254 [DOI] [PubMed] [Google Scholar]
- 11. Lada O, Benhamou Y, Cahour A, Katlama C, Poynard T, Thibault V. 2004. In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir. Ther. 9:353–363 [PubMed] [Google Scholar]
- 12. Delaney WEt., Ray AS, Yang H, Qi X, Xiong S, Zhu Y, Miller MD. 2006. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob. Agents Chemother. 50:2471–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang H, Qi X, Sabogal A, Miller M, Xiong S, Delaney WEt. 2005. Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antivir. Ther. 10:625–633 [PubMed] [Google Scholar]
- 14. Brunelle MN, Lucifora J, Neyts J, Villet S, Holy A, Trepo C, Zoulim F. 2007. In vitro activity of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]-pyrimidine against multidrug-resistant hepatitis B virus mutants. Antimicrob. Agents Chemother. 51:2240–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. 1973. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 60:646–649 [DOI] [PubMed] [Google Scholar]
- 16. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al. 1995. Histological grading and staging of chronic hepatitis. J. Hepatol. 22:696–699 [DOI] [PubMed] [Google Scholar]
- 17. Manns M, Jeffers L, Dalekos G, Berg T, Trepo C, Roberts S, Prieto M, Rizzetto M, Sorbel J, Anderson J, Mondou E, Rousseau F. 2008. The antiviral response to tenofovir disoproxil fumarate (TDF) is comparable in lamivudine (LAM)-naïve and LAM-experienced subjects treated for chronic hepatitis B (CHB). J. Hepatol. 48:S33–S33 [Google Scholar]
- 18. Snow-Lampart A, Chappell B, Curtis M, Zhu Y, Myrick F, Schawalder J, Kitrinos K, Svarovskaia ES, Miller MD, Sorbel J, Heathcote J, Marcellin P, Borroto-Esoda K. 2011. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology 53:763–773 [DOI] [PubMed] [Google Scholar]
- 19. Degertekin B, Hussain M, Tan J, Oberhelman K, Lok AS. 2009. Sensitivity and accuracy of an updated line probe assay (HBV DR v. 3) in detecting mutations associated with hepatitis B antiviral resistance. J. Hepatol. 50:42–48 [DOI] [PubMed] [Google Scholar]
- 20. Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D, Sorbel J, Borroto-Esoda K, Oldach D, Rousseau F. 2010. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology 139:1207–1217 [DOI] [PubMed] [Google Scholar]
- 21. Hussain M, Fung S, Libbrecht E, Sablon E, Cursaro C, Andreone P, Lok AS. 2006. Sensitive line probe assay that simultaneously detects mutations conveying resistance to lamivudine and adefovir. J. Clin. Microbiol. 44:1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussain M, Chu CJ, Sablon E, Lok AS. 2003. Rapid and sensitive assays for determination of hepatitis B virus (HBV) genotypes and detection of HBV precore and core promoter variants. J. Clin. Microbiol. 41:3699–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brunelle MN, Jacquard AC, Pichoud C, Durantel D, Carrouee-Durantel S, Villeneuve JP, Trepo C, Zoulim F. 2005. Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology 41:1391–1398 [DOI] [PubMed] [Google Scholar]
- 24. Sheldon J, Camino N, Rodes B, Bartholomeusz A, Kuiper M, Tacke F, Nunez M, Mauss S, Lutz T, Klausen G, Locarnini S, Soriano V. 2005. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir. Ther. 10:727–734 [PubMed] [Google Scholar]


