Abstract
We evaluated the effect of tigecycline (50-mg and 200-mg doses) on corrected QT (QTc) intervals and assessed safety and tolerability in a randomized, placebo-controlled, four-period crossover study of 48 (44 male) healthy volunteers aged 22 to 53 years. Fed subjects received tigecycline (50 mg or 200 mg) or placebo in a blinded fashion or an open-label oral dose of moxifloxacin (400 mg) after 1 liter of intravenous fluid. Serial electrocardiograms were recorded before, and for 96 h after, dosing. Blood samples for tigecycline pharmacokinetics were collected after each recording. QTc intervals were corrected using Fridericia's correction (QTcF). Pharmacokinetic parameters were calculated using noncompartmental methods with potential relationships examined using linear mixed-effects modeling. Adverse events were recorded. The upper limits of the 90% confidence interval for the mean difference between both tigecycline doses and placebo for all time-matched QTcF interval changes from baseline were <5 ms. The tigecycline concentrations initially declined rapidly and then more slowly. In the group given 50 mg of tigecycline, the pharmacokinetic parameters and means were as follows: maximum concentration of drug in serum (Cmax), 432 ng/ml; area under the concentration-time curve from time zero extrapolated to infinity (AUC0–∞), 2,366 ng · h/ml; clearance (CL), 21.1 liters/h; volume of distribution at steady state (Vss), 610 liters; and terminal half-life (t1/2), 22.1 h. Proportional or similar values were found for the group given 200 mg of tigecycline. Linear mixed-effects modeling failed to show an effect on QTcF values by tigecycline concentrations (P = 0.755). Tigecycline does not prolong the QTc interval in healthy subjects. This study has been registered at ClinicalTrials.gov under registration no. NCT01287793.
INTRODUCTION
Tigecycline is a broad-spectrum glycylcycline that binds to the 30S ribosomal subunit blocking entry of amino-acyl tRNA and preventing peptide chain elongation. Tigecycline overcomes two major mechanisms of tetracycline resistance, ribosomal protection and efflux; however, resistance via nonspecific multidrug efflux pumps has been demonstrated (1). Its in vitro spectrum of activity includes many Gram-positive and Gram-negative organisms, anaerobes, atypical organisms, and some multidrug-resistant pathogens (2–5). These attributes suggest a role for tigecycline in the empirical treatment of susceptible and resistant pathogens and the definitive treatment of tigecycline-susceptible resistant bacteria.
Tigecycline is approved for the treatment of complicated skin and skin structure infections, complicated intra-abdominal infections, and community-acquired bacterial pneumonia (6). The efficacy and safety of tigecycline for these indications have been demonstrated in phase 3 clinical trials (7–12). The recommended treatment regimen for tigecycline includes an initial infusion of 100-mg dose followed by 50 mg every 12 h (6), although in practice some clinicians have acknowledged using higher doses (13).
In a pooled analysis of phase 3 and 4 studies in patients with a variety of infections, an increase in all-cause mortality was found in tigecycline-treated patients versus comparators. In some other classes of antibiotics, such as macrolides and fluoroquinolones, mortality rates may be adversely affected by prolongation of the cardiac QTc (corrected QT) interval (14, 15).
Thorough QT studies were not routinely performed at the time of the clinical development of tigecycline, and until now, a QT study of tigecycline has not been performed due to the potential impact of vomiting on QTc measurement. The incidences of nausea and vomiting reported in clinical trials with tigecycline are 26% and 18%, respectively (6), although limited data suggest a potential role for food and hydration to improve tolerability (16, 17).
Nausea and vomiting are the major dose-related adverse events (AEs) associated with tigecycline treatment. A wider range of tigecycline doses, 12.5 to 300 mg, has been evaluated by logistic regression analysis in healthy volunteers which predicted incidences of 26% for nausea and 7.5% for vomiting in the group given 50 mg and ∼60% for nausea and ∼40% for vomiting in those treated with 200 mg (18).
The primary objective of this study was to evaluate the effect of tigecycline (50-mg and 200-mg dose intravenous [i.v.] infusions) relative to placebo on the QTc interval in healthy subjects. The secondary objectives were to evaluate the pharmacokinetics (PK) of tigecycline and assess the tolerability of tigecycline after administration with food and hydration in these subjects.
MATERIALS AND METHODS
Study design.
This was a single-dose, randomized, placebo-controlled, four-period crossover study in healthy volunteers conducted at a single center in the United States between January and May 2011. The protocol was approved by the institutional review board, and the study was performed in compliance with all International Conference on Harmonization (ICH) good clinical practice guidelines. Written informed consent was received from all subjects. This study has been registered at ClinicalTrials.gov under registration no. NCT01287793.
Entry criteria.
Men and women were eligible for entry into the study if they were between the ages of 18 and 55 years with a body mass index of 17.5 to 30.5 kg/m2 and a body weight of >50 kg. To ensure a healthy subject population, subjects underwent screening which included a detailed medical history, physical examination, including vital sign measurement, 12-lead electrocardiogram (ECG), and clinical laboratory tests.
Exclusion criteria.
Subjects were excluded from the study for the following: evidence or history of clinically significant hematological, renal, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, psychiatric, neurologic, or allergic diseases (including drug allergies but excluding untreated, asymptomatic, seasonal allergies at time of dosing), which would potentially interfere with the conduct or interpretation of the study; recent diarrhea (2 weeks); use of oral antibiotics (2 weeks) or i.v. antibiotics (2 to 3 months); any condition affecting drug absorption; use of tobacco/nicotine products; history of excessive alcohol consumption or positive urine drug screen; treatment with an investigational drug within 30 days or 5 half-lives; pregnancy, nursing, or unwilling/unable to use nonhormonal contraception; QTc > 450 ms or QRS interval > 120 ms; risk factors for QT prolongation or torsades de pointes including organic heart disease, congestive heart failure, hypokalemia, hypomagnesemia, congenital long QT syndrome, myocardial ischemia or infarction, and syncope; personal or family history of congenital deafness; use of prescription drugs or dietary supplements within 7 days or 5 half-lives prior to the first study dose; blood donation (∼500 ml) within 56 days prior to the first dose; or history of sensitivity to heparin or heparin-induced thrombocytopenia.
Treatment regimen.
After baseline ECG measurements and a standard breakfast followed by 1 liter of 0.9% NaCl administered i.v. over 1 h, subjects received 50 mg of tigecycline, 200 mg of tigecycline, or placebo administered i.v. over 30 min in a blinded fashion, or an open-label oral dose of moxifloxacin (400 mg) (positive control for QTc prolongation) (19) with 0.9% NaCl given i.v. over 30 min. The sequence of treatment periods was randomized (Williams square), and each treatment period was separated by a washout period of ≥1 week.
ECGs and PK sampling.
Triplicate 12-lead ECGs (separated by 2- to 4-min intervals) were collected at screening; 2.5, 2.0, and 1.5 h predose; 0 h (baseline); immediately after infusion (0.5 h postdose); and 1, 2, 3, 4, 8, 12, 16, 24, 48, 72, and 96 h postdose. When the timing of ECG measurements coincided with blood collection, the ECG measurement was performed prior to blood collection. Blood samples (5 ml) for PK analysis were collected at 0 h (baseline) and 0.5, 1, 2, 3, 4, 8, 12, 16, 24, 48, 72, and 96 h postdose. Serum samples were separated by centrifugation after clotting at room temperature, and serum samples were stored at approximately −20°C within 1 h of collection and assayed within the 617 days of established stability data. Tigecycline serum samples were assayed using a validated high-performance liquid chromatography tandem mass spectrometry method with a lower limit of quantification (LLOQ) of 10 ng/ml. Calibration standard responses were linear over the range of 10.0 ng/ml to 2,000 ng/ml, using a linear least-squares regression (liter/concentration squared). Clinical specimens with serum tigecycline concentrations below the LLOQ were reported as below the lower limit of quantification. The between-day assay accuracy, expressed as percent relative error (%RE), for quality control (QC) concentrations, ranged from 2.5% to 3.3% for the low, medium, high, and diluted QC samples. Assay precision, expressed as the between-day percent coefficients of variation (%CV) of the mean estimated concentrations of QC samples was ≤8.4%CV for low (25.0-ng/ml), medium (200-ng/ml), high (1,500-ng/ml), and diluted (20,000-ng/ml) concentrations.
Statistical analysis of QT/QTc.
In this study, the lack of an effect of tigecycline on QTc intervals was concluded if the upper bound of the two-sided 90% confidence interval (90% CI; equivalent to one-sided 95% CI) for all the time-matched mean difference between tigecycline and placebo was <10 ms (20). To demonstrate the sensitivity of the measure of QTc intervals, moxifloxacin was used as a positive control (19).
A sample size of 40 completers provided 99% power to exclude a 10-ms mean difference between tigecycline and placebo and to detect a mean difference of ≥5 ms between moxifloxacin and placebo at the historical 3-h median time to maximum drug concentration (Tmax) for moxifloxacin (positive control, as per ICH guidance) (20). To allow for dropouts, 48 subjects were recruited into the study (12 per sequence). The average of the triplicate ECG measurements collected predose on day 1 of each period served as the baseline QTc measurement for each subject.
Postdose QTc intervals were analyzed using a mixed-effects model with sequence, period, treatment, time, and treatment-by-time interaction as fixed effects, subject within sequence as a random effect, and baseline QTc as a covariate. Estimates of the adjusted mean differences (test reference), and the two-sided 90% CIs for each treatment and time, were obtained from the model and plotted against postdose time by treatments.
PK-pharmacodynamic (PD) analyses.
Tigecycline PK parameters were calculated for each subject and treatment using noncompartmental analysis of concentration-time data. A linear mixed-effects modeling approach was undertaken to characterize the relationship between the concentrations of tigecycline in serum and the appropriate QTc (QT corrected using Fridericia's correction [QTcF] or Bazett's correction [QTcB]) using a linear model: QTc = BSL + (SLP × CONC), where QTc was the dependent variable, BSL was the baseline QTc value, CONC was the serum tigecycline concentration observed at the same time at QTc, and SLP was the slope of the relationship between QTc and CONC. Full block (unstructured) variance/covariance matrices for the interindividual random effects (additive error models) on BSL and SLP were examined, and an additive residual error model was examined. The magnitude of change (mean and 90% CI) in QTc for the observed mean maximum concentration of drug in serum (Cmax) was calculated.
Safety and tolerability.
Each patient who received at least one dose of study drug was evaluated for safety. Safety assessments included recording AEs, vital signs, and laboratory measurements. For all AEs (including abnormal test findings, clinically significant signs or symptoms, changes in physical examination findings, hypersensitivity, progression or worsening of disease, and the signs or symptoms resulting from drug use and exposure during pregnancy), the investigator characterized the severity and the potential for a relationship to the study drug. Serious AEs (SAEs) included those that were life-threatening; required hospitalization; resulted in persistent or significant disability/incapacity, congenital abnormality or birth defect; or caused death.
RESULTS
Demographics.
In total, 48 subjects were randomized, treated, and had ≥1 postdose ECG measurement. One subject discontinued treatment after receiving placebo only, and one subject was removed from the study because of an AE (throat irritation) after receiving tigecycline (50 mg) only. The remaining 46 subjects received moxifloxacin, placebo, and both tigecycline doses and were included in the PK analyses, whereas all 48 subjects were included in safety and PD analyses. Most subjects were male, and most were black (Table 1).
Table 1.
Demographic characteristics
| Demographic characteristic | Value for demographic characteristic for: |
||
|---|---|---|---|
| Males (n = 44) | Females (n = 4) | All subjects (n = 48) | |
| Age, yr | |||
| Mean (SD) | 37.3 (8.8) | 37.0 (11.3) | 37.3 (8.9) |
| Range | 22–53 | 22–48 | 22–53 |
| Race, n | |||
| White | 12 | 1 | 13 |
| Black | 25 | 1 | 26 |
| Other | 7 | 2 | 9 |
| Wt, kg [mean (SD)] | 82.8 (9.7) | 76.4 (7.3) | 82.3 (9.7) |
| Body mass index, kg/m2 [mean (SD)] | 26.6 (2.6) | 28.7 (1.8) | 26.8 (2.6) |
ECG.
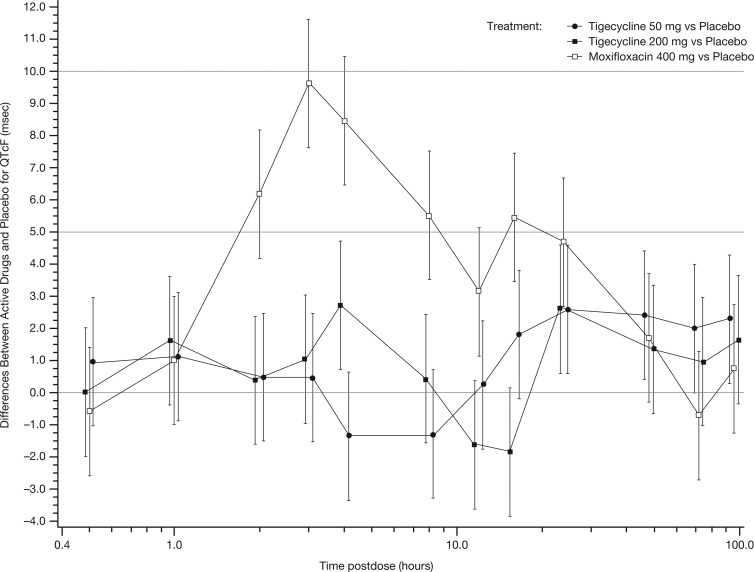
At all time points, for both tigecycline doses, the upper limits of the 90% CI for the mean differences between both tigecycline and placebo in change from baseline of QTcF interval were <5 ms, thereby meeting the criteria for a negative QT/QTc study and the absence of clinically significant prolongation of the QT interval by tigecycline (Fig. 1). The lower bound of the 90% CI for the mean differences between moxifloxacin and placebo was >5 ms at 3 h postdose (Fig. 1), indicating that the study had sufficient assay sensitivity to detect QT/QTc prolongation.
Fig 1.
Estimated mean differences between treatment and placebo postdose for QTcF with 90% CI. The change in QTcF is shown in milliseconds.
PK parameters.
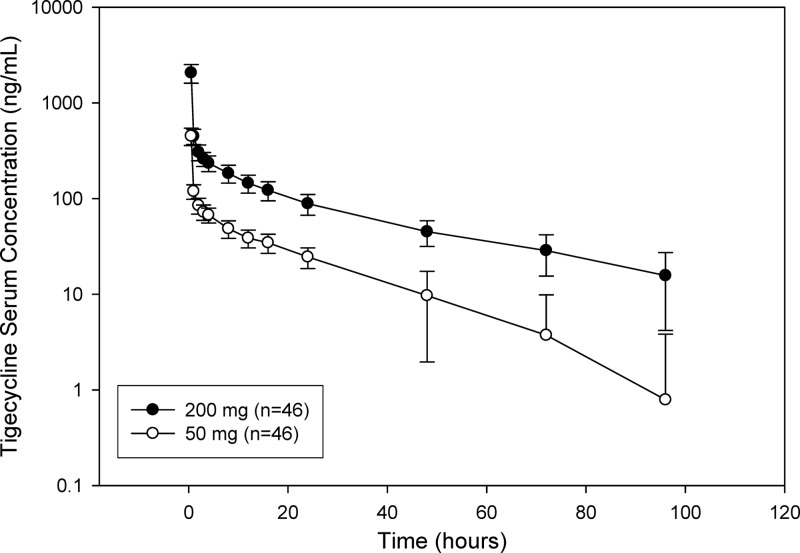
The PK parameters for tigecycline are summarized in Table 2. The concentrations of tigecycline in serum initially declined rapidly from peak concentrations observed at the end of the infusions (0.533 to 0.650 h across both doses), with a slower decline following distribution of drug into tissues (Fig. 2). The 4-fold increase in dose between the 50-mg and 200-mg doses was accompanied by a slightly greater than 4-fold mean increase in Cmax and a slightly smaller than 4-fold mean increase in area under the concentration-time curve from time zero extrapolated to infinity (AUC0–∞). Mean terminal elimination half-life (t1/2) values and clearance values (CL) were similar between the 50-mg and 200-mg treatments, with a slightly higher mean Vss value observed for the 200-mg dose compared with the 50-mg dose.
Table 2.
Tigecycline PK parameters following single 50-mg and 200-mg 30-min i.v. infusions in healthy volunteers
| PK parametera | Valueb for PK parameter for volunteers given the following dose: |
|
|---|---|---|
| 50 mg (n = 47)c | 200 mg (n = 46)c | |
| nd | 46 | 46 |
| ne | 26 | 46 |
| Cmax, ng/ml (%CV) | 432 (23) | 1964 (27) |
| Tmax, h (range) | 0.533 (0.533–0.650) | 0.533 (0.533–0.633) |
| t1/2, h | 22.1 ± 8.8 | 26.5 ± 5.1 |
| AUC0–∞, ng · h/ml (%CV) | 2,366 (26) | 8,237 (22) |
| CL, liter/h | 21.1 (28) | 24.3 (20) |
| Vss, liter | 610 (18) | 749 (17) |
Abbreviations: AUC0–∞, area under the concentration-time curve from time zero extrapolated to infinity; CL, clearance; Cmax, maximum serum drug concentration; CV, coefficient of variation; t1/2, terminal half-life; Tmax, time to maximum drug concentration; Vss, volume of distribution at steady state.
The values are geometric means, except the median values are given for Tmax and arithmetic mean ± standard deviation are given for t1/2.
Number of subjects in the treatment group in the indicated population.
Number of subjects contributing to the mean.
Number of subjects with reportable AUC0–∞, t1/2, CL, and Vss values.
Fig 2.
Serum tigecycline concentration following single 30-min i.v. 50-mg and 200-mg infusions. The means ± standard errors (SE) (error bars) are shown.
PK-PD modeling.
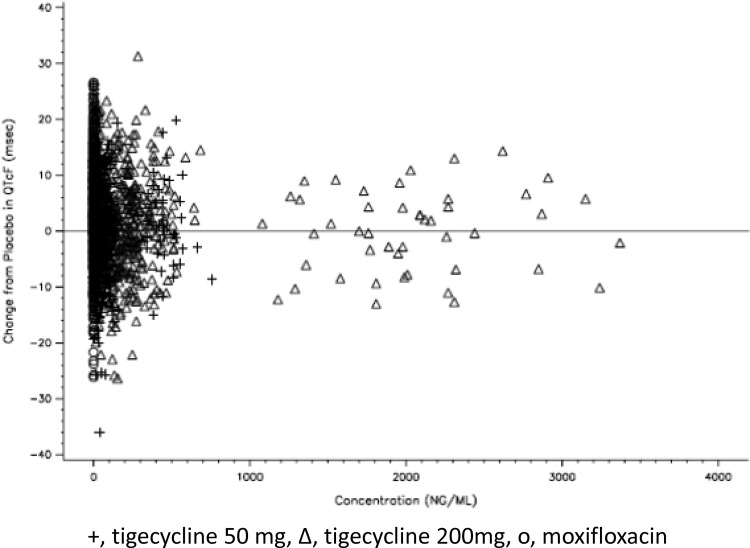
Linear mixed-effects modeling failed to show an effect on QTcF values by tigecycline concentrations (P = 0.755). A plot of individual study drug concentrations versus changes from placebo in QTcF interval showed no relationship between tigecycline concentrations and QTcF interval (Fig. 3).
Fig 3.
Plot of tigecycline concentrations versus change in QTcF. The tigecycline concentration is shown in nanograms per milliliter, and the change in QTcF is shown in milliseconds.
Safety and tolerability.
A summary of AEs is provided in Table 3. There were no SAEs, dose reductions, or temporary discontinuations of treatment due to an AE. One patient (2.1%) was removed from the study following administration of 50-mg tigecycline because of throat irritation, which was considered by the investigator to be moderate and related to treatment.
Table 3.
Adverse events
| Adverse event | No. of subjects (%) with the indicated AE given the following: |
|||
|---|---|---|---|---|
| Tigecycline |
Moxifloxacin (400 mg) (n = 46)a | Placebo (n = 47) | ||
| 50 mg (n = 47) | 200 mg (n = 46)a | |||
| Any AE | 23 (48.9) | 34 (73.9) | 21 (45.7) | 18 (38.3) |
| Most frequent AEsb | ||||
| Nausea | 9 (19.1) | 26 (56.5) | 3 (6.5) | 2 (4.3) |
| Vomiting | 2 (4.3) | 10 (21.7) | 0 | 0 |
| Decreased appetite | 0 | 6 (13.0) | 2 (4.3) | 1 (2.2) |
| Diarrhea | 4 (8.5) | 5 (10.9) | 3 (6.5) | 0 |
| Headache | 3 (6.4) | 3 (6.5) | 3 (6.5) | 4 (8.7) |
| Abdominal pain | 2 (4.3) | 4 (8.7) | 2 (4.3) | 1 (2.2) |
| Contusion | 3 (6.4) | 3 (6.5) | 1 (2.2) | 0 |
| Constipation | 3 (6.4) | 2 (4.3) | 1 (2.2) | 2 (4.3) |
| Dizziness | 0 | 3 (6.5) | 1 (2.2) | 0 |
One patient was removed from the study after receiving 50 mg tigecycline.
Occurring in ≥5% of subjects with any treatment.
Nausea and vomiting were the most frequent AEs, and the incidences were greater and related to the dose of tigecycline compared to either moxifloxacin or placebo. Compared with what was predicted in previous analyses in healthy volunteers who were not fed (i.e., fasting) and not provided with additional hydration, the incidence of nausea was similar to what was predicted, but the incidence of vomiting was reduced by approximately 44% for both doses administered.
Abnormal laboratory values were observed in 14 subjects. These abnormal values are shown in Table 4. The changes were generally sporadic increases and decreases in parameters and were mild in severity. No dose-related or treatment-related trends were evident, and all resolved without sequelae.
Table 4.
Laboratory abnormalities during each treatment period
| Subject (age [yr], gender)a | Lab test (baseline value; most abnormal value)b for subject during each treatment period |
|||
|---|---|---|---|---|
| Tigecycline |
Moxifloxacin (400 mg) | Placebo | ||
| 50 mg | 200 mg | |||
| 002 (40, M) | Eosinophils [%] | Eosinophils [%] | Eosinophils [%] | Eosinophils [%] |
| (9.4*; 10.4) | (9.4*; 9.9) | (9.4*; 10.3) | (9.4*; 10.3) | |
| 004 (35, F) | Urine blood/Hgb [qual] | |||
| (0; 1) | ||||
| Urine RBC [/HPF] | ||||
| (NR; 6) | ||||
| 005 (43, F) | Urine blood/Hgb [qual] (1*; 1) | |||
| 010 (39, M) | MCH [pg] (24.2*; 23.8) | Total neutrophils [1,000/mm3] | MCH [pg] (24.2*; 24.1) | |
| Total neutrophils [1,000/mm3] | (1.60; 1.00) | |||
| (1.60; 1.10) | ||||
| Monocytes [%] | ||||
| (13.9*;17.3) | ||||
| 016 (28, M) | Urine bilirubin [qual] | |||
| (0/1) | ||||
| 017 (46, M) | Basophils [%] | |||
| (2.1*; 3.4) | ||||
| 018 (36, M) | Urine blood/Hgb [qual] | |||
| (1*; 1) | ||||
| 020 (37, M) | ALT [IU/liter] | |||
| (37; 266) | ||||
| 034 (27, M) | Lymphocytes [%] | |||
| (47.3*; 54.5) | ||||
| 038 (44, M) | Lymphocytes [%] | Basophils [%] | ||
| (27.0; 8.7) | (2.0/2.6) | |||
| 040 (50, M) | MCH [pg] | MCH [pg] | MCH [pg] | MCH [pg] |
| (22.7*; 21.3) | (22.7*; 22.8) | (22.7*; 22.9) | (22.7*; 22.7) | |
| MCHC [g/dl] | Monocytes [%] | Basophils [%] | ||
| (30.5*; 28.5) | (8.2; 16.5) | (1.5; 2.7) | ||
| 041 (48, F) | Potassium [mEq/liter] | |||
| (4.8; 5.7) | ||||
| 043 (41, M) | Total bilirubin [mg/dl] | Total bilirubin [mg/dl] | Total bilirubin [mg/dl] | Total bilirubin [mg/dl] |
| (2.5*; 2.2) | (2.5*; 3.2) | (2.5*; 2.3) | (2.5*; 2.0) | |
| Indirect bilirubin | Indirect bilirubin | Indirect bilirubin | Indirect bilirubin | |
| [mg/dl] | [mg/dl] | [mg/dl] | [mg/dl] | |
| (2.6*; 2.3) | (2.6*; 3.5) | (2.6*; 2.4) | (2.6*; 2.1) | |
| 048 (22, M) | Urine bilirubin [qual] | |||
| (0; 1) | ||||
M, male; F, female.
Baseline values that are abnormal are indicated by an asterisk. Abbreviations: ALT, alanine aminotransferase; Hgb, hemoglobin; HPF, high-power field; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; NR, not reported; qual, qualitative; RBC, red blood cells.
DISCUSSION
A thorough QT study, which had not been conducted previously, was considered necessary following the identification of all-cause mortality increase in the tigecycline clinical program. In this study, tigecycline did not cause prolongation of the QT interval at either the approved dose (50 mg) or at the higher dose (200 mg), a dose that has been evaluated as a loading dose in a phase 2 study exploring higher doses in the treatment of hospital-acquired pneumonia (16). The threshold level of concern, as established in the ICH guideline E14, is a QTc value of around 5 ms with an upper bound 95% CI of 10 ms (20). For both tigecycline doses explored, no time-matched difference using the upper bound of a two-sided 90% CI exceeded 5 ms. The study was sufficiently sensitive to detect any potential effect of tigecycline on QT prolongation, and no relationship between serum tigecycline concentration and QTc interval was evident.
The results of this study are in agreement with prior observations in healthy subjects. Zimmerman and colleagues demonstrated that, 24 h after the administration of tigecycline to healthy men, a single 100-mg dose (loading dose) did not affect the QT interval, and the standard dose (50 mg every 12 h) did not prolong the QTc interval (21). These results are also consistent with results from the tigecycline clinical program in patients. In a phase 3 study, which evaluated 150 mg of tigecycline given once daily in the treatment of diabetic foot infections, a median QTcF change 60 min after a 30-min infusion of 5.5 ms was observed (Pfizer Inc., data on file). The upper bound of the 90% CI being <10 ms at this higher daily dose supports the low potential of tigecycline to cause clinically significant events in patients. The AEs defined by the ICH E14 as signals of potential proarrhythmic effects include torsades de pointes, sudden death, ventricular tachycardia, ventricular fibrillation and flutter, syncope, and seizures. None of these AEs alone, or in combination, were significantly elevated relative to comparator (Pfizer Inc., data on file).
Pfizer has conducted both study-level and patient-level analysis on all-cause mortality in the phase 3 and 4 clinical program (22). In general, the deaths appear related to worsening or complications of the infection or underlying comorbidities. Despite an association with increased mortality, causality is difficult to conclude across the entire clinical program. Substantial differences were observed among evaluated infection types. For example, hospital-acquired pneumonia (HAP), particularly in subjects with ventilator-associated pneumonia, had the highest mortality rates with decreased efficacy, the likely explanation for the observed excess mortality in this indication. A phase 2 study looking at higher doses of tigecycline in the treatment of HAP was stopped early due to enrollment difficulties (16). Although additional confirmation is needed, the data on the higher doses suggested a possible benefit without additional safety concerns. This study adds to an evolving safety profile at higher tigecycline doses by demonstrating no QT prolongation at the 200-mg dose in healthy volunteers.
The PK parameters observed in the current study are consistent with previous studies (17, 21, 23). As noted previously, the concentration-time profile after tigecycline administration includes a rapid distribution phase and an extensive elimination phase (17), which we observed in this study. Dose proportionality has previously been demonstrated for doses ranging between 12.5 mg and 300 mg (17), which is beyond the dose range used in this study. The modest departures from linearity observed in this study are not considered clinically relevant. PK parameters of tigecycline have been shown to be unaffected by subject age, gender, renal function, concomitant medications, or food status (fasting or fed) (17, 23); therefore, the parameters observed in this study can be extrapolated to what would have been observed in the phase 3 and 4 clinical trials.
Efforts to mitigate nausea and vomiting have been part of the clinical experience with tigecycline. Based on a small series of cases, it has been suggested that the incidence of nausea and vomiting, at least with higher doses, might be reduced by increasing the infusion volume; no nausea and vomiting was observed when tigecycline was given in a volume of 400 ml (13).
Administration with food to reduce nausea and vomiting has been proposed as another strategy. In a study of healthy subjects, a maximum tolerated dose of 100-mg tigecycline in subjects who were not fed (i.e., fasting) was increased to 200 mg when the subjects were fed prior to the infusion, but infusion in a volume of 250 ml did not appear to change the incidence of nausea and vomiting (17). The PK of tigecycline was unaffected in fed subjects compared with subjects who were not fed (17). Although not well controlled, this study suggests that in the absence of administration of antiemetics, additional hydration and food prior to tigecycline treatment may decrease the incidence of vomiting but will be unlikely to have an effect on nausea.
This study provides further support for the safety of tigecycline by excluding the likelihood of effects on the cardiac QTc interval. Given these results, a role for QT prolongation seems unlikely to be the explanation for the increase in all-cause mortality in tigecycline-treated subjects in the clinical program.
ACKNOWLEDGMENTS
This study was funded by Pfizer Inc., Collegeville, PA. Editorial support was provided by Ed Parr and Sharmy Blows of UBC Scientific Solutions and was funded by Pfizer Inc.
Footnotes
Published ahead of print 12 February 2013
REFERENCES
- 1. Dean CR, Visalli MA, Projan SJ, Sum PE, Bradford PA. 2003. Efflux-mediated resistance to tigecycline (GAR-936) in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 47:972–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gales AC, Jones RN. 2000. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn. Microbiol. Infect. Dis. 36:19–36 [DOI] [PubMed] [Google Scholar]
- 3. Milatovic D, Schmitz FJ, Verhoef J, Fluit AC. 2003. Activities of the glycylcycline tigecycline (GAR-936) against 1,924 recent European clinical bacterial isolates. Antimicrob. Agents Chemother. 47:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen PJ, Bradford PA, Weiss WJ, Murphy TM, Sum PE, Projan SJ. 2002. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant Gram-positive pathogens. Antimicrob. Agents Chemother. 46:2595–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petersen PJ, Jacobus NV, Weiss WJ, Sum PE, Testa RT. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wyeth Pharmaceuticals Inc 2012. Tygacil prescribing information. Wyeth Pharmaceuticals Inc., Philadelphia, PA: [Online.] http://labeling.pfizer.com/showlabeling.aspx?id=491 [Google Scholar]
- 7. Babinchak T, Ellis-Grosse E, Dartois N, Rose GM, Loh E. 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin. Infect. Dis. 41(Suppl 5):S354–S367 [DOI] [PubMed] [Google Scholar]
- 8. Bergallo C, Jasovich A, Teglia O, Oliva ME, Lentnek A, de Wouters L, Zlocowski JC, Dukart G, Cooper A, Mallick R. 2009. Safety and efficacy of intravenous tigecycline in treatment of community-acquired pneumonia: results from a double-blind randomized phase 3 comparison study with levofloxacin. Diagn. Microbiol. Infect. Dis. 63:52–61 [DOI] [PubMed] [Google Scholar]
- 9. Breedt J, Teras J, Gardovskis J, Maritz FJ, Vaasna T, Ross DP, Gioud-Paquet M, Dartois N, Ellis-Grosse EJ, Loh E. 2005. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob. Agents Chemother. 49:4658–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliva ME, Rekha A, Yellin A, Pasternak J, Campos M, Rose GM, Babinchak T, Ellis-Grosse EJ, Loh E, 301 Study Group 2005. A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744]. BMC Infect. Dis. 5:88 doi:10.1186/1471-2334-5-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacchidanand S, Penn RL, Embil JM, Campos ME, Curcio D, Ellis-Grosse E, Loh E, Rose G. 2005. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int. J. Infect. Dis. 9:251–261 [DOI] [PubMed] [Google Scholar]
- 12. Tanaseanu C, Milutinovic S, Calistru PI, Strausz J, Zolubas M, Chernyak V, Dartois N, Castaing N, Gandjini H, Cooper CA, 313 Study Group 2009. Efficacy and safety of tigecycline versus levofloxacin for community-acquired pneumonia. BMC Pulm. Med. 9:44 doi:10.1186/1471-2466-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunha BA. 2009. Pharmacokinetic considerations regarding tigecycline for multidrug-resistant (MDR) Klebsiella pneumoniae or MDR Acinetobacter baumannii urosepsis. J. Clin. Microbiol. 47:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo D, Cai Y, Chai D, Liang B, Bai N, Wang R. 2010. The cardiotoxicity of macrolides: a systematic review. Pharmazie 65:631–640 [PubMed] [Google Scholar]
- 15. Mehlhorn AJ, Brown DA. 2007. Safety concerns with fluoroquinolones. Ann. Pharmacother. 41:1859–1866 [DOI] [PubMed] [Google Scholar]
- 16. Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. 2013. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin against hospital-acquired pneumonia. Antimicrob. Agents Chemother. 57:1756–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. 2005. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 49:220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Passarell J, Ludwig E, Liolios K, Meagher AK, Grasela TH, Babinchak T, Ellis-Grosse EJ. 2009. Exposure-response analyses of tigecycline tolerability in healthy subjects. Diagn. Microbiol. Infect. Dis. 65:123–129 [DOI] [PubMed] [Google Scholar]
- 19. Bloomfield DM, Kost JT, Ghosh K, Hreniuk D, Hickey LA, Guitierrez MJ, Gottesdiener K, Wagner JA. 2008. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin. Pharmacol. Ther. 84:475–480 [DOI] [PubMed] [Google Scholar]
- 20. International Conference on Harmonization 2005. E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129357.pdf [PubMed] [Google Scholar]
- 21. Zimmerman JJ, Harper DM, Matschke K, Speth JL, Raible DG, Fruncillo RJ. 2007. Absence of an interaction between tigecycline and digoxin in healthy men. Pharmacotherapy 27:835–844 [DOI] [PubMed] [Google Scholar]
- 22. McGovern P, Wible M, El-Tahtawy A, Biswas P, Meyer D. 2010. Mortality imbalance in the tigecycline phase 3 and 4 clinical trials. Crit. Care Med. 38(Suppl 12):A302. [DOI] [PubMed] [Google Scholar]
- 23. Muralidharan G, Fruncillo RJ, Micalizzi M, Raible DG, Troy SM. 2005. Effects of age and sex on single-dose pharmacokinetics of tigecycline in healthy subjects. Antimicrob. Agents Chemother. 49:1656–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]