Abstract
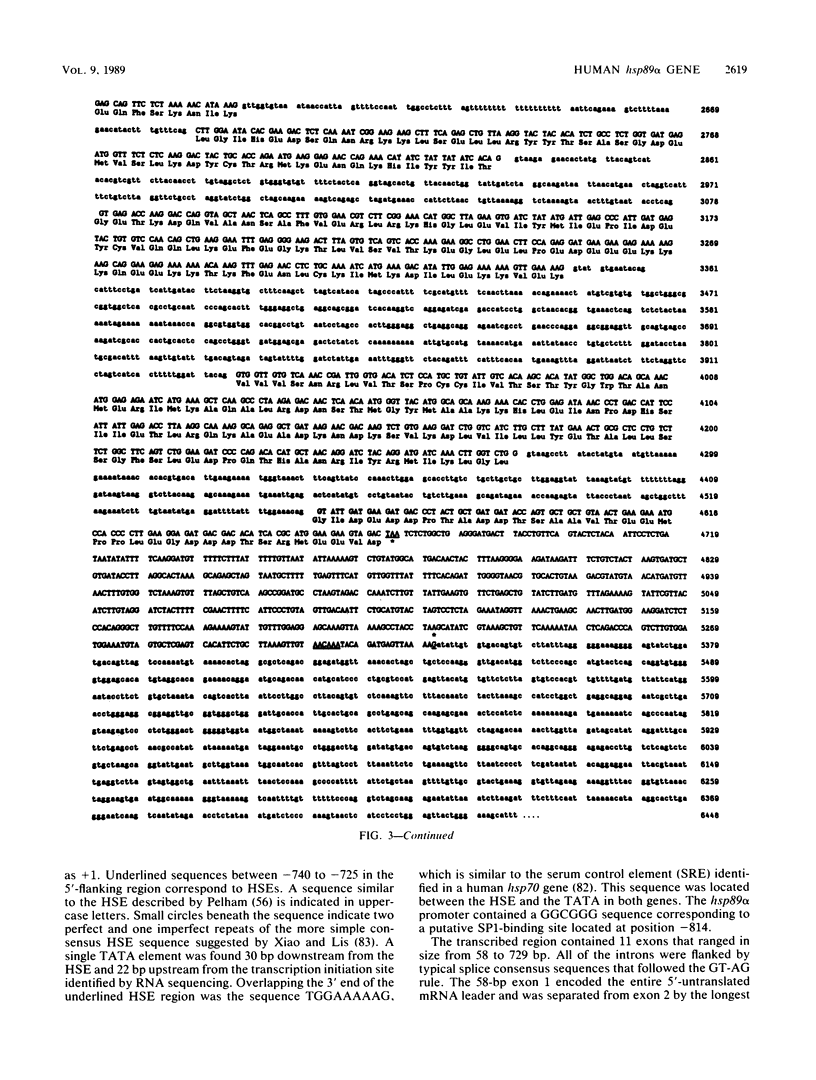
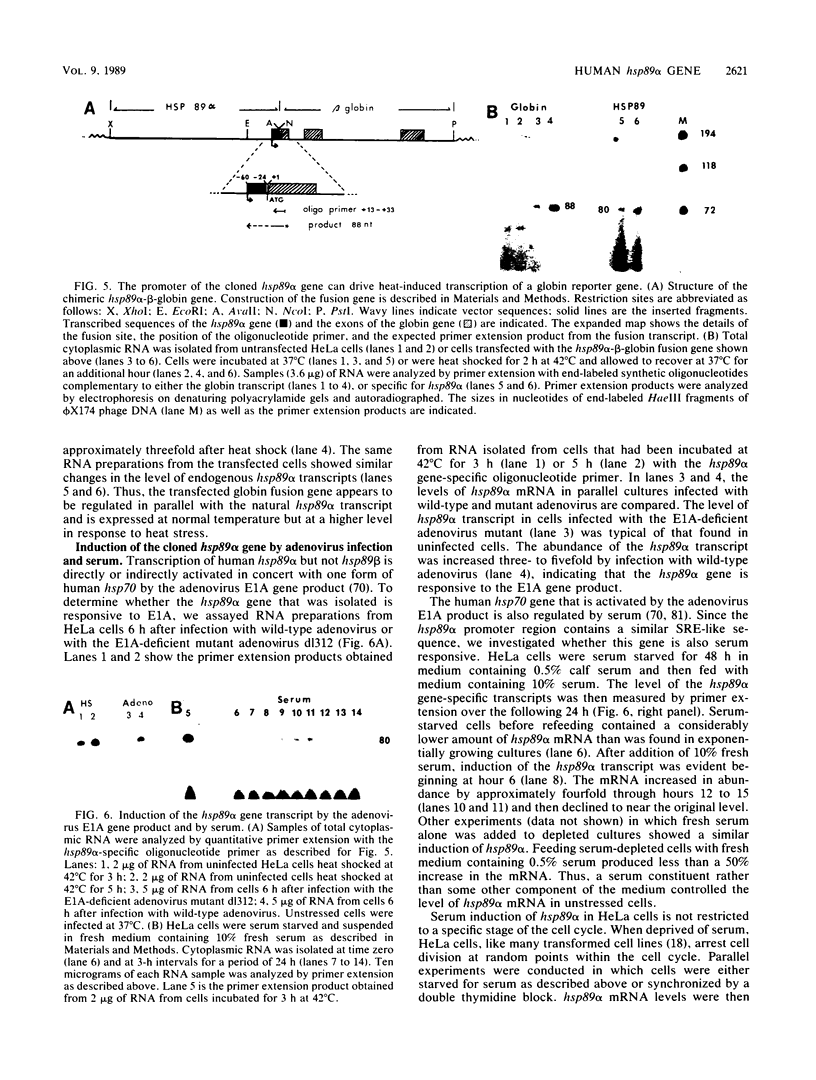
Vertebrate cells synthesize two forms of the 82- to 90-kilodalton heat shock protein that are encoded by distinct gene families. In HeLa cells, both proteins (hsp89 alpha and hsp89 beta) are abundant under normal growth conditions and are synthesized at increased rates in response to heat stress. Only the larger form, hsp89 alpha, is induced by the adenovirus E1A gene product (M. C. Simon, K. Kitchener, H. T. Kao, E. Hickey, L. Weber, R. Voellmy, N. Heintz, and J. R. Nevins, Mol. Cell. Biol. 7:2884-2890, 1987). We have isolated a human hsp89 alpha gene that shows complete sequence identity with heat- and E1A-inducible cDNA used as a hybridization probe. The 5'-flanking region contained overlapping and inverted consensus heat shock control elements that can confer heat-inducible expression on a beta-globin reporter gene. The gene contained 10 intervening sequences. The first intron was located adjacent to the translation start codon, an arrangement also found in the Drosophila hsp82 gene. The spliced mRNA sequence contained a single open reading frame encoding an 84,564-dalton polypeptide showing high homology with the hsp82 to hsp90 proteins of other organisms. The deduced hsp89 alpha protein sequence differed from the human hsp89 beta sequence reported elsewhere (N. F. Rebbe, J. Ware, R. M. Bertina, P. Modrich, and D. W. Stafford (Gene 53:235-245, 1987) in at least 99 out of the 732 amino acids. Transcription of the hsp89 alpha gene was induced by serum during normal cell growth, but expression did not appear to be restricted to a particular stage of the cell cycle. hsp89 alpha mRNA was considerably more stable than the mRNA encoding hsp70, which can account for the higher constitutive rate of hsp89 synthesis in unstressed cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell J. C., Craig E. A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnier J. V., Bensaude O., Morange M., Babinet C. Mouse 89 kD heat shock protein. Two polypeptides with distinct developmental regulation. Exp Cell Res. 1987 May;170(1):186–194. doi: 10.1016/0014-4827(87)90128-5. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. Steroid hormone antagonists at the receptor level: a role for the heat-shock protein MW 90,000 (hsp 90). J Cell Biochem. 1987 Oct;35(2):161–174. doi: 10.1002/jcb.240350209. [DOI] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. EMBO J. 1984 Nov;3(11):2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman R. K., Meselson M. Interspecific nucleotide sequence comparisons used to identify regulatory and structural features of the Drosophila hsp82 gene. J Mol Biol. 1986 Apr 20;188(4):499–515. doi: 10.1016/s0022-2836(86)80001-8. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 1988 Nov;7(11):3509–3518. doi: 10.1002/j.1460-2075.1988.tb03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. The chicken ubiquitin gene contains a heat shock promoter and expresses an unstable mRNA in heat-shocked cells. Mol Cell Biol. 1986 Dec;6(12):4602–4610. doi: 10.1128/mcb.6.12.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J., Yonemoto W., Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983 Jan;3(1):9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard P. A., Brandhorst B. P. Translational activation of maternal mRNA encoding the heat-shock protein hsp90 during sea urchin embryogenesis. Dev Biol. 1986 Sep;117(1):286–293. doi: 10.1016/0012-1606(86)90371-4. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Carbajal M. E., Duband J. L., Lettre F., Valet J. P., Tanguay R. M. Cellular localization of Drosophila 83-kilodalton heat shock protein in normal, heat-shocked, and recovering cultured cells with a specific antibody. Biochem Cell Biol. 1986 Aug;64(8):816–825. doi: 10.1139/o86-110. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier N. C., Schlesinger M. J. The dynamic state of heat shock proteins in chicken embryo fibroblasts. J Cell Biol. 1986 Oct;103(4):1495–1507. doi: 10.1083/jcb.103.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragon E. A., Sias S. R., Kato E. A., Gabe J. D. The genome of Trypanosoma cruzi contains a constitutively expressed, tandemly arranged multicopy gene homologous to a major heat shock protein. Mol Cell Biol. 1987 Mar;7(3):1271–1275. doi: 10.1128/mcb.7.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrow R., Riddle V. G., Pardee A. B. Different responses to drugs and serum of cells transformed by various means. Cancer Res. 1979 Jul;39(7 Pt 1):2718–2726. [PubMed] [Google Scholar]
- Dworniczak B., Mirault M. E. Structure and expression of a human gene coding for a 71 kd heat shock 'cognate' protein. Nucleic Acids Res. 1987 Jul 10;15(13):5181–5197. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrelly F. W., Finkelstein D. B. Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem. 1984 May 10;259(9):5745–5751. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Polvere R. I., Van der Ploeg L. H. Conserved sequences and transcription of the hsp70 gene family in Trypanosoma brucei. Mol Cell Biol. 1986 Dec;6(12):4657–4666. doi: 10.1128/mcb.6.12.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haire R. N., Peterson M. S., O'Leary J. J. Mitogen activation induces the enhanced synthesis of two heat-shock proteins in human lymphocytes. J Cell Biol. 1988 Mar;106(3):883–891. doi: 10.1083/jcb.106.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A. Modulation of heat-shock polypeptide synthesis in HeLa cells during hyperthermia and recovery. Biochemistry. 1982 Mar 30;21(7):1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Sadis S., Smale G., Weber L. A. Molecular cloning of sequences encoding the human heat-shock proteins and their expression during hyperthermia. Gene. 1986;43(1-2):147–154. doi: 10.1016/0378-1119(86)90018-1. [DOI] [PubMed] [Google Scholar]
- Howard K. J., Distelhorst C. W. Effect of the 90 kDa heat shock protein, HSP90, on glucocorticoid receptor binding to DNA-cellulose. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1226–1232. doi: 10.1016/s0006-291x(88)80497-2. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- Kay R. J., Russnak R. H., Jones D., Mathias C., Candido E. P. Expression of intron-containing C. elegans heat shock genes in mouse cells demonstrates divergence of 3' splice site recognition sequences between nematodes and vertebrates, and an inhibitory effect of heat shock on the mammalian splicing apparatus. Nucleic Acids Res. 1987 May 11;15(9):3723–3741. doi: 10.1093/nar/15.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsek D. A., Beattie W. G., Tsai M. J., O'Malley B. W. Molecular cloning of a steroid-regulated 108K heat shock protein gene from hen oviduct. Nucleic Acids Res. 1986 Dec 22;14(24):10053–10069. doi: 10.1093/nar/14.24.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., Nishida E., Kadowaki T., Matsuzaki F., Iida K., Harada F., Kasuga M., Sakai H., Yahara I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8054–8058. doi: 10.1073/pnas.83.21.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lagrimini L. M., Brentano S. T., Donelson J. E. A DNA sequence analysis package for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):605–614. doi: 10.1093/nar/12.1part2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai B. T., Chin N. W., Stanek A. E., Keh W., Lanks K. W. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984 Dec;4(12):2802–2810. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J. Purification and characterization of a major component from the cytoplasmic matrix of cultured murine L cells. Biochim Biophys Acta. 1979 May 23;578(1):1–12. doi: 10.1016/0005-2795(79)90106-5. [DOI] [PubMed] [Google Scholar]
- Lanks K. W. Metabolite regulation of heat shock protein levels. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5325–5329. doi: 10.1073/pnas.80.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lees-Miller S. P., Anderson C. W. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem. 1989 Feb 15;264(5):2431–2437. [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lowe D. G., Fulford W. D., Moran L. A. Mouse and Drosophila genes encoding the major heat shock protein (hsp70) are highly conserved. Mol Cell Biol. 1983 Aug;3(8):1540–1543. doi: 10.1128/mcb.3.8.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. G., Moran L. A. Molecular cloning and analysis of DNA complementary to three mouse Mr = 68,000 heat shock protein mRNAs. J Biol Chem. 1986 Feb 15;261(5):2102–2112. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moore S. K., Kozak C., Robinson E. A., Ullrich S. J., Appella E. Cloning and nucleotide sequence of the murine hsp84 cDNA and chromosome assignment of related sequences. Gene. 1987;56(1):29–40. doi: 10.1016/0378-1119(87)90155-7. [DOI] [PubMed] [Google Scholar]
- Morange M., Diu A., Bensaude O., Babinet C. Altered expression of heat shock proteins in embryonal carcinoma and mouse early embryonic cells. Mol Cell Biol. 1984 Apr;4(4):730–735. doi: 10.1128/mcb.4.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida E., Koyasu S., Sakai H., Yahara I. Calmodulin-regulated binding of the 90-kDa heat shock protein to actin filaments. J Biol Chem. 1986 Dec 5;261(34):16033–16036. [PubMed] [Google Scholar]
- Ogden R. C., Adams D. A. Electrophoresis in agarose and acrylamide gels. Methods Enzymol. 1987;152:61–87. doi: 10.1016/0076-6879(87)52011-0. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt W. B. Transformation of glucocorticoid and progesterone receptors to the DNA-binding state. J Cell Biochem. 1987 Sep;35(1):51–68. doi: 10.1002/jcb.240350105. [DOI] [PubMed] [Google Scholar]
- Ramachandran C., Catelli M. G., Schneider W., Shyamala G. Estrogenic regulation of uterine 90-kilodalton heat shock protein. Endocrinology. 1988 Aug;123(2):956–961. doi: 10.1210/endo-123-2-956. [DOI] [PubMed] [Google Scholar]
- Ratajczak T., Brockway M. J., Hähnel R., Moritz R. L., Simpson R. J. Sequence analysis of the nonsteroid binding component of the calf uterine estrogen receptor. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1156–1163. doi: 10.1016/s0006-291x(88)80487-x. [DOI] [PubMed] [Google Scholar]
- Rebbe N. F., Ware J., Bertina R. M., Modrich P., Stafford D. W. Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene. 1987;53(2-3):235–245. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose D. W., Wettenhall R. E., Kudlicki W., Kramer G., Hardesty B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry. 1987 Oct 20;26(21):6583–6587. doi: 10.1021/bi00395a003. [DOI] [PubMed] [Google Scholar]
- Russnak R. H., Candido E. P. Locus encoding a family of small heat shock genes in Caenorhabditis elegans: two genes duplicated to form a 3.8-kilobase inverted repeat. Mol Cell Biol. 1985 Jun;5(6):1268–1278. doi: 10.1128/mcb.5.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadis S., Hickey E., Weber L. A. Effect of heat shock on RNA metabolism in HeLa cells. J Cell Physiol. 1988 Jun;135(3):377–386. doi: 10.1002/jcp.1041350304. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Meshinchi S., Tienrungroj W., Schlesinger M. J., Toft D. O., Pratt W. B. Relationship of the 90-kDa murine heat shock protein to the untransformed and transformed states of the L cell glucocorticoid receptor. J Biol Chem. 1987 May 25;262(15):6986–6991. [PubMed] [Google Scholar]
- Sanchez E. R., Redmond T., Scherrer L. C., Bresnick E. H., Welsh M. J., Pratt W. B. Evidence that the 90-kilodalton heat shock protein is associated with tubulin-containing complexes in L cell cytosol and in intact PtK cells. Mol Endocrinol. 1988 Aug;2(8):756–760. doi: 10.1210/mend-2-8-756. [DOI] [PubMed] [Google Scholar]
- Schwindinger W. F., Warner J. R. DNA sequence analysis on the IBM-PC. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):601–604. doi: 10.1093/nar/12.1part2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Kitchener K., Kao H. T., Hickey E., Weber L., Voellmy R., Heintz N., Nevins J. R. Selective induction of human heat shock gene transcription by the adenovirus E1A gene products, including the 12S E1A product. Mol Cell Biol. 1987 Aug;7(8):2884–2890. doi: 10.1128/mcb.7.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Cloning and expression of a gene encoding hsc73, the major hsp70-like protein in unstressed rat cells. EMBO J. 1987 Apr;6(4):993–998. doi: 10.1002/j.1460-2075.1987.tb04850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Theodorakis N. G., Morimoto R. I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987 Dec;7(12):4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S. J., Robinson E. A., Law L. W., Willingham M., Appella E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc Natl Acad Sci U S A. 1986 May;83(10):3121–3125. doi: 10.1073/pnas.83.10.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. I., Hunt T., Jackson R. J., Anderson C. W. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985 Jan;4(1):139–145. doi: 10.1002/j.1460-2075.1985.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. A., Nilsen T., Baglioni C. Isolation of histone messenger RNA and its translation in vitro. Methods Cell Biol. 1978;19:215–236. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Wu B. J., Kingston R. E., Morimoto R. I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Morimoto R. I. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B. J., Williams G. T., Morimoto R. I. Detection of three protein binding sites in the serum-regulated promoter of the human gene encoding the 70-kDa heat shock protein. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2203–2207. doi: 10.1073/pnas.84.8.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. Germline transformation used to define key features of heat-shock response elements. Science. 1988 Mar 4;239(4844):1139–1142. doi: 10.1126/science.3125608. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Catelli M. G., Joab I., Moncharmont B. Association of the heat shock protein hsp90 with steroid hormone receptors and tyrosine kinase oncogene products. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1298–1307. doi: 10.1016/s0006-291x(86)80424-7. [DOI] [PubMed] [Google Scholar]
- van Bergen en Henegouwen P. M., Berbers G., Linnemans W. A., van Wijk R. Subcellular localization of the 84,000 dalton heat-shock protein in mouse neuroblastoma cells: evidence for a cytoplasmic and nuclear location. Eur J Cell Biol. 1987 Jun;43(3):469–478. [PubMed] [Google Scholar]