Abstract
Recently, we have demonstrated that the cognate regulatory locus of the mecA gene in methicillin-resistant Staphylococcus aureus (MRSA) is in fact a three-component system containing the novel mecR2 gene coding for an antirepressor. MecR2 interacts with the repressor MecI, disturbing its binding to the mecA promoter and fostering its proteolysis. Here, we engineered a point mutation in the putative cleavage site of MecI and demonstrated that MecI proteolysis is strictly required for the optimal expression of β-lactam resistance.
TEXT
In response to β-lactam chemotherapy, Staphylococcus aureus has acquired two resistance mechanisms: the production of a β-lactamase, coded by blaZ, which confers resistance to penicillins, and the production of an extra penicillin-binding protein (PBP2a), coded by mecA, with reduced affinity to virtually all other β-lactams. Until recently, the transcriptional control of both resistance genes was thought to be regulated by homologous two-component systems consisting of a bifunctional sensor-inducer, BlaR1 or MecR1, respectively, and a transcriptional repressor, BlaI or MecI, respectively. However, we have recently demonstrated that the mecA regulatory locus is in fact a three-component system that also contains the mecR2 gene, coding for an antirepressor. MecR2 interacts directly with MecI, disturbing its binding to the mecA promoter and fostering its proteolysis (1). Surprisingly, in sharp contrast to what has been described for the bla system (2, 3), the MecI proteolysis occurs in the absence of MecR1 and, as such, must be mediated by native cytoplasmatic proteases. Here, we thought to clarify the role of MecI proteolysis in the expression of β-lactam resistance by methicillin-resistant Staphylococcus aureus (MRSA). For that purpose, we engineered a point mutation in the putative cleavage site of MecI, N101-F (4). The MecI variant was then introduced into prototype MRSA strain N315 (5), which has been used in many studies addressing the transcriptional control of mecA and in which we described the function of the mecR2 gene. The phenotypic expression of β-lactam resistance, as well as the proteolysis of MecI, were then evaluated in a recombinant variant of strain N315 overexpressing the MecI variant.
To generate the MecI mutant variant (MecIMUT) we used the QuikChange site-directed mutagenesis strategy (6). Briefly, using as the template DNA a recombinant high-copy-number Escherichia coli-S. aureus shuttle plasmid overexpressing a wild-type copy of mecI, pGC2::mecI (7), complementary mutagenic primer sequences (MecI-SDM1, 5′ GGT TTC AAT TCA CTT GTC TTG CAG CTT GTA GAA AAA GAA GAT CTA TC 3′, and MecI-SDM2, 5′ GAT AGA TCT TCT TTT TCT ACA AGC TGC AAG ACA AGT GAA TTG AAA CC 3′; miss-priming positions are underlined) were extended with Phusion high-fidelity DNA polymerase (New England BioLabs). Parental methylated and hemimethylated DNA was digested with DpnI (New England BioLabs) overnight, followed by enzyme inactivation at 80°C for 20 min and dialysis through a 0.025 μM membrane (Millipore) at 1:1,000 for 30 min. Mutated plasmid DNA was then transformed into XL10 Gold ultracompetent E. coli cells (Agilent Technologies). The DNA sequence of the MecIMUT insert was confirmed after PCR amplification with two flanking primers (MecI-P1, 5′ GC ACA ACA AAT TTC TGA GCG 3′, and MecI-P2, 5′ TC AAC GAC TTG ATT GTT TCC 3′). Finally, the recombinant plasmid pGC2::mecIMUT was introduced into the restriction-deficient strain RN4220 (R. Novick) by electroporation and then transduced into the prototype MRSA strain N315, as previously described (8, 9). The phenotypic expression of β-lactam resistance was evaluated with 1-mg-oxacillin diffusion disks prepared in-house (10). The proteolysis of MecI under induction conditions (sub-MIC oxacillin concentration of 0.05 mg/liter) was analyzed by Western blotting with a polyclonal antibody raised against purified MecI (Eurogentec), as previously described (1).
In previous studies, we observed that, contrary to what was expected, the overexpression in trans of MecI in a representative collection of prototype MRSA strains did not cause significant effects on the expression of β-lactam resistance in most cases (7). Later, we demonstrated that the “MecI overexpression protection effect” was due to the presence of a third regulator in the mecA locus, MecR2, acting as a potent antirepressor and fostering the proteolysis of MecI (1). As shown by the results in Fig. 1, when prototype MRSA strain N315, which is mecR2 positive, was transformed with the recombinant plasmid overexpressing the MecI mutant variant with a mutation in the putative cleavage site [N315(pGC2::mecIMUT)], the levels of resistance to oxacillin decreased much more substantially than when strain N315 was transformed with wild-type MecI [N315(pGC2::mecI)]. These observations suggest that the protective function of the anti-repressor MecR2 is lost in the presence of a MecI variant that is resistant to proteolysis. In order to further explore these observations, we have evaluated the proteolysis of MecI for the parental strain N315 and for both recombinant strains, overexpressing wild-type and mutant MecI [N315(pGC2::mecI) and N315(pGC2::mecIMUT)]. Previously, we have observed that the presence of MecR2 was required for the accumulation of cleaved MecI under induction conditions (1). As shown by the results in Fig. 2, in recombinant strain N315(pGC2::mecIMUT), MecI accumulates in its intact form, explaining the poor expression of oxacillin resistance in this recombinant strain.
Fig 1.
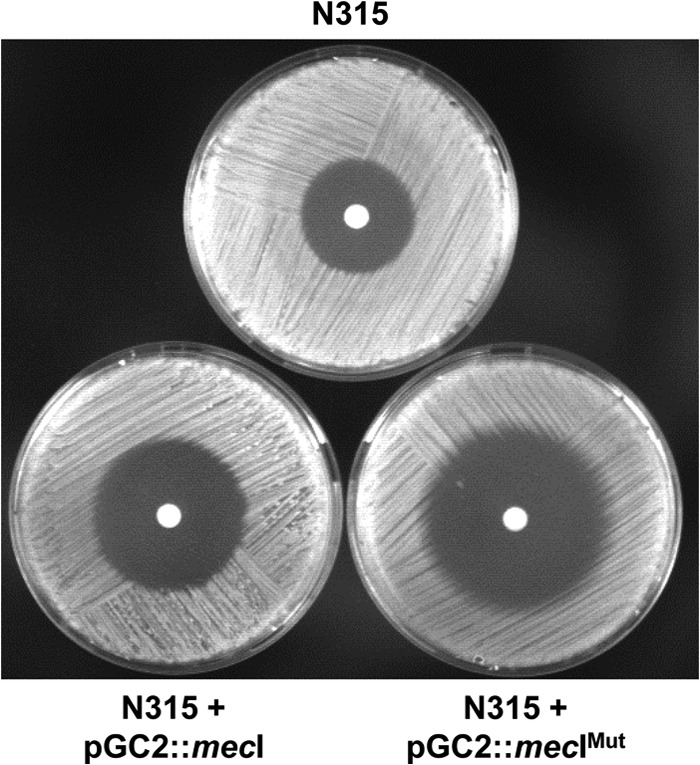
Analysis of the phenotypic expression of β-lactam resistance. The phenotypic expression of β-lactam resistance in prototype strain N315 and recombinant strains overexpressing wild-type and mutant MecI variants [N315(pGC2::mecI) and N315(pGC2::mecIMUT)] was evaluated with diffusion disks containing 1 mg of oxacillin.
Fig 2.
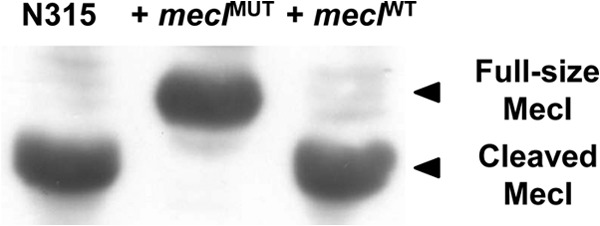
Analysis of the MecI proteolysis by Western blotting. The proteolysis of MecI under induction conditions (sub-MIC of oxacillin at 0.05 mg/liter) was evaluated for prototype strain N315 and recombinant strains overexpressing wild-type and mutant MecI variants [N315(pGC2::mecI) and N315(pGC2::mecIMUT)] using a polyclonal antibody raised against purified MecI.
Together, these observations confirm that the proteolysis of MecI occurs at position N101-F and demonstrate that it is strictly necessary for the optimal induction of β-lactam resistance in MRSA strains. Therefore, similar to what has been demonstrated for the bla system of S. aureus (2), the proteolysis of the repressor protein is required for proper induction of mecA and is not a secondary event, as described for the homologous pen system of Bacillus licheniformis (11). However, contrary to what happens for the bla system (2, 3), the proteolysis of MecI is independent of the sensor-inducer MecR1 and, presumably, is mediated by native cytoplasmatic proteases (1). The elucidation of the fine molecular details of the induction mechanism underlying the expression of the mecA gene may pave the way for the design of alternative therapeutic strategies targeting the MRSA phenotype and, hence, contribute to attenuation of the burden of MRSA infections.
ACKNOWLEDGMENTS
We thank K. Hiramatsu for the kind gift of prototype MRSA strain N315 used in this study.
Partial support for this study was provided by projects POCI/BIA-MIC/60320/2004 and PTDC/BIA-MIC/64071/2006 from Fundação para a Ciência e Tecnologia (FCT), Lisbon, Portugal, awarded to D.C.O., and P.A. was supported by grant SFRH/BD/38316/2007 from FCT.
Footnotes
Published ahead of print 12 February 2013
REFERENCES
- 1. Arede P, Milheirico C, De Lencastre H, Oliveira DC. 2012. The anti-repressor MecR2 promotes the proteolysis of the mecA repressor and enables optimal expression of β-lactam resistance in MRSA. PLoS Pathog. 8:e1002816 doi:10.1371/journal.ppat.1002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang HZ, Hackbarth CJ, Chansky KM, Chambers HF. 2001. A proteolytic transmembrane signaling pathway and resistance to beta-lactams in staphylococci. Science 291:1962–1965 [DOI] [PubMed] [Google Scholar]
- 3. Llarrull LI, Toth M, Champion MM, Mobashery S. 2011. Activation of BlaR1 protein of methicillin-resistant Staphylococcus aureus, its proteolytic processing, and recovery from induction of resistance. J. Biol. Chem. 286:38148–38158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García-Castellanos R, Mallorquí-Fernández G, Marrero A, Potempa J, Coll M, Gomis-Rüth FX. 2004. On the transcriptional regulation of methicillin resistance: MecI repressor in complex with its operator. J. Biol. Chem. 279:17888–17896 [DOI] [PubMed] [Google Scholar]
- 5. Hiramatsu K, Asada K, Suzuki E, Okonogi K, Yokota T. 1992. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS Lett. 298:133–136 [DOI] [PubMed] [Google Scholar]
- 6. Papworth C, Bauer JC, Braman J, Wright DA. 1996. QuikChange site-directed mutagenesis. Strategies 9:3–4 [Google Scholar]
- 7. Oliveira DC, de Lencastre H. 2011. Methicillin-resistance in Staphylococcus aureus is not affected by the overexpression in trans of the mecA gene repressor: a surprising observation. PLoS One 6:e23287 doi:10.1371/journal.pone.0023287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kraemer GR, Iandolo JJ. 1990. High-frequency transformation of Staphylococcus aureus by electroporation. Curr. Microbiol. 21:373–376 [Google Scholar]
- 9. Oshida T, Tomasz A. 1992. Isolation and characterization of a Tn551-autolysis mutant of Staphylococcus aureus. J. Bacteriol. 174:4952–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Lencastre H, Sa Figueiredo AM, Urban C, Rahal J, Tomasz A. 1991. Multiple mechanisms of methicillin resistance and improved methods for detection in clinical isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 35:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filee P, Benlafya K, Delmarcelle M, Moutzourelis G, Frere JM, Brans A, Joris B. 2002. The fate of the BlaI repressor during the induction of the Bacillus licheniformis BlaP beta-lactamase. Mol. Microbiol. 44:685–694 [DOI] [PubMed] [Google Scholar]


